
J. Cent. South Univ. (2020) 27: 3567-3580
DOI: https://doi.org/10.1007/s11771-020-4569-6
Recovery of lead and silver from zinc acid-leaching residue via a sulfation roasting and oxygen-rich chlorination leaching method
WANG Rui-xiang(王瑞祥), YANG Yu-dong(杨裕东), LIU Cha-xiang(刘茶香), ZHOU Jie(周杰),
FANG Zhuang(方壮), YAN Kang(严康), TIAN Lei(田磊), XU Zhi-feng(徐志峰)
School of Metallurgical and Chemical Engineering, Jiangxi University of Science and Technology, Ganzhou 341000, China
 Central South University Press and Springer-Verlag GmbH Germany, part of Springer Nature 2020
Central South University Press and Springer-Verlag GmbH Germany, part of Springer Nature 2020
Abstract:
A large amount of acid-leaching residue is produced during the conventional Zn hydrometallurgy process, and this residue has a large concentration of a variety of valuable metals. The purpose of this study was to evaluate the ability of a procedure that entails the use of sulfation roasting, water leaching, and chlorination leaching (blowing oxygen technique) to recover Pb and Ag, followed by cooling crystallization and the replacement of Ag with lead sheet, to realize the full recovery of all valuable metals from zinc acid-leaching residue; consequently, good results were achieved. The best results were obtained under the following conditions: a sulfuric acid at 70% of the raw material quality, roasting temperature of 300 °C and roasting time of 2 h, followed by the process of leaching the roasted residue for 1 h by applying a water-to-solid ratio of 5:1 at room temperature. The recovery rates of Zn and Fe were 98.69% and 92.36%, respectively. The main parameters of the chlorine salt leaching system were as follows: Cl- concentration of 300 g/L, Fe3+ concentration of 25 g/L, acid concentration of 2 mol/L, liquid-to-solid ratio of 9 mL:1 g, temperature of 90 ℃, and leaching time of 0.5 h; this leaching process was followed by filtration separation. These conditions resulted in high extents of leaching for Pb and Ag (i.e., 98.87% and 96.74%, respectively). Finally, the kinetics of the process of Ag leaching using Cl- ions in an oxygen-rich medium was investigated. It was found that the leaching process was controlled by the diffusion of the product layers, and the activation energy was 19.82 kJ/mol.
Key words:
acid-leaching residue; sulfation roasting; chlorine salt; Pb, Ag; kinetics;
Cite this article as:
WANG Rui-xiang, YANG Yu-dong, LIU Cha-xiang, ZHOU Jie, FANG Zhuang, YAN Kang, TIAN Lei, XU Zhi-feng. Recovery of lead and silver from zinc acid-leaching residue via a sulfation roasting and oxygen-rich chlorination leaching method [J]. Journal of Central South University, 2020, 27(12): 3567-3580.
DOI:https://dx.doi.org/https://doi.org/10.1007/s11771-020-4569-61 Introduction
The process of conventional zinc hydrometallurgy produces a large amount of acid-leaching residue [1-3], which not only contains Cu, Pb, Zn, Fe, and other elements, but also precious metals such as Ag. It is still common in industry to apply the technique of stockpiling to acid-leaching residue [4]; however, this technique generates secondary resource waste and heavily pollutes the environment [5-7]; therefore, it is of practical significance to investigate the recovery of valuable metals in Zn acid-leaching residue at a time when mineral resources are becoming increasingly exhausted.
Recently, treatment methods have been developed for the recovery of valuable metals from Zn acid-leaching residue [8]; generally, these methods entail the use of hydrometallurgical, pyrometallurgical, flotation, and pyro- hydrometallurgical processes, as well as the dressing-metallurgy combination process. ZHANG et al [9] studied the leaching behavior of lead and silver from lead sulfate residues in NaCl-CaCl2- NaClO3 media; the extents of leaching for lead and silver were more than 98% and 95%. APARAJITH et al [10] applied acid roasting, warm-water leaching, alkali roasting, and washing to a Ag concentrate obtained via froth flotation to obtain an enriched Pb-Ag residue that could be consumed by a Pb smelter to eventually produce metallic Pb and Ag. ZHENG et al [11] obtained concentrates by performing reduction roasting with coal powder, followed by a flotation treatment. The Pb, Zn, and Ag recover rates were 48.38%, 68.23%, and 77.41%, respectively. XING et al [12] investigated the asynchronous leaching process by using acid-chlorination leaching; consequently, the extents of leaching for Pb and Ag reached 97% and 85%, respectively. BEHNAJADY et al [13] used sodium chloride and calcium hypochlorite as leaching and oxidizing reagents, respectively. Under optimized conditions, this resulted in Pb and Ag extraction rates of 93.60% and 49.21%, respectively. RODRIGUEZ et al [14] developed a novel process to recover Pb and Ag from zinc leaching residue by using methanesulfonic acid; under optimized conditions, nearly 80% of lead and silver can be recovered. Regarding the pyro-hydrometallurgical process, JIANG et al [15] employed a combination of sulfation roasting and water leaching to recover the valuable metals from Zn leaching residue, but this process did not involve the recovery of Pb or Ag. The pyrogenic process for acid-leaching residue typically feeds directly into a Pb smelting system, primarily via the rotary kiln volatilization method; therefore, the percentage of Pb recovery can exceed 80%, whereas only approximately 35% of the total amount of Ag can be recovered.
With the above-mentioned processes, it is difficult to concurrently achieve high recovery rates for Pb and Ag. This is especially true for pyrometallurgical processes, which tend to focus on the recovery of Pb, resulting in a recovery rate for Ag that is too low; furthermore, the cost of equipment and energy consumption related to this process is relatively high. With hydrometallurgical processes, although the recovery of Ag is comparatively better, the process is cumbersome, costly, and time-consuming. Therefore, based on the phase characteristics of acid-leaching residue, the authors have developed a method that entails the following processes: sulfation roasting, water leaching, and oxygen-rich chlorination leaching.
2 Experimental
2.1 Materials
The zinc acid-leaching residue used in this study was obtained from a Zn hydrometallurgy factory in Northern China; the particle size was 74-110 μm. It is referred to in this study as Pb-Ag residue; its composition and main elements are detailed in Table 1.
Table 1 Main elemental composition of Pb-Ag residue (mass fraction, %)

From Table 1, it can be seen that the contents of Fe, Pb, and Zn were high, and thus recyclable. In addition, the content of Ag was sufficient to satisfy industrial recycling requirements.
