
Preparation of Ti-based amorphous brazing alloy
ZOU Jia-sheng(邹家生)1,2, JIANG Zhi-guo(蒋志国)1, XU Zhi-rong(许志荣)1, CHEN Guang(陈 光)2
1. Provincial Key Lab of Advanced Welding Technology,
Jiangsu University of Science and Technology, Zhenjiang 212003, China;
2. Department of Materials Science and Engineering,
Nanjing University of Science and Technology, Nanjing 210094,China
Received 10 April 2006; accepted 25 April 2006
Abstract:
A new kind of amorphous active brazing alloy foil with the composition of Ti40Zr25Ni15Cu20 was successfully synthesized using melt spinning in roll forging machine in argon atmosphere. The amorphous structure and composition were examined by X-ray diffraction, differential thermal analysis and energy dispersive X-ray detector. The results show that the Ti40Zr25Ni15Cu20 amorphous alloy foil has excellent wettability on Si3N4 ceramic and demonstrate a strong glass forming ability. The reduced glass transition temperature (Trg) and the temperature interval of supercooled liquid region before crystallization are 0.76 and 78 K, respectively.
Key words:
Si3N4 ceramic; Ti based brazing alloy; glass forming ability; glass transition temperature;
1 Introduction
Si3N4 is an important engineering and structural ceramic that has been widely used in the areas of thermal machine, wear resistant elements and heat exchangers[1]. Si3N4 ceramic bonding technique was extensively studied in order to use ceramics more effectively in industry applications. Because of simple craft, high brazing strength, good repetitiveness, perfect adaptability of joint size and form, and low costs, active brazing is the best choice in ceramic/metal bonding methods. However, the study and development of high temperature active brazing filler in this field is the key problem[2].
In recent years, instead of crystal alloy fillers, amorphous active solder has become one of the most important headings in the fields of ceramic/ceramic and ceramic/metal bonding. And it has attracted extensive attention[3-5].
Amorphous alloy, a material at unsteady state, can boost atomic diffusion and surface reaction during the high temperature brazing process. In addition, it can decrease brazing temperature so as to reduce residual stress in the joint and enhances the joint strength. But it doesn’t decrease heat-resisting temperature after brazing. Active elements such as Ti added into amorphous alloy filler can strengthen diffusion. Through adjusting of the components in amorphous, active elements can diffuse to the surface and be consumed, which avoids formation of brittle compounds and improve the joint strength. Furthermore, amorphous foils can solve the problem of machining of brittle brazing alloy.
In this work, high temperature active Ti-Zr-Ni-Cu amorphous brazing foils were produced by melt spinning. The structure, property and composition of the brazing alloys were examined by X-ray diffraction (XRD), differential thermal analysis (DTA) and energy dispersive X-ray spectroscope(EDX).
2 experimental
2.1 Constituent selection of amorphous active brazing material
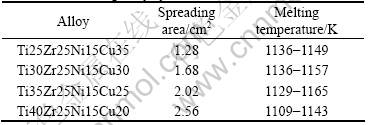
In high-temperature active brazing foils, Ti and Zr elements are the most effective active element. Combined with the former studies, Cu-Ti(Zr) was used to be master alloy system in this work. In order to improve the heat-resistant property of the joint, Ni was added into the Cu-Ti(Zr) alloy system. In addition, previous results showed that Cu-Ti, Cu-Zr, Ni-Zr, Ni-B,Ti-Cu-Ni melts could be solidified to form an amorphous alloy under certain condition[6]. So, Ti-Zr-Ni-Cu alloy system was selected, and its component is shown in Table 1. Master alloy was smelted in the non-consumable vacuum melting furnace. After Cu-Ti(Zr) alloys dispersed, spreading experiments were carried out with 0.1 mg massive solder, respectively, to value the infiltrative of solder on Si3N4. When brazing temperature was 1323K and holding time was 15 min, the spreading area and fusing temperature of solders are shown in Table 1. Ti40Zr25Ni15Cu20 brazing alloy has the largest spreading area.
