Trans. Nonferrous Met. Soc. China 27(2017) 908-916
Effects of iron-containing phases on transformation of sulfur-bearing ions in sodium aluminate solution
Xiao-bin LI, Fei NIU, Gui-hua LIU, Tian-gui QI, Qiu-sheng ZHOU, Zhi-hong PENG
School of Metallurgy and Environment, Central South University, Changsha 410083, China
Received 20 May 2016; accepted 23 November 2016
Abstract:
Sulfides in the high-sulfur bauxite lead to serious steel equipment corrosion and alumina product degradation via the Bayer process, owing to the reactions of sulfur and iron-containing phases in the sodium aluminate solution. The effects of iron-containing phases on the transformation of sulfur-bearing ions (S2-,  ,
,  and
and  ) in sodium aluminate solution were investigated. Fe, Fe2O3 and Fe3O4 barely react with
) in sodium aluminate solution were investigated. Fe, Fe2O3 and Fe3O4 barely react with  and
and  , but all of them, particularly Fe, can promote the conversion of
, but all of them, particularly Fe, can promote the conversion of  to
to  and S2- in sodium aluminate solution. Fe can convert to
and S2- in sodium aluminate solution. Fe can convert to 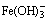 in solution at elevated temperatures, and further react with S2- to form FeS2, but Fe2O3 and Fe3O4 have little influence on the reaction behavior of S2- in sodium aluminate solution. Increasing temperature, duration, dosage of Fe, mole ratio of Na2Ok to Al2O3 and caustic soda concentration are beneficial to the transformation of
in solution at elevated temperatures, and further react with S2- to form FeS2, but Fe2O3 and Fe3O4 have little influence on the reaction behavior of S2- in sodium aluminate solution. Increasing temperature, duration, dosage of Fe, mole ratio of Na2Ok to Al2O3 and caustic soda concentration are beneficial to the transformation of  to
to  and S2-. The results may contribute to the development of technologies for alleviating the equipment corrosion and reducing caustic consumption during the high-sulfur bauxite treatment by the Bayer process.
and S2-. The results may contribute to the development of technologies for alleviating the equipment corrosion and reducing caustic consumption during the high-sulfur bauxite treatment by the Bayer process.
Key words:
high-sulfur bauxite; sodium aluminate solution; sulfur-bearing ion; iron-containing phase; transformation;
1 Introduction
More than 560 million tons of high-sulfur diasporic bauxite resources have not been effectively utilized in China [1,2]. The main sulfide mineral in the bauxite is pyrite (FeS2) which readily reacts with alkaline solution in the Bayer process, resulting in the increase of caustic consumption, serious equipment corrosion, and Fe-contamination of alumina product [2-4]. In order to resolve such problems, many scholars have conducted researches which focused on: 1) pyrite removal from bauxite by pretreatments, such as roasting [4], flotation [5], and bio-beneficiation [6]; 2) removal of S2- from Bayer liquor, such as through formation of ZnS by adding Zn(II) [3,7] or NaFeS2·2H2O by adding fresh iron hydroxides [8]; 3) removal of  from Bayer liquor through formation of BaSO4 by adding barium compounds [9]; 4) conversion of S2- to
from Bayer liquor through formation of BaSO4 by adding barium compounds [9]; 4) conversion of S2- to  during the Bayer digestion process by addition of oxidants, such as O2 [10,11] and NaNO3 [12]. However, inefficient desulfurization, high cost and complicated operation limit the practical application of these methods. Therefore, developing new methods to minimize the impact of sulfur-containing minerals is crucial for utilization of high-sulfur bauxite.
during the Bayer digestion process by addition of oxidants, such as O2 [10,11] and NaNO3 [12]. However, inefficient desulfurization, high cost and complicated operation limit the practical application of these methods. Therefore, developing new methods to minimize the impact of sulfur-containing minerals is crucial for utilization of high-sulfur bauxite.