2.2 Experimental procedure
2.2.1 Process and reactions
The experiments for the extraction of Zn, Fe, Pb and Ag from the zinc acid-leaching residue were carried out by a sulfation roasting and oxygen-rich chlorination leaching. The process flow sheet of the experiments is shown in Figure 1.
This study was aimed to entails the use of sulfation roasting, water leaching, and chlorination leaching (blowing oxygen technique) to recover Zn, Fe, Pb and Ag, followed by cooling crystallization and the replacement of Ag with lead sheet, to realize the full recovery of all valuable metals from zinc acid-leaching residue.
The zinc ferrite and sulfide which were difficult to decompose in zinc acid-leaching residue react with concentrated sulfuric acid to form the corresponding sulfate by sulfate roasting.
After the roasted residue was washed with water, zinc and iron enter the solution, and lead and silver were enriched in the residue in the form of sulfate. For the chlorination leaching process, hydrochloric acid is the main leaching reagent, ferric chloride is the oxidizing reagent, and sodium chloride provides excess Cl- ions. The main reactions are as follows:
3PbSO4+2FeCl3=3PbCl2↓+Fe2(SO4)3 (1)
3Ag2SO4+2FeCl3=6AgCl↓+Fe2(SO4)3 (2)
Ag+Fe3++Cl-=AgCl+Fe2+ (3)
The chloride precipitate generated in the solution and the excess chloride ion will form a coordination complex which is stable in the solution, as follows:
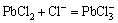 (4)
(4)
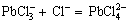 (5)
(5)
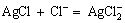 (6)
(6)
 (7)
(7)
2.2.2 Sulfation roasting and water leaching
The sulfation roasting testing was carried out in a muffle furnace. The zinc acid-leaching residue and concentrated sulfuric acid were uniformly mixed in a stainless steel bowl according to a certain liquid-solid ratio. Next, the mixture was crushed, placed in a muffle furnace; then the temperature was heated to the appropriate temperature at a rate of 5 °C/min, and kept for a set time. Oxygen was added into the furnace to keep oxygen atmosphere. After finishing the roasting process, the air continued to be forced into the muffle furnace until the roasting temperature naturally dropped to 50 °C. Finally, the residue was taken out of the muffle furnace for water leaching. The water leaching residue was sent for sample analysis to investigate the effects of sulfation roasting on the leaching of Zn and Fe.
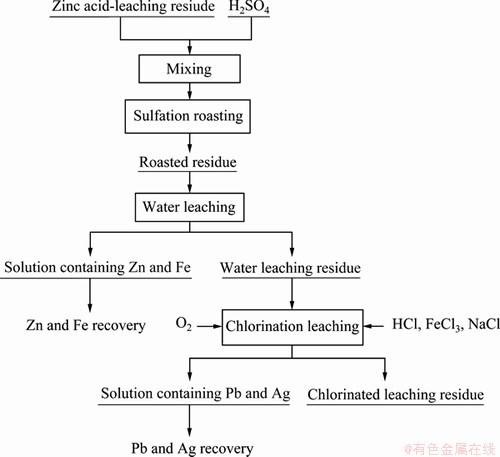
Figure 1 Diagram of experimental procedure
2.2.3 Oxygen-rich chlorination leaching
The oxygen-rich chlorination leaching experiment was conducted in a beaker. Heating and stirring were provided by a thermostat water bath. Typically, a known amount of leaching solution (FeCl3+HCl+NaCl system) was heated to the desired temperature, and oxygen was bubbled into the solution using an air pump. Then a certain amount of water washing residue was slowly added to the solution with stirring. Record the reaction time, as zero when all the washing residue has been added.
After the leaching process, the slurry was vacuum filtered immediately while hot. The filter cake was washed with hot deionized water to remove soluble salts. Collect the filtrate and record the volume. The chlorination residue was dried in a vacuum drying oven. After drying, samples were sent for analysis to investigate the influence of the oxygen-rich chlorination leaching system on the leaching of Pb and Ag.
2.3 Analytical methods
The chemical compositions of leach residues and solutions were analyzed by ICP-AES unless otherwise stated. The silver was analyzed by atomic absorption spectrometry (AAS). The X-ray diffraction (XRD) and scanning electron microscopy (SEM) analyses were performed to characterize the solid residues prior to and after each step.
The dissolution rates of Zn and Fe, and extents of leaching for Pb and Ag were calculated as follows:
α=(m0w0-m1w1)/(m0w0)×100% (8)
where α is the leaching rate of the metal; m0 is the mass of the material used in the experiment, or the mass of the residue used for chlorination; m1 is the mass of the dry residue after matured water leaching or chlorinated leaching; w0 is the mass fraction of the metal in the raw material, or the mass fraction of the metal used in the chlorinated residue; w1 is the mass fraction of the metal in the matured water-leaching residue, or the mass fraction of the metal in the chlorinated leaching residue.
In the kinetic experiments, the silver concentration was analyzed by atomic absorption spectrometry (AAS). The leaching ratio of silver was calculated as follows:
α=(C1×V1)/(m0×x0)×100% (9)
where C1 is the concentration of silver in the solution; V1 is the volume of the solution; m0 is the quality of the materials; x0 is the valuable element content.
3 Results and discussion
3.1 Sulfation roasting
3.1.1 Influence of roasting temperature
The influence of roasting temperature on Zn and Fe dissolution was investigated by introducing sulfuric acid at 70% of the raw material quality under the conditions of atmospheric pressure, heating rate of 5 °C/min and a roasting time of 120 min. Additionally, 100 g of raw material was used, the ratio of fluid to solid during water leaching was 5, and the stirring duration, temperature and speed for water immersion were 1 h, 24 °C and 500 r/min, respectively.
From the results shown in Figure 2(a), it is clear that the dissolution rates of Zn and Fe were maximized when the temperature was 300 °C, whereas the dissolution rates were not preferable when the temperature was lower than 300 °C. The reason for this may be that the zinc ferrite in the residue does not readily completely decompose; these conditions consequently reduce the dissolution rate of the metal [16]. When the temperature was higher than 300 °C, the dissolution rates of Zn and Fe decreased because the temperature was too high; these conditions caused the concentrated sulfuric acid to decompose, and weakened its effects, thereby making it difficult to convert Zn and Fe into soluble sulfates, and consequently generating new insoluble ferrates and sulfates at higher temperatures.
