J. Cent. South Univ. Technol. (2007)05-0651-05
DOI: 10.1007/s11771-007-0125-x ![]()
Modification of natural graphite using pitch through
dynamical melt-carbonization
ZHOU You-yuan(周友元), LI Xin-hai(李新海), GUO Hua-jun (郭华军),
WANG Zhi-xing(王志兴), YANG Yong(杨 勇), XIE Qiao-ling(谢巧玲)
(School of Metallurgical Science and Engineering, Central South University, Changsha 410083, China)
Abstract:The graphite was modified using pitch through dynamical melt-carbonization, and the effects of modification temperature and the amount of pitch on the characteristics of graphite were investigated. The structure and characteristics of the graphite were determined by X-ray diffractometry(XRD), scanning electron microscopy(SEM), particle size analysis and electrochemical measurements. The results show that the modified graphite has a disordered carbon/graphite composite structure, larger average particle diameter, greater tap density, and better electrochemical characteristics than the untreated graphite. The sample coated with 10% pitch dynamical melt-carbonized at 400 ℃ for 3 h and heat-treated at 850 ℃ for 2 h has better electrochemical performances with a reversible capacity of 360.5 mA?h/g, a irreversible capacity of 41.0 mA?h/g, and an initial coulombic efficiency of 89.8% compared with natural graphite and disordered carbon. The cycling stability of the Li/C cell with modified graphite as anodes is improved, and its capacity retention ratio at the 30th cycle is up to 94.37%.
Key words:
lithium ion battery; graphite; dynamical melt-carbonization; anode ;
1 Introduction
Lithium ion batteries have attracted worldwide attention and been developed rapidly due to their high energy density, good charge-discharge performances and long cycle life[1-4]. Worldwide efforts have been devoted to the study of carbon materials as anodes in these batteries[5-6]. Graphite is an attractive material for anodes of lithium ion batteries for its high reversible capacity (372 mA?h/g in theory), low and flat discharge potential (0.1-0.2 V(vsLi))[7-10]. Nevertheless, there are still some problems in the application with respect to a low practically available capacity, and a large irreversible capacity during the initial charge/discharge cycle and the poor cycling performance[11-12]. Much effort has been focused on improving capacity of carbon anodes during the past few years[13-15].
In the present work, disordered carbon/graphite composite materials were obtained by coating natural graphite with the pitch and following with heat-treatment in an argon gas atmosphere. The structure and performances of the composite graphite material as anodes in Li/C cells were investigated.
2 Experimental
2.1 Thermal gravimetric analysis of pitch
A Mettler Toledo TGA/ SDTA851e thermal
gravimetric analyzer was used to measure the relative mass loss of the pitch vs. temperature during carbonization. The sample was held in a platinum pan and heated up to 800 ℃ at 10 ℃/min in a nitrogen flow of 30 mL/min.
2.2 Preparation of composite graphite and disordered
carbon
In a kneader, the finely ground natural graphite powder was well mixed with pitch (10 % for sample A and 15% for sample B). Samples A and B were rotated constantly and heat-treated at 400 ℃ for 3 h in air, and then heat-treated at 850 ℃ for 2 h in argon gas atmosphere and furnace-cooled to room temperature.
For comparison, the disordered carbon sample was prepared using pitch treated at 850 ℃ for 2 h in argon gas atmosphere.
2.3 Characterization of composite graphite
XRD measurements were made with a Rigaku diffractometer equipped with Cu Kα radiation. SEM images were obtained with a JEOL JSM6380 spectrometer. The average particle diameters of the graphite samples were measured using Malvern Mastersizer particle size analyzer. Tap density was measured using a vessel by constantly vibrating.
The composite materials, acetylene black as electric conductor and poly vinylidene difluoride (PVDF) as binder were mixed. The graphite anodes were prepared by spreading the above mixture onto a copper foil sub- strate. And then the anodes were dried overnight at 105 ℃ in vacuum. A Celgard 2300 porous membrane of 20 μm thickness was used as a separator, and the electrolyte was 1 mol/L LiPF6 dissolved in a mixture of ethylene carbonate (EC) and dimethylcarbonate(DMC) with a volume ratio of 1?1. Li/C cells were assembled in an argon-filled glove box. The electrochemical characteristics of the graphite anodes in Li/C cells were tested at a constant current density of 0.1 mA/cm2 between 0.001 and 2.000 V.
For comparison, the natural graphite and disordered carbon anodes were prepared by the same method above.