Table 1 Spreading area and melting temperature of Ti-Zr-Ni-Cu brazing alloy system
2.2 Preparation of amorphous brazing alloy
Amorphous Ti40Zr15Ni25Cu20 alloy foil was prepared using HVDS-Ⅱplaner flow casting method in which the foil was made by rapid thermal conductivity of cooled roller with a high speed chill roll to draw the alloy into thin film. According to the orientation of fused mass alloy to cool roller, it can be classified as two types, i.e. free-jet melt spinning (FJMS) and planar flow casting (PFC). The main difference between these two techniques is that in the former, the crucible nozzle is located relatively near from the wheel. With rapider cooling rate, the thinner foil can be produced, but it only suites the narrowband. On the contrary, in the latter, the outlet of alloy liquidness is nearer to the roller so that fused bath is formed between the single roller and muzzle. The fused bath has buffer effect on the liquidness in order to get the more homogeneous foil. Consequently, PFC was used in this work.
After master alloy was dispersed, about 20 g was added into quartz glass tub of PFC. Homogeneous molten alloy, obtained by heating the melt to 1 773- 1 873 K using high frequency induction, was sprayed onto the surface of cool roller with high velocity to form a foil due to down quenching. The schematic diagram of injection casting using single copper roller is shown in Fig.1.
To get the good quality of amorphous brazing materials, the key is to control the thickness of spraying foil. According to empirical equation[7]:
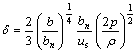 (1)
(1)
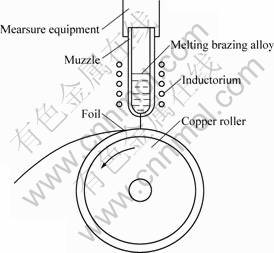
Fig.1 Schematic diagram of injection casting using single copper roller
where δ is the thickness of foil, bn is the separation between surface of roller and air spout, b is the clearance width of air spout, us is linear speed of roller, p is argon pressure when ejecting, ρ is superheating density at 373-473 K. From Eqn.(1), we can get that δ can be decreased by controlling bn, b, p and the increment of us. The optimized processing parameters for getting Ti40Zr25Ni15Cu20 amorphous foil can be obtained by experiments: us=30 m/s; b=3-5 mm; bn=1.0 mm; p= 48 kPa.
Under those conditions, the thickness of foil is (0.040±0.002) mm(average value sample of 10 times) according to the above technological operation. And it has perfect degree of finish on the surface, bilateral smooth and good ductility.
3 Results and discussion
3.1 Components
Since 1988, Inoue studied glass forming ability (GFA) of multi-alloy, i.e. 1)multi-component alloy systems consisting of more than three elements; 2) significantly different atomic size ratios above about 12% among the main constituent elements, 3) negative heats of mixing among the elements. The base composition in the presented alloys is the Ti-Zr-Ni-Cu system, which has higher negative heats of mixing[8]. Atomic radius of every element is shown in Table 2.
3.2 Technological conditions
According to Eqn.(1), an, δ, us and p need to be adjusted in response to the thickness and width of the amorphous alloy foil. Besides the above parameters, the temperature of the melting alloy and the time of muzzle dropped from heating position to the job position have very great influence on the amorphous forming ability. With higher heating temperature and the shorter time, super-cooled result can be strengthened and the thickness of the foil can be reduced further. This research succeeded in making the amorphous alloy of Ti40Zr25Ni15Cu20 under the situation of the temperature of the melting alloy between 1 773-1 873 K and the time less than 1 s.
Table 2 Comparation with atomic radius

3.3 Discussion
Fig.2 shows the XRD pattern of Ti40Zr25Ni15Cu20 alloy. There is only a spacious diffraction peak on the curve and no obvious diffraction peak corresponding to any crystallization is observed. After polishing this sample surface, XRD pattern of the centre region is the same as the former, so it indicates the whole sample is amorphous alloy.
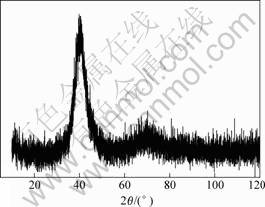
Fig.2 XRD pattern of Ti40Zr25Ni15Cu20 alloy
The DTA curve of Ti40Zr25Ni15Cu20 alloy is shown in Fig.3. It can be seen that the solidus temperature Ts is 1 119 K and the liquidus temperature Tl is 1 143 K.