3.1.2 Influence of sulfuric acid dosage
The influence of sulfuric acid dosage on Zn and Fe dissolution was investigated under the conditions of atmospheric pressure, a roasting temperature of 300 °C, heating rate 5 °C/min and a roasting time of 120 min. Additionally, 100 g of raw material was used, the ratio of fluid to solid during water leaching was 5, and the stirring duration, temperature and speed for water immersion were 1 h, 24 °C and 500 r/min, respectively.
The data presented in Figure 2(b) show that, as the sulfuric acid content increased, the dissolution rates of Zn and Fe correspondingly significantly increased. When the sulfuric acid content exceeded 70%, the metal dissolution rate tended to be stable; moreover, the dissolution rate of Fe reached 92.36%, and the dissolution rate of Zn reached 98.69%. For the process of sulfation roasting, the proportion of concentrated sulfuric acid must be sufficient to convert the Zn and Fe compounds, which are only weakly soluble in water, into more readily soluble sulfates [17]. Therefore, in this study, the dissolution rates of Zn and Fe significantly increased with increasing sulfuric acid dosage in a certain range.
3.1.3 Influence of roasting time
The influence of roasting time on Zn and Fe dissolution was investigated under the conditions of atmospheric pressure, a sulfuric acid content of 70%, heating rate 5 °C/min and a roasting temperature of 300 °C. Additionally, 100 g of raw material was used, the ratio of fluid to solid during water leaching was 5, and the stirring duration, temperature and speed for water immersion were 1 h, 24 °C and 500 r/min, respectively.
It can be seen in Figure 2(c) that, as the roasting time was increased, the dissolution rates of Zn and Fe gradually increased, and the recovery rates were maximized at 120 min. Specifically, the dissolution rate of Zn was found to be strongly dependent on the roasting time, and significantly increased after 90 min; additionally, the dissolution rate of Fe increased as the roasting time was increased. At a certain temperature, a longer roasting time allowed the reaction to reach completion, and resulted in more sulfation [18].
3.2 Characterization of raw material and sulfation roasting residue
By analyzing the X-ray diffraction (XRD) patterns (Figure 3) obtained before and after roasting, it was found that the Pb in the raw material mainly existed in the form of lead sulfate, but there was also a small amount of plumbojarosite. Additionally, Zn mainly existed in the form of zinc ferrite, and Fe mainly existed in the form of zinc ferrite, but there were small amount of plumbojarosite. No significant diffraction peaks were observed for Ag compounds because of the low Ag content of the raw materials (210 g/t). The Pb in the roasting residue mainly existed in the form of lead sulfate and lead sulfide, and Ag, Fe, and Zn were all present in their corresponding sulfate forms.
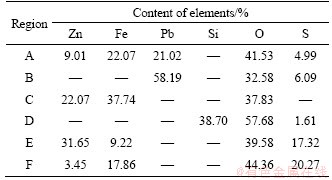
The scanning electron microscopy (SEM) (Figure 4) images and elemental data (Table 2) obtained before and after roasting were analyzed; in Table 2, the main components of each region are labeled as a, b, c, d, e, and f, and the Pb and Ag residue particles in these region are massive, and the structure is dense, indicating a high degree of dispersion. The valuable metal minerals could not be easily distinguished from gangue and impurities.
Moreover, the Pb and Ag exposure was low, with most of the Pb and Ag being wrapped by zinc ferrite and iron alum, which formed complexes with the gangue minerals in the sample. After sulfation roasting, the zinc ferrite and plumbojarosite in the residue were found to have been decomposed as a result of high temperature exposure; furthermore, many of the enveloping structures were destroyed, and valuable metals such as Pb and Ag were exposed. Alternatively, Zn and Fe underwent reactions during water leaching to respectively form iron sulfate and zinc sulfate. The particles on the surface of the sampled minerals after roasting were less densely packed than those observed on the surface before roasting. Additionally, after roasting, the dispersion was low, the granules occurred as flakes and layers, and the pores between the particles were distinct, which is the result of high-temperature oxidation.
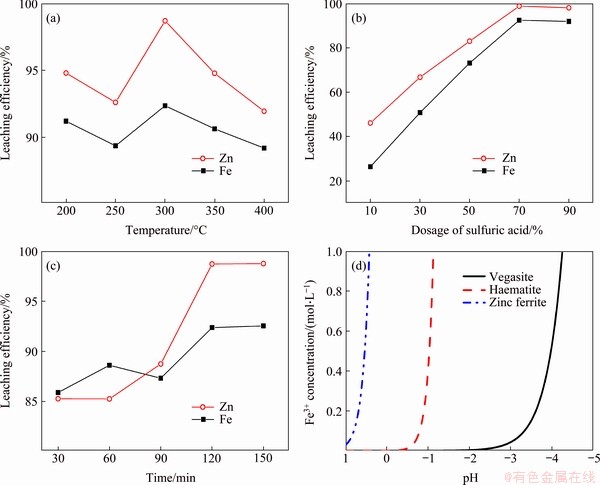
Figure 2 Effects of leaching temperature (a), sulfuric acid dosage (b), and roasting time (c) on metal leaching efficiency, and relationship between Fe3+ concentration and pH (d)
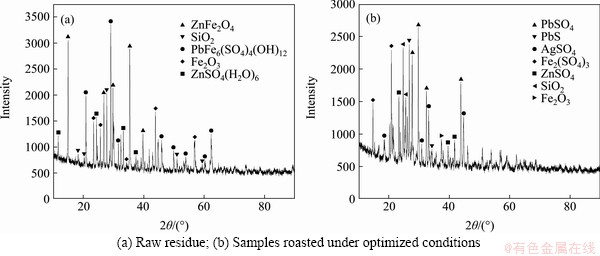
Figure 3 XRD patterns of residue:
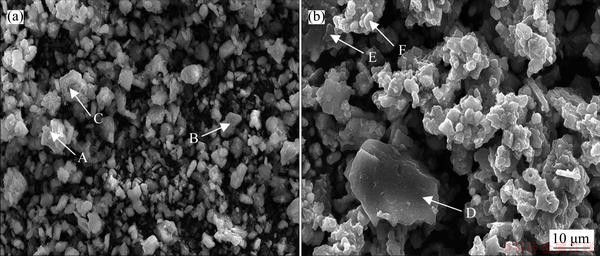
Figure 4 SEM images of Pb-Ag residue before (a) and after (b) roasting (A-PbFe6(SO4)4(OH)12; B-PbSO4; C-ZnFe2O4; D-SiO2; E-ZnSO4; F-Fe2(SO4)3)
Table 2 Elemental content in mass within each region
3.3 Oxygen-rich chlorination leaching
3.3.1 Effects of Cl- ion concentration
After the sulfuric acid-water leaching experiment, the Ag content in the residue was found to have increased from 0.021% (original sample) to 0.076%, and the Pb content increased from 16.3% (original sample) to 50.3%.