3 Results and discussion
3.1 Thermo-gravimetric analysis of pitch
Thermo gravimetric(TG) and differential thermal analysis (DTA) data for primary pitch are shown in Fig.1. The sample was heated in N2 atmosphere at 10 ℃/min. There are several mass loss steps for the primary pitch in the temperature range of 25-800 ℃. The endotherm and the corresponding mass loss centered around 200 ℃ in Fig.1 are due to the loss of superficial moisture and organic solvent from the pitch. A second endotherm appears between 200 and 380 ℃, and it is accompanied by a mass loss associated with the carbonization of the pitch. At 380-470 ℃, a plateau appears, which shows that the pitch is carbonized and the oxidation of carbon is not so obvious. The mass loss occurs mostly at 470-670 ℃, which is indicated by the intense peak of the DTA curve in Fig.1. They are attributed to the oxidation and burning of carbons.
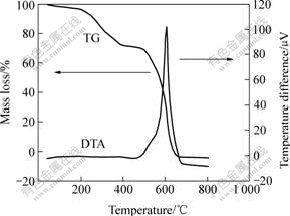
Fig.1 TG-DTA curves for pitch
3.2 Characterization of composite graphite
Fig.2 shows XRD patterns of natural graphite and the modified graphite heat-treated at 400 ℃. The results show the existence of disordered carbon in the composite graphite materials. For samples A and B, the intensity ratios of (100) and (101) peaks to those of (002) and (004) peaks are larger compared to sample S0, indicating that the stacking of carbon on the surface of graphite is decreased because of a thin film of disordered carbon coated on the graphite.

Fig.2 XRD patterns for natural graphite (sample S0) and composite graphite materials (samples A and B)
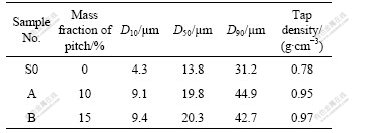
Table 1 shows the characteristic particle size and tap density of the samples after heat-treated at 400 ℃. The average diameters of composite graphite materials are larger than that of the untreated natural graphite. The increase of average diameter is attributed to the reunion of some graphite particles and the reduction of small particles resulted from the modification. The formation of the disordered carbon film on the surface of graphite results in much larger surface area. The composite graphite has greater tap density for its smooth surface morphology and suitable particle size distribution, which are favorable for tight stack.
Table 1 Characteristic particle size and tap density of samples after heat-treated at 400 ℃ for 3 h
SEM images of the untreated natural graphite and composite graphite sample are shown in Fig.3. It can be seen that surface morphology of the graphite is retained and becomes smooth after disordered carbon coating. Furthermore, the amount of small particles decreases and the particle sizes increase after disordered carbon coating, which is also shown in Table 1. Therefore, a thin film of disordered carbon is formed on the surface of graphite by carbonization of the pitch after treated at 400 ℃. In other words, a new graphite material with disordered carbon/graphite composite structure is obtained.
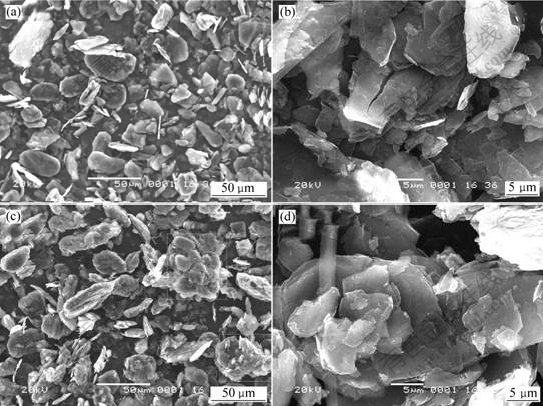
Fig.3 SEM images of natural graphite and composite graphite material
(a) Natural graphite, low magnification; (b) Natural graphite, high magnification;
(c) Composite graphite material, low magnification; (d) Composite graphite material, high magnification
3.3 Electrochemical performances
Fig.4 shows the first discharge/charge properties of natural graphite anode, composite graphite anode treated at 400 ℃ for 3 h and disordered carbon anode. Compared with the untreated graphite sample and the disordered carbon sample, the composite graphite samples have larger discharge capacity (lithium intercalation capacity, Cins), larger charge capacity (reversible capacity for lithium de-intercalation, Crev), higher coulombic efficiency(Crev/Cins) and smaller irreversible capacity(Cirrev, the difference between Cins and Crev). Among the graphite samples, the composite graphite of 10% pitch treated at 400 ℃ for 3 h has the best electrochemical performances with a reversible capacity of 377.6 mA?h/g, a irreversible capacity of 90.9 mA?h/g, and a initial coulombic efficiency of 80.6%, while those of the untreated graphite are 352.5 mA?h/g, 104.1 mA?h/g and 77.2%, and those of the disordered carbon are 319.9 mA?h/g, 161.9 mA?h/g, and 66.4%, respectively.