TURNBULL[9] suggested a method to value amorphous forming ability according to the classical forming core theory. He used the ratio Trg=Tg/Tm to evaluate the amorphous forming ability of alloy system, Tg is the glass changing temperature and Tm is the melting temperature of the alloy. This ratio comes from dynamics mechanism of amorphous forming, and means that viscidity of the melting alloy must be great enough in the temperature range between Tg and Tm in order to reduce the nucleation rate and growth rate. The larger Trg,

Fig.3 DTA curve for Ti40Zr25Ni15Cu20 alloy
the heavier viscidity of alloy in the nose of the TTT or CCT curve, i.e. the easier in forming amorphous alloy. If Trg >2/3, the average nucleation rate of the alloy will become much lower in the district of super-cooling liquid, thus bulk amorphous alloy could be obtained. From Fig.3, Tg and Trg are estimated to be 813K and 0.76, respectively. INOUE et al[10-12] used difference (ΔTx=Txl-Tg) between crystal temperature and glass changing temperature, i.e. the width of the liquid phase region, to define the stability of the super-cooling liquid. Investigations in recent years demonstrated that almost all the bulk amorphous alloy systems had very large ΔT which is greater than 50 K. Fig.3 displays the Txl=891 K and ΔTx=78 K, indicating that Ti40Zr25Ni15Cu20 alloy has strong amorphous forming ability.
EDX analysis of the amorphous brazing foil was also carried out at three random points. The results are listed in Table 3. It clearly shows that the amorphous brazing foil has very good composition homogeneity.
Table 3 Component of Ti40Zr25Ni15Cu20 brazing alloys at different three points

4 Conclusions
1) Composition Ti40Zr25Ni15Cu20 is confirmed as the amorphous brazing alloy according to the brazing ability of Si3N4 and the requirement of the amorphous forming ability.
2) Brazing ribbon sample of Ti40Zr25Ni15Cu20 alloy was produced by melt spinning in roll forging machine in argon atmosphere. This sample is proved to be amorphous after examined by XRD, DTA and EDX.
3) The reduced glass transition temperature (Trg) and the temperature interval of super-cooled liquid region before crystallization are 0.76 and 78 K, respectively, so it has good GFA.
References[1] HUANG Yong, WU Jian-guang. The current situation and development trend of the high-performance structure ceramic[J]. Material Science Progress, 1990, 4(2): 150-160.(in Chinese)
[2] REN Jia-lie, WU Ai-ping. Connection of the Advanced Material[M]. Beijing: China Machine Press, 2000.( in Chinese)
[3] ZOU Jia-sheng, XU Zhi-rong, CHEN Guang. Characteristic and application of the amouphous welding materials[J]. Material Report, 2004, 18(4): 51-53( in Chinese)
[4] NAKA M, TANAKA T, OKAMOTO I. Application of amorphous Cu-Ti filler metal to joining of silicon nitride[J]. Transactions of the Japan Welding Society, 1990, 21(1): 66-72.(in Japanese)
[5] REN Jia-lie, ZHAI Yang. Research of supercooled material used for connecting ceramic[J]. Material Craft of Space Flight, 1995(3): 11-13.( in Chinese)
[6] CHEN Guang, FU Heng-zhi. Solidify the New-Type Metal Material of Non-Equilibriumly[M]. Beijing: Science Press, 2004.(in Chinese)
[7] LI Yue-zhu. Fast Solidification Technology And Materials[M]. Beijing: National Defense Industry Press, 1993.(in Chinese)
[8] LI Wei-huo. Research of Multielement Amorphous Forming Ability And Mechanics Performance[D]. Shanghai: Shanghai University, 2002.
[9] TURNBULL D. Dependence of crystallization rate on amorphous structure[J]. Journal of Non-crystalline Solids, 1985, 75(1-3): 197-207.
[10] INOUE A, KOSHIBA H, ZHANG T, MAKINO T. Thermal and magnetic properties of Fe50Co7Ni7Zr10-xNbxB20 amorphous alloys with wide supercooled liquid range[J]. Mater Trans, 1997, 38: 577-582.(in Japanese)
[11] INOUE A, GOOK J S. Multicomonent Fe-based glassy alloys with wide supercooled liquid region before crystallization[J]. Mater Trans, 1995, 36: 1180-1183.
[12] INOUE A, ZHANG T, ITOI T, TAKEUCHI A. New Fe-Co-Ni-B amorphous alloys with wide supercooled liquid region and good soft magnetic properties[J]. Mater Trans, 1997, 38: 359-362.(in Japanese)
Foundation item: Project(BK2003045) supported by the Natural Science Foundation of Jiangsu Province, China; Project(03KJB430006) supported by the Natural Science Foundation of High School of Jiangsu Province
Corresponding author: ZOU Jia-sheng; Tel: +86-13305280882; E-mail: zjzoujs@public.zj.js.cn