The effects of Cl- ion concentration on the extents of leaching for Pb and Ag were investigated in a leaching system under the following conditions: ρ(Fe3+) of 25 g/L, acidity of 2 mol/L, liquid-to-solid ratio of 9:1, temperature of 90 °C, leaching time of 0.5 h, and stirring speed of 500 r/min.
According to the data shown in Figure 5(a), the concentration of Cl- ions in the leaching reagent system significantly affected the Pb and Ag extents of leaching. The Pb and Ag extents of leaching reached their highest values when the concentration of Cl- ions reached 300 g/L.
When there is an excess of Cl- ions in the solution, the Pb and Ag metals form coordination complexes with the Cl- ions in the solution. Thus, the solution must have a higher Cl- ion concentration to increase the Pb and Ag extents of leaching [18]. The initial Pb extent of leaching was much lower than the initial Ag extent of leaching. This may be because the Pb content in the leaching residue was much higher than that of Ag, and the ratio of concentration to leaching reagent was small. Regarding the reaction kinetics, the reaction driving force was small, so the extent of leaching was slow [19].
3.3.2 Effects of liquid-to-solid ratio
The effects of the liquid-to-solid ratio on the leaching rate of metal were investigated in a leaching system under the following conditions: ρ(Fe3+) 25 g/L, ρ(Cl-) 300 g/L, acidity 2 mol/L, temperature 90 °C, leaching time 0.5 h, and stirring speed 500 r/min.
It can be seen in Figure 5(b) that, as the liquid-to-solid ratio increased, the of Pb and Ag significantly increased. When the liquid-to-solid ratio was increased to 9 mL:1 g, the Pb and Ag extents of leaching reached their highest values. Thus, liquid-to-solid ratio of 9 mL:1 g was chosen as an invariable quantity for the subsequent experiments.
3.3.3 Effects of Fe3+ ion concentration
The effects of Fe3+ concentration on the Pb and Ag extents of leaching were investigated in a leaching system under the following conditions: ρ(Cl-) 300 g/L, acidity 2 mol/L, liquid-to-solid ratio 9 mL:1 g, temperature 90 °C, leaching time 0.5 h, and stirring speed 500 r/min.
As can be seen in Figure 5(c), varying the Fe3+ ion concentration in the leaching reagent minimally affected the Pb extent of leaching, but significantly affected the Ag extent of leaching. When the Fe concentration in the leaching reagent was 10 g/L,the Pb extent of leaching reached 97.19%; however, increasing the Fe3+ concentration significantly increased the Ag extent of leaching, with the maximum of 96.74% corresponding to a 25 g/L Fe3+ concentration.
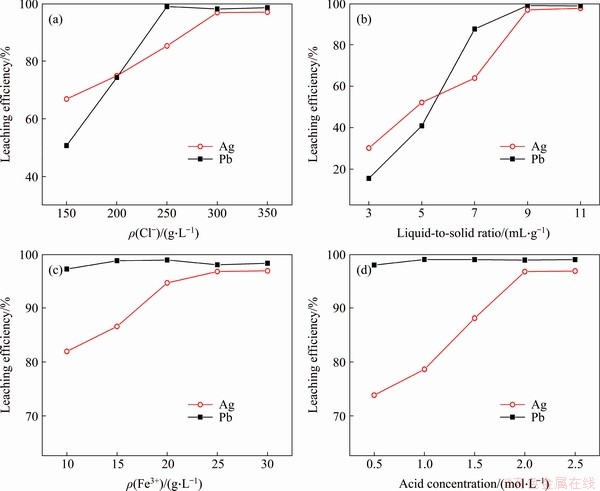
Figure 5 Respective effects of Cl- ion concentration (a), liquid-to-solid ratio (b), Fe3+ ion concentration (c), and acid concentration (d) on metal leaching efficiency
FeCl3 was used as the auxiliary leaching reagent; under acidic conditions, their ability to oxidize elemental Ag and Ag2S to 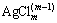 is much better than that of O2, which yields more desirable effects. O2 is used to maintain the concentration of Fe3+ in the leaching system during leaching. During the leaching process, not only did complexes form between Pb, Ag with Cl-, but the oxidation number of elemental Ag also changed; changing the Fe3+ concentration in the leaching reagent significantly affected the Ag leaching process. Therefore, to investigate the effects of other parameters, 25 g/L was chosen.
is much better than that of O2, which yields more desirable effects. O2 is used to maintain the concentration of Fe3+ in the leaching system during leaching. During the leaching process, not only did complexes form between Pb, Ag with Cl-, but the oxidation number of elemental Ag also changed; changing the Fe3+ concentration in the leaching reagent significantly affected the Ag leaching process. Therefore, to investigate the effects of other parameters, 25 g/L was chosen.
3.3.4 Effects of acidity
The effects of acid concentration on the Pb and Ag extents of leaching were investigated in a leaching system under the following conditions: ρ(Cl-) 300 g/L, ρ(Fe3+) 25 g/L, liquid-to-solid ratio 9 mL:1 g, temperature 90 °C, leaching time 0.5 h, and stirring speed 500 r/min.
It can be seen in Figure 5(d) that the effects of acidity on the Pb and Ag extents of leaching are similar to the effects of Fe3+ ions on the extents of leaching. Specifically, acidity minimally affected the Pb extent of leaching, but significantly affected the Ag extent of leaching. Furthermore, the Ag extent of leaching increased with increasing acidity, reaching 96.74% when the acidity was 2 mol/L. To achieve a high metal extent of leaching during chlorination leaching [6], the leaching process must be carried out in an acidic environment, yet the extra hydrochloride was not economical in industrial production practice. Therefore, a acid concentration of 2 mol/L was used in further experiments.
3.3.5 Characterization of chlorination leaching residue
Inductive coupled plasma analysis of chlorinated leaching residue was carried out after subjecting the samples to drying and fine grinding; the resulting composition and main elements are shown in Table 3.
From Table 3, it can be seen that the silicon content in the residue was high and recyclable. Conversely, the contents of Zn, Pb, and Ag were all relatively low.