During the Bayer digestion process, sulfur- containing minerals in bauxite inevitably react with the alkaline solution to form S2-,  ,
,  and
and  etc., and these sulfur-bearing ions would further react with Fe (in steel equipment), Fe2O3 or Fe3O4 (in bauxite). The adverse effects of sulfur-containing species on the alumina production process are generally believed to be caused by these various reactions. KUZNETSOV et al [13] and LI et al [14] investigated the reactions of FeS2 in sodium aluminate solution at elevated temperatures, and proposed that the soluble iron-sulfur complex generated by sulfur and iron-containing phases was the main reason for Fe-contamination of the product. XIE et al [15-17] studied the influence of S2- on corrosion of steels in sodium aluminate solution and suggested that S2- could react with steel to generate iron-sulfur compounds with loose structure and thus accelerate the corrosion. XIE et al [16] also reported that the corrosion of steels could be decelerated by
etc., and these sulfur-bearing ions would further react with Fe (in steel equipment), Fe2O3 or Fe3O4 (in bauxite). The adverse effects of sulfur-containing species on the alumina production process are generally believed to be caused by these various reactions. KUZNETSOV et al [13] and LI et al [14] investigated the reactions of FeS2 in sodium aluminate solution at elevated temperatures, and proposed that the soluble iron-sulfur complex generated by sulfur and iron-containing phases was the main reason for Fe-contamination of the product. XIE et al [15-17] studied the influence of S2- on corrosion of steels in sodium aluminate solution and suggested that S2- could react with steel to generate iron-sulfur compounds with loose structure and thus accelerate the corrosion. XIE et al [16] also reported that the corrosion of steels could be decelerated by  anion in sodium aluminate solution, while WENSLEY and CHARLTON [18] found that both S2- and
anion in sodium aluminate solution, while WENSLEY and CHARLTON [18] found that both S2- and  anions were the corrosion activators for steels in alkaline solution. Compared with the low-valence sulfur ions,
anions were the corrosion activators for steels in alkaline solution. Compared with the low-valence sulfur ions,  and
and  were believed to be harmless to the corrosion of steel equipment and Fe-contamination of alumina products.
were believed to be harmless to the corrosion of steel equipment and Fe-contamination of alumina products.
In view of different impacts of various sulfur- bearing ions, the conversions of sulfur-bearing ions in aluminate solution have received considerable research attention. ABIKENOVA et al [19], and HU and CHEN [10] investigated the transformation of S2- in sodium aluminate solution in the presence of oxidants, and demonstrated that the transformation process among sulfur-bearing ions, i.e., S2- was first oxidized to  and then to
and then to  or
or  which was finally converted to
which was finally converted to  . However, the conversion behaviors of sulfur-bearing ions during the reactions of them with iron-bearing substances were not taken into consideration.
. However, the conversion behaviors of sulfur-bearing ions during the reactions of them with iron-bearing substances were not taken into consideration.
In sum, understanding the reaction behaviors of the iron-containing phases and the sulfur-bearing ions as well as the transformation of the sulfur-bearing ions is essential to develop new technologies for high-sulfur bauxite utilization. Unfortunately, the previous researches paid much attention to the transformation of iron-bearing species in the sulfur-containing solutions, while the effects of iron-containing phases on transformation of the sulfur-bearing ions have been scarcely reported. In view of this, this work focused on the dependence of sulfur-bearing ion (S2-,  ,
,  and
and  ) transformation on iron-containing phases (Fe, Fe2O3 and Fe3O4) in sodium aluminate solution. We attempted to provide the fundamental basis for taking some measures to reduce the steel equipment corrosion, caustic soda loss and the product Fe-contamination during the high-sulfur bauxite treatment by the Bayer process.
) transformation on iron-containing phases (Fe, Fe2O3 and Fe3O4) in sodium aluminate solution. We attempted to provide the fundamental basis for taking some measures to reduce the steel equipment corrosion, caustic soda loss and the product Fe-contamination during the high-sulfur bauxite treatment by the Bayer process.
2 Experimental
2.1 Materials
Sodium aluminate solutions were prepared by dissolving industrial grade aluminum hydroxide (Aluminum Corporation of China) into hot sodium hydroxide solution. Various sulfur-bearing ion solutions were obtained by adding analytical grade of Na2S·9H2O (Xilong Chemical Co., Ltd.), Na2S2O3·5H2O, Na2SO3 or Na2SO4 (Sinopharm Chemical Reagent Co., Ltd.) of a defined dosage into the prepared sodium aluminate solution. Both iron powder (Kermel Chemical Reagent Corporation of Tianjing, China) and Fe2O3 powder (Sinopharm Chemical Reagent Co., Ltd.) were analytical grade reagents, Fe3O4 powder (Sinopharm Chemical Reagent Co., Ltd.) was chemically pure reagent, and no other phases were detected in these iron-containing phases (Fig. 1).