Fig.4 Initial charge/ discharge curves of natural graphite, composite graphite and disordered carbon anodes
1―10% pitch, discharge; 2―10% pitch, charge; 3―15% pitch, discharge; 4―15% pitch, charge; 5―Natural graphite, discharge; 6―Natural graphite, charge; 7―Disordered carbon, discharge; 8―Disordered carbon, charge
Electrochemical performances of composite graphite anodes at different temperatures are shown in Fig.5. After being heat-treated, the discharge capacity and charge capacity of the graphite anodes decrease a little, but the change of discharge capacity is much faster than that of charge capacity. Therefore, irreversible capacity of the graphite anodes decreases and the initial coulombic efficiency rises. The discharge capacity of the composite graphite anodes treated at 850 ℃ for 2 h is 401.5 mA?h/g for sample A and 426.3 mA?h/g for sample B, respectively, and the irreversible capacity reduces to 41.0 mA?h/g and 59.7 mA?h/g, while the initial coulombic efficiency increases to 89.8% and 86.0%, respectively. Furthermore, the graphite anode treated at higher temperature has lower average charge potential with charge capacity increasing in the low potential range and reducing in the high potential range. The ratio of charge capacity near 0.2 V to the total charge capacity for the graphite anode rises.

Fig.5 Initial charge/discharge curves of composite graphite anodes treated at different temperatures
(a) 10% pitch; (b) 15% pitch
1―400℃, discharge; 2―400 ℃, charge;
3―850℃, discharge; 4―850 ℃, charge
Cycling performances of natural graphite, disordered carbon and composite graphite samples treated at 850 ℃ for 2 h are shown in Fig.6. It shows that the composite graphite anodes have improved cycling performance. After 30 charge/discharge cycles, the composite graphite coated with 10% pitch after heat-treated at 850 ℃ for 2 h has the best electrochemical characteristics among the graphite anodes. The capacity retention ratios of natural graphite
and composite graphite anodes are 68.96% and 94.37%, respectively. The improved performances are attributed to the better solid electrolyte inter-face(SEI) film formed on the surface of composite graphite anode at the first charge/discharge cycle. The electrochemical performance of composite graphite anode is remarkably affected by the structure and characteristics of the SEI film. It is reported that the edge sites of graphite hexagonal arrays are the more active (catalytic) sites for electrolyte decomposition than those at basal plane[16-18]. In the case of graphite, the hexagonal arrays form well-developed layer structures, and there are many differences between the basal plane and the edge sites. As to the composite graphite materials, the graphite is coated with a layer of disordered carbon, and the uniformity of surface structure is improved because of the well-distributed edge sites. Therefore, a thin, even and stable SEI film can be formed for the composite graphite during the first charge/discharge cycling, which depresses the decomposition of electrolyte and keeps structure of graphite from deterioration. So low irreversible capacity loss and improved cycling capability are obtained for the composite graphite anodes.
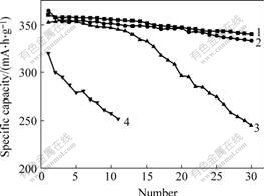
Fig.6 Cycling performances of natural graphite, disordered carbon and composite graphite
1—Sample B, 850 ℃; 2—Sample A, 850 ℃; 3—Natural graphite; 4—Disordered carbon
4 Conclusions
1) The graphite was modified using pitch through dynamical melt-carbonization. After the modification, the graphite has larger particle sizes and greater tap density than the untreated graphite.
2) The electrochemical properties of the graphite are improved obviously by the modification. The modified graphite coated with 10% pitch heat-treated at 850 ℃ for 2 h has better electrochemical performances with a reversible capacity of 360.5 mA?h/g, irreversible capacity of 41.0 mA?h/g, and initial coulombic efficiency of 89.8% compared with natural graphite and disordered carbon.
3) The Li/C cells with modified graphite as anodes can improve the cycling stability. After 30 charge/ discharge cycles, the capacity retention ratio of composite graphite anode reaches 94.37%.