Table 3 Main elements in chlorinated leaching residue (mass fraction, %)

By analyzing the XRD patterns (Figure 6) of the chlorinated leaching residue, the main phases in the residue were determined to be silica, and small amounts of lead sulfate, zinc ferrite, and lead chloride. No significant diffraction peaks were observed owing to the low content of Ag in the residue. It was also found that, during the process of oxygen-rich chlorination leaching, the structure of silica is not destroyed, but is enriched in the residue; additionally, a trace amount of zinc ferrite does not completely decompose, some of the lead sulfate does not completely react, and a small amount of lead chloride remains in the filter residue following filtration.
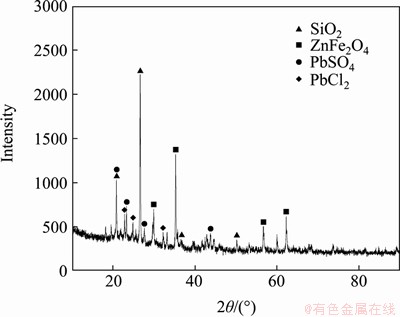
Figure 6 XRD pattern for chlorinated leaching residue
3.4 Kinetics of oxygen-rich silver chloride
The Ag in the zinc acid-leaching residue mainly existed in the form of Ag2SO4 after sulfation roasting and water leaching. The leaching that occurred in the FeCl3+HCl+NaCl system is found to be a complex fluid–solid heterogeneous reaction process. In the leaching process, solid particles almost remain unchanged in size during the heterogeneous reaction, if the water leaching residue contains a large amount of silica, which remain as a nonflaking residual so called solid layer. The thickness of insoluble solid residual layer progressively increases as the reaction proceeds, while the size of unreacted inner core decreases. It results in the reduction of the reactant surface and the increase in path length for the diffusion of ions [20]. In order to determine the kinetic parameters and rate control steps of the silver leaching process, we analyzed the water leaching residue and chlorinated leaching residue by scanning electron microscopy (SEM), and the results showed that there was little difference in their sizes. This suggests that the leaching process proceeded according to the shrinking core model with a constant particle size [21]. According to the model, the leaching reaction first occurs on the outer surface of the solid particles. As the reaction progresses, the leaching solution gradually diffuses toward the center of the particles, and at the same time the surface shrinks toward the center of the sphere, leaving a solid layer around the unreacted shrinking core.
If the reaction rate is controlled by the diffusion of the product layers, it will be an integrated rate equation, as follows [22, 23]:
1-2/3x-(1-x)2/3=kdt (10)
where x is the leaching rate of Ag, %; kd is the kinetic parameter for diffusion control; t is the reaction time, min.
If the chemical reaction is the rate-controlling step, then the following expression of the shrinking particle model can be used to represent the dissolution kinetics of the process [24, 25]:
1-(1-x)1/3=kct (11)
where kc is the kinetic parameter for chemical reaction control.
3.4.1 Effects of various factors on Ag extent of leaching
The influence of various factors on the Ag extent of leaching was investigated under the condition of liquid-to-solid ratio of 50 mL:1 g.
Temperature strongly influenced the Ag extent of leaching. As shown in Figure 7, at the same temperature, the Ag extent of leaching significantly increased with increasing processing time, and eventually stabilized at a constant rate. Alternatively, a higher temperature was found to correspond to a higher Ag leaching rate. Similarly, the concentration of chloride strongly influenced the Ag extent of leaching. Specifically, under the condition of the same leaching duration, a higher concentration of chloride resulted in a higher Ag extent of leaching. At the same chloride concentration, the Ag extent of leaching increased in response to an extension of the leaching duration. Analysis of the acidity and Fe3+ ion concentration investigations also revealed a similar trend. Particularly, the Ag extent of leaching significantly increased in response to higher acidity and Fe3+ ion concentration in the leaching system.
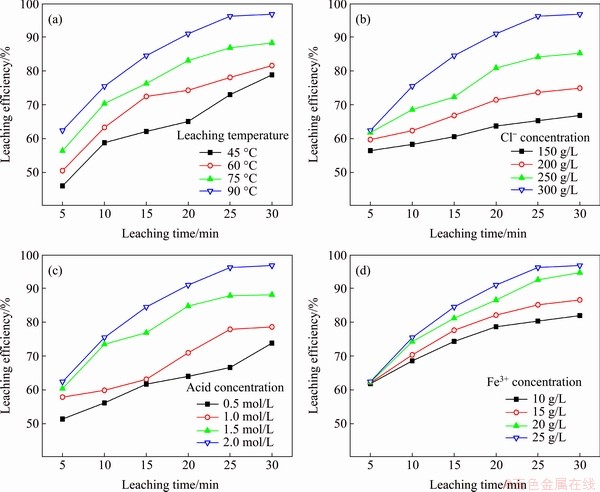
Figure 7 Respective effects of leaching temperature (a), Cl- ion concentration (b), acid concentration (c), and Fe3+ ion concentration (d) on Ag extent of leaching
3.4.2 Determination of control steps
The extent of leaching data for silver at different temperatures were substituted into the reaction rate expressions given by Eqs. (10) and (11) for fitting, and the fitting results are shown in Figure 8. The results show that the linear relationship between 1-2/3x-(1-x)2/3 and time t is significantly more accurate than that between time t and 1-(1-x)1/3. Therefore, it can be considered that the leaching of silver in the FeCl3+HCl+NaCl system is controlled by diffusion. Since the experiment used magnetic stirring to assist the leaching, the influence of the external diffusion can be eliminated, so the rate-controlling step of the leaching experiment is the internal diffusion process.
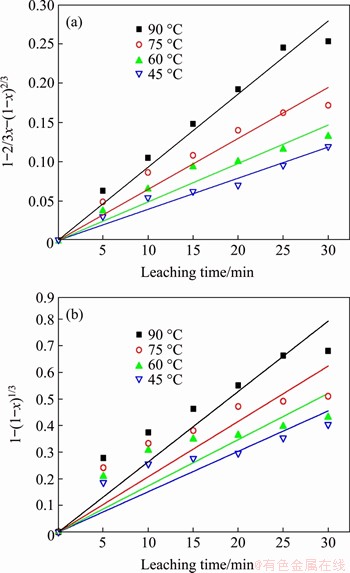
Figure 8 Relationship between 1-2/3x-(1-x)2/3 and time (a), 1-(1-x)1/3 and time (b)
In addition, the Arrhenius equation can be used to calculate the activation energy according to the slope of the line, as follows:
kd=A0exp[-E/(RT)] (12)
where A0 is the pre-exponential factor; E is the activation energy; R is the gas constant,8.314 J/(mol·K); and T is the temperature.
This relationship was used to plot kd as a function of 1/T; the result was then fitted to a straight line (see Figure 9), and the linear slope (-E/(RT)) was calculated. This linear equation was used to calculate the apparent activation energy of the leaching reaction, which was E=19.82 kJ/mol; this result further verifies that the leaching reaction process was controlled by the internal diffusion process.
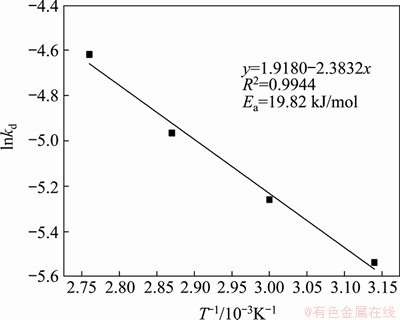
Figure 9 Dependence of lnkd on T-1/10-3K-1
The Ag extents of leaching under the conditions of different concentrations of Cl- ions, acid, and Fe3+ ions were substituted into Eq. (10), and a linear fit was obtained, as shown in Figure 10. For all conditions, a good linear relationship was observed. These results also indicate that the chlorine leaching process for silver sulfate in the residue was controlled by internal diffusion.

 The natural logarithm of the apparent rate constant (lnk) as a function of the natural logarithms of the Cl- ion concentration, acidity, and Fe3+ ion concentration (ln[Cl-], ln[H+], and ln[Fe3+]) is shown in Figure 11.
The natural logarithm of the apparent rate constant (lnk) as a function of the natural logarithms of the Cl- ion concentration, acidity, and Fe3+ ion concentration (ln[Cl-], ln[H+], and ln[Fe3+]) is shown in Figure 11.
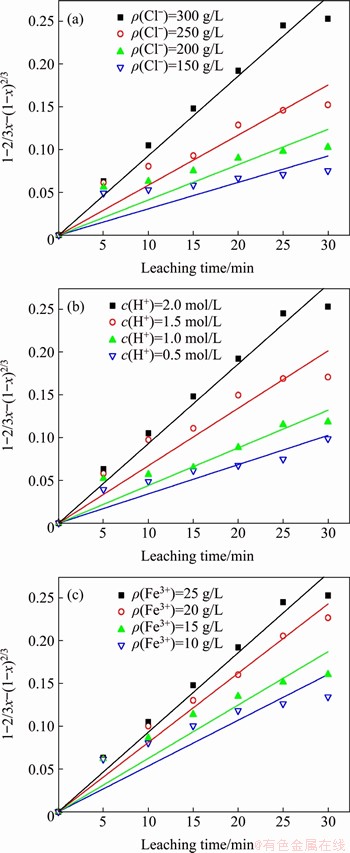
Figure 10 Variation of 1-2/3x-(1-x)2/3 as a function of time for various Cl- ion concentrations (a), acid concentration (b), and Fe3+ ion concentrations (c)
From Figure 11, the kinetic equations that respectively describe the effects of Cl- ion concentration, acidity, and Fe3+ ion concentration on the Ag extent of leaching are given as Eqs. (13)-(15):
lnk=2.98-1.52×ln[Cl-] (13)
lnk=-5.73+1.19×ln[H+] (14)
lnk=-6.90 + 0.70×ln[Fe3+] (15)

Figure 11 Natural logarithm of apparent rate constant as a function of natural logarithm of Cl- ion concentration (a), acid concentration (b), and Fe3+ ion concentration (c)
3.4.3 Establishment of mathematical kinetics model
From the above-mentioned analysis, the kinetic equations for Ag leaching can be expressed as follows:
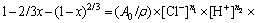
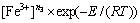 (16)
(16)
where x and other previously presented terms have the same meanings; and n1, n2, and n3 respectively represent the reaction order for the Cl- ions, acid, and Fe3+ ions.
The kinetic parameters were substituted into Eq. (16), and the relationship between 1-2/3x- (1-x)2/3 and [Cl-]-1.52×[H+]1.19×[Fe3+]0.7×exp (-E/(RT)) is illustrated in Figure 12 for Ag leaching. Although some degree of scatter is obvious, a straight line with a correlation coefficient (R2) above 0.96 was fitted to the data. Therefore, the data presented in Figure 12 were used to determine that the A0/ρ constant for Ag leaching was 20.56; thus, the kinetic equation for Ag leaching can be expressed as follows:
1-2/3x-(1-x)2/3=20.56×[Cl-]-1.52×[H+]1.19×[Fe3+]0.7×exp[-19820/(RT)] (17)
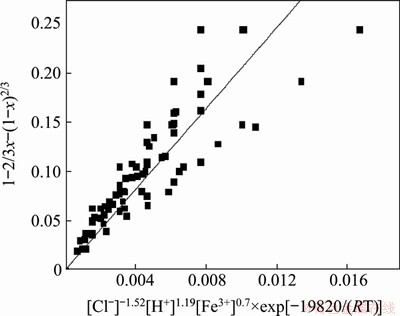
Figure 12 Relationship between 1-2/3x-(1-x)2/3 and [Cl-]-1.52×[H+]1.19×[Fe3+]0.7×exp(-19820/(RT)) during Ag leaching process
4 Conclusions
It is feasible to fully recover each valuable metal from zinc acid-leaching residue by sulfation roasting and oxygen-rich chlorination leaching method:
1) After sulfation roasting, the ZnFe2O4 and plumbojarosite in the zinc acid-leaching residue were decomposed by high temperature, the zinc and iron were converted into corresponding sulfates and enter the solution during water leaching. The optimum roasting and leaching parameters were determined to be a sulfuric acid at 70% of the raw material quality, roasting temperature of 300 ℃ and roasting time of 2 h, followed by the process of leaching the roasted residue for 1 h by applying water under the conditions of a water-to-solid ratio of 5 mL/g at room temperature. Under these experimental conditions, the recovery rates for Zn and Fe, are, 98.69% and 92.36%, respectively.
2) The PbSO4 and Ag2SO4 in the water leaching residue were leached by the chlorine salt leaching and combined with chloride ion to transform into complexes, which were stable in the solution. The optimal chlorine salt leaching parameters were determined to be as follows: Cl- concentration 300 g/L, Fe3+ concentration 25 g/L, acid concentration 2 mol/L, liquid-to-solid ratio 9 mL:1 g, temperature 90 °C, and leaching time 0.5 h. Filtration separation was performed after leaching, and Pb and Ag extents of leaching as high as 98.87% and 96.74%, respectively, were achieved.