References
[1] GUO Hua-jun, LI Xin-hai, WANG Zhi-xing, et al. Mild oxidation treatment of graphite anode for Li-ion batteries[J]. J Cent South Univ Technol, 2005, 12(1): 50-54.
[2] HONGYU W, MASAKI Y, TAKESHI A, et al. Characterization of carbon-coated natural graphite as a lithium ion battery anode material[J]. J Electrochem Soc, 2002, 149(4): 499-503.
[3] GUO Hua-jun, LI Xin-hai, WANG Zhi-xing, et al. Effect of lithium or aluminum substitution on the characteristics of graphite for anode of lithium ion batteries[J]. Rare Metals, 2003, 22(4): 280-284.
[4] KATSUNORI Y, ATSUSHI Y, YOSHINORI K, et al. Carbon hybrids graphite hard carbon and graphite coke as negative electrode batteries materials for lithium secondary batteries charge/discharge characteristics[J]. J Electrochem Soc, 2002, 149(7): A804-A807.
[5] HOSSAIN S, YONGKYU K, SALEH Y, et al. Comparative studies of MCMB and C-C composite as anodes for lithium-ion systems[J]. J Power Sources, 2003, 114(2): 264-276.
[6] FEY T K, LEE D C, LIN Y Y, et al. High-capacity disordered carbons derived from peanut shells as lithium-intercalating anode materials[J]. Synthetic Metals, 2003, 139(1): 71-78.
[7] SHI Hang. Coke vs. graphite as anodes for lithium-ion batteries[J]. J Power Sources, 1998, 75(1): 64-72.
[8] MENACHEM C, WANG Y, FLOWERS J, et al. Characterization of lithiated natural graphite before and after mild oxidation[J]. J Power Sources, 1998, 76(2): 180-185.
[9] HUANG Hong, LIU Wei-teng, HUANG Xue-jie, et al. Effect of a rhombohedral phase on lithium intercalation capacity in graphite[J]. Solid State Ionics, 1998, 110(3/4): 173-178.
[10] SUZUKI K, HAMADA T, SUGIURA T. Effect of graphite structure on initial irreversible reaction in graphite anodes[J]. J Electrochem Soc, 1999, 146(3): 890-897.
[11] BEGUIN F, CHEVALLIER F, VIX C, et al. A better understanding of the irreversible lithium insertion mechanisms in disordered carbons[J]. Journal of Physics and Chemistry of Solids, 2004, 65(2/3): 211-217.
[12] YOSHIO M, WANG H, FUKUDA K, et al. Effect of carbon coating on electrochemical performance of treated natural graphite as lithium-ion battery anode material[J]. J Electrochem Soc, 2000, 147(4): 1245-1250.
[13] DING Y S, LI W N, SANTO I, et al. Characteristics of graphite anode modified by CVD carbon coating[J]. Surface & Coatings Technology, 2006, 200(9): 3041-3048.
[14] SHU Jie, LI Hong, YANG Rui-zhi, et al. Cage-like carbon nanotubes/Si composite as anode material for lithium ion batteries[J]. Electrochemistry Communications, 2006, 8(1): 51-54.
[15] GUO Hua-jun, LI Xin-hai, WANG Zhi-xing, et al. Si-doped composite carbon as anode of lithium ion batteries[J]. Transactions of Nonferrous Metals Society of China, 2003,13(5): 1062-1065.
[16] CHUNG G C, JUN S H, LEE K Y, et al. Effect of surface structure on the irreversible capacity of various graphitic carbon electrodes[J]. J Electrochem Soc, 1999, 146(5): 1664-1671.
[17] ZAGHIB K, NADEAU G, KINOSHITA K. Effect of graphite particle size on irreversible capacity loss[J]. J Electrochem Soc, 2000, 147(6): 2110-2115.
[18] WANG Guo-ping, ZHANG Bo-lan, YUE Min, et al. A modified graphite anode with high initial efficiency and excellent cycle life expectation[J]. Solid State Ionics, 2005, 176(9/10): 905-909.
Foundation item: Project(50302016) supported by the National Natural Science Foundation of China
Received date: 2006-10-20; Accepted date: 2006-12-23
Corresponding author: LI Xin-hai, PhD, Professor; Tel: +86-731-8836633; E-mail: xhli@mail.csu.edu.cn
(Edited by ZHAO Jun)