3) Leaching process is controlled by internal diffusion, and the activation energy of the leaching reaction was 19.82 kJ/mol. The equation for the kinetics model was established as
1-2/3x-(1-x)2/3=20.56×[Cl-]-1.52×[H+]1.19×[Fe3+]0.7×exp(-19820/(RT)).
Contributors
WANG Rui-xiang conducted conceptualization, methodology, data curation, writing-original draft, writing-review and editing; YANG Yu-dong and LIU Cha-xiang finished methodology, investigation and data curation; ZHOU Jie and FANG Zhuang helped to perform the analysis with constructive discussions; YAN Kang conducted data curation, software and validation; TIAN Lei and XU Zhi-feng provided writing-review and editing, resources, project administration and funding acquisition.
Conflict of interest
All authors declare that they have no conflict of interest.
References
[1] DUTRIZAC J E, DINARDO O. The co-precipitation of copper and zinc with lead jarosite [J]. Hydrometallurgy, 1983, 11(1): 61-78. DOI: 10.1016/0304-386X(83)90016-6.
[2] LUO W, FENG Q, OU L, ZHANG G, CHEN Y. Kinetics of saprolitic laterite leaching by sulphuric acid at atmospheric pressure [J]. Minerals Engineering, 2010, 23(6): 458-462. DOI: 10.1016/j.mineng.2009.10.006.
[3] LI Chun-cheng, XIE Feng-chun, MA Yang, CAI Ting-ting, LI Hai-ying, HUANG Zhi-yuan, YUAN Gao-qing. Multiple heavy metals extraction and recovery from hazardous electroplating sludge waste via ultrasonically enhanced two-stage acid leaching [J]. Journal of Hazardous Materials, 2010, 178(1-3): 823-833. DOI: 10.1016/ j.jhazmat.2010.02.013.
[4] NAGIB S, INOUE K. Recovery of lead and zinc from fly ash generated from municipal incineration plants by means of acid and/or alkaline leaching [J]. Hydrometallurgy, 2000, 56(3): 269-292. DOI: 10.1016/S0304-386X(00)00073-6.
[5] ZVERD A, ERDEM M. Environmental risk assessment and stabilization/solidification of zinc extraction residue: I. Environmental risk assessment [J]. Hydrometallurgy, 2010, 100(3-4): 103-109. DOI: 10.1016/j.hydromet.2009.10.011.
[6] TURAN M D, ALTUNDOGAN H S, TUMEN F. Recovery of zinc and lead from zinc plant residue [J]. Hydrometallurgy, 2004, 75(1): 169-176. DOI: 10.1016/j.hydromet.2004.07. 008.
[7] STEER J M, GRIFFITHS A J. Investigation of carboxylic acids and non-aqueous solvents for the selective leaching of zinc from blast furnace dust slurry [J]. Hydrometallurgy, 2013: 34-41. DOI: 10.1016/j.hydromet.2013.08.011.
[8] RUSEN A, SUNKAR A S, TOPKAYA Y A. Zinc and lead extraction from Cinkur leach residues by using hydrometallurgical method [J]. Hydrometallurgy, 2008, 93(1, 2): 45-50. DOI: 10.1016/j.hydromet.2008.02.018.
[9] ZHANG Yu-hui, JIN Bing-jie, SONG Qing-he, CHEN Bu-ming, WANG Cheng-yan. Leaching behavior of lead and silver from lead sulfate hazardous residues in NaCl-CaCl2- NaClO3 media [J]. JOM, 2019, 71(7): 2388-2395. DOI: 10.1007/s11837-019-03472-1.
[10] APARAJITH B, MOHANTY D B, GUPTA M L. Recovery of enriched lead-silver residue from silver-rich concentrate of hydrometallurgical zinc smelter [J]. Hydrometallurgy, 2010, 105(1, 2): 127-133. DOI: 10.1016/j.hydromet.2010.08.010.
[11] ZHENG Yong-xing,LV Jin-fang,LIU Wei,QIN Wen-qing, WEN Shu-ming. An innovative technology for recovery of zinc, lead and silver from zinc leaching residue [J]. Physicochemical Problems of Mineral Processing, 2016, 52(2): 943-954. DOI: 10.5277/ppmp160233.
[12] XING Peng, MA Bao-zhong, ZENG Peng, WANG Cheng-yan, WANG Ling, ZHANG Yong-lu, CHEN Yong-qiang, WANG Shuo, WANG Qiu-yin. Deep cleaning of a metallurgical zinc leaching residue and recovery of valuable metals [J]. International Journal of Minerals, Metallurgy, and Materials, 2017, 24(11): 1217-1227. DOI: 10.1007/s12613-017-1514-2.
[13] BEHNAJADY B, MOGHADDAM J. Optimization of lead and silver extraction from zinc plant residues in the presence of calcium hypochlorite using statistical design of experiments [J]. Metallurgical and Materials Transactions B, 2014, 45(6): 2018-2026. DOI: 10.1007/s11663-014-0130-z.
[14] RODRIGUEZ N, ONGHENA B, BINNEMANS K. Recovery of lead and silver from zinc leaching residue using methanesulfonic acid [J]. ACS Sustainable Chemistry & Engineering, 2019, 7(24): 19807-19815. DOI: 10.1021/acssuschemeng.9b05116.
[15] JIANG Guo-min, PENG Bing, LIANG Yan-jie, CHAI Li-yuan, WANG Qing-wei, LI Qing-zhu, HU Ming. Recovery of valuable metals from zinc leaching residue by sulfate roasting and water leaching [J]. Transactions of Nonferrous Metals Society of China, 2017, 27(5): 1180-1187. DOI: 10.1016/S1003-6326(17)60138-9.
[16] HAN Hai-sheng, SUN Wei, HU Yue-hua, JIA Bao-liang, TANG Hong-hu. Anglesite and silver recovery from jarosite residues through roasting and sulfidization-flotation in zinc hydrometallurgy [J]. Journal of Hazardous Materials, 2014, 278: 49-54. DOI: 10.1016/j.jhazmat.2014.05.091.
[17] NADIROV R K. Recovery of valuable metals from copper smelter slag by sulfation roasting [J]. Transactions of the Indian Institute of Metals, 2018, 72(3): 603-607. DOI: 10.1007/s12666-018-1507-5.
[18] LIU W F, YANG T Z, XIA X. Behavior of silver and lead in selective chlorination leaching process of gold-antimony alloy [J]. Transactions of Nonferrous Metals Society of China, 2010, 20(2): 322-329. DOI: 10.1016/S1003-6326 (09)60141-2.
[19] ZHANG Ya-li, YU Xian-jin, LI Xiao-bin. Leaching of silver and lead by chloride simultaneously from residue after zinc extraction of low-grade zinc oxide ores [J]. The Chinese Journal of Nonferrous Metals, 2012, 22(1): 296-303. (in Chinese)
[20] WANG Rui-xiang, TANG Mo-tang, YANG Sheng-hai, ZHAGN Wen-hai, TANG Chao-bo, HE Jing, YANG Jian-guang. Leaching kinetics of low grade zinc oxide ore in NH3-NH4Cl-H2O system [J]. Journal of Central South University of Technology, 2008, 15(5): 679-683. DOI: 10.1007/s11771-008-0126-4.
[21] JU Shao-hua, TANG Mo-tang, YANG Sheng-hai, LI Ying-nian. Dissolution kinetics of smithsonite ore in ammonium chloride solution [J]. Hydrometallurgy, 2005, 80(1): 67-74. DOI: 10.1016/j.hydromet.2005.07.003.
[22] LIU Jia-nan, ZHAI Yu-chun, WU Yan, ZHANG Jun, SHEN Xiao-yi. Kinetics of roasting potash feldspar in presence of sodium carbonate [J]. Journal of Central South University, 2017, 24(7): 1544-1550. DOI: 10.1007/s11771-017-3559-9.
[23] WANG Hai-dong, ZHOU An-an, GUO Hui, LU Meng-hua, YU Hai-zhao. Kinetics of leaching lithium from lepidolite using mixture of hydrofluoric and sulfuric acid [J]. Journal of Central South University, 2020, 27(1): 27-36. DOI: https://doi.org/10.1007/s11771-020-4275-4.
[24] GUO Yue-dong. Complex gold ore roasting-acid leaching-fluoride salt pretreatment-cyanidaton process[D]. Ganzhou: Jiangxi University of Science and Technology, 2019: 42-43. (in Chinese)
[25] YANG Hui-bin, PAN Xiao-lin, YU Hai-yan, TU Gan-feng, SUN Jun-min. Effect of ferrite content on dissolution kinetics of gibbsitic bauxite under atmospheric pressure in NaOH solution [J]. Journal of Central South University, 2017, 24(3): 489-495. DOI: 10.1007/s11771-017-3451-7.
(Edited by YANG Hua)
中文导读
湿法酸浸锌渣硫酸熟化-富氧氯化浸出提取铅、银的实验研究
摘要:常规湿法炼锌过程中会产生大量的酸浸渣,该残渣含有大量各种有价值的金属。本研究的目的是评估使用硫酸熟化、水浸和氯化浸出(吹氧技术)回收铅和银的能力,接着通过冷却结晶并用铅片置换出银,实现酸浸锌渣中所有有价金属的全面回收,该工艺取得了良好的效果。条件实验研究得出最佳工艺条件如下:硫酸用量为原样质量的70%,焙烧温度300 °C,焙烧时间2 h,焙烧渣按液固比5:1室温水浸1 h,此时锌、铁的浸出率最高(锌:98.69%,铁:92.36%)。氯化浸出系统主要参数如下:Cl-浓度300 g/L,Fe3 +浓度25 g/L,酸浓度2 mol/L,液固比9 mL:1 g,温度90 °C,浸出时间0.5 h,该条件下铅和银浸出率分别高达98.87%和96.74%;最后,对在富氧介质下使用Cl-浸出银进行动力学研究,发现浸出过程受内扩散控制,活化能为19.82 kJ/mol。
关键词:酸浸渣;硫酸熟化;氯盐;铅; 银;动力学
Foundation item: Projects(51804136, 52064021, 52074136, 51564021, 52064022) supported by the National Natural Science Foundation of China; Projects(2019T120625, 2019M652276) supported by the China Postdoctoral Science Foundation; Project(20202ACB213002) supported by the Jiangxi Province Science Fund for Distinguished Young Scholars, China; Project(2019KY09) supported by the Program for Excellent Young Talents,JXUST Young Jinggang Scholars of Jiangxi Province, Merit-based Postdoctoral Research in Jiangxi Province, China; Projects supported by the Distinguished Professor Program of Jinggang Scholars, Chinain Institutions of Higher Learning, Jiangxi Province, China
Received date: 2020-04-18; Accepted date: 2020-10-15
Corresponding author: TIAN Lei, PhD, Associate Professor; E-mail: tianleijx@163.com; ORCID: https://orcid.org/0000-0002-5510- 5315; XU Zhi-feng, PhD, Professor; E-mail: xzf_1@163.com; ORCID: https://orcid.org/0000-0003-0851-9598
Abstract: A large amount of acid-leaching residue is produced during the conventional Zn hydrometallurgy process, and this residue has a large concentration of a variety of valuable metals. The purpose of this study was to evaluate the ability of a procedure that entails the use of sulfation roasting, water leaching, and chlorination leaching (blowing oxygen technique) to recover Pb and Ag, followed by cooling crystallization and the replacement of Ag with lead sheet, to realize the full recovery of all valuable metals from zinc acid-leaching residue; consequently, good results were achieved. The best results were obtained under the following conditions: a sulfuric acid at 70% of the raw material quality, roasting temperature of 300 °C and roasting time of 2 h, followed by the process of leaching the roasted residue for 1 h by applying a water-to-solid ratio of 5:1 at room temperature. The recovery rates of Zn and Fe were 98.69% and 92.36%, respectively. The main parameters of the chlorine salt leaching system were as follows: Cl- concentration of 300 g/L, Fe3+ concentration of 25 g/L, acid concentration of 2 mol/L, liquid-to-solid ratio of 9 mL:1 g, temperature of 90 ℃, and leaching time of 0.5 h; this leaching process was followed by filtration separation. These conditions resulted in high extents of leaching for Pb and Ag (i.e., 98.87% and 96.74%, respectively). Finally, the kinetics of the process of Ag leaching using Cl- ions in an oxygen-rich medium was investigated. It was found that the leaching process was controlled by the diffusion of the product layers, and the activation energy was 19.82 kJ/mol.