Fig. 1 XRD patterns of iron-containing phases
2.2 Methodology
The digestion experiments were performed in a self-designed autoclave, in which the sealed stainless bombs (150 mL) were heated in either molten salts (>160 °C) or glycerol (<140 °C). A given mass of iron-containing substance and 100 mL sulfur-bearing sodium aluminate solution were added into a 150 mL bomb, together with four steel balls (two 18 mm- diameter and two 8 mm-diameter) for improved agitation. The sealed bomb was fixed in a rotating device (rotation speed of 120 r/min), immersed in the heating medium and was retained for fixed duration at the designated temperature. The resultant slurry obtained was filtered and washed using hot water. The filtrate was collected for sulfur-bearing ions analysis, and the residue was dried at (50±1) °C for 24 h.
The sodium aluminate solutions were characterized by Na2Ok concentration and caustic molar ratio (αk). The Na2Ok represents caustic soda as Na2O in solution, and the αk refers to the molar ratio of Na2Ok to Al2O3. The concentrations of Na2Ok and Al2O3 were determined by titration [20]. The concentrations of sulfur-bearing ions were characterized by the mass concentration of element sulfur, and the total sulfur concentration (ST) is the summation of concentrations of S2-,  ,
,  and
and  in solution. The concentrations of
in solution. The concentrations of  ,
,  and
and  were simultaneously analyzed by an ion chromatograph (ICS-90, Dionex, USA) and designated respectively as x2, x3 and x4. The total concentration (x) of S2-,
were simultaneously analyzed by an ion chromatograph (ICS-90, Dionex, USA) and designated respectively as x2, x3 and x4. The total concentration (x) of S2-,  and
and  was measured by titration [21]. Hence, the S2- concentration (x1) can be calculated by subtraction, i.e., x1=x-x2-x3. Every digestion experiment was conducted at least twice to verify the variation of the sulfur-bearing ions, average concentrations of sulfur-bearing ions were recorded. X-ray diffraction (D/MAAX2500, Rigaku Corporation, Japan) was applied to characterizing the residues using Cu Kα radiation at a scanning speed of 8 (°)/min. The contents of sodium and sulfur in residues were identified by flame photometer (AP1302, Shanghai Aopu Analytical Instruments Corporation, China) and sulfur analyzer (HDS3000, Hunan Huade Electronics Corporation, China), respectively.
was measured by titration [21]. Hence, the S2- concentration (x1) can be calculated by subtraction, i.e., x1=x-x2-x3. Every digestion experiment was conducted at least twice to verify the variation of the sulfur-bearing ions, average concentrations of sulfur-bearing ions were recorded. X-ray diffraction (D/MAAX2500, Rigaku Corporation, Japan) was applied to characterizing the residues using Cu Kα radiation at a scanning speed of 8 (°)/min. The contents of sodium and sulfur in residues were identified by flame photometer (AP1302, Shanghai Aopu Analytical Instruments Corporation, China) and sulfur analyzer (HDS3000, Hunan Huade Electronics Corporation, China), respectively.
3 Results and discussion
3.1 Effects of iron-containing phases on reaction behavior of sulfur-bearing ions
The digestion of diasporic bauxite was generally conducted at 260-280 °C with the Na2Ok concentration of about 230 g/L and αk of 3.0 in the alumina refineries. The main iron-containing phases involved in the digestion process were Fe (steel equipment) and iron oxides (main iron minerals in bauxite and the passivation coating of the steel equipment). In order to reveal the effects of iron-containing phases (Fe, Fe2O3 and Fe3O4) on the transformation of sulfur-bearing ions, the concentration variation of sulfur-bearing ions was investigated in sodium aluminate solution during the digestion process with adding various iron-bearing phases. Blank experiments without the addition of the iron-containing phases were also conducted for comparison. Moreover, in order to react with the sulfur-bearing ions adequately during the digestion, and the initial dosage of iron-containing phases was determined with the mole ratio of Fe to S being 1.5:1.
3.1.1 Effects of iron-containing phases on reaction behavior of S2-
When high-sulfur bauxite is treated using the Bayer process, the sulfur exists in solution predominately in the form of S2-. The influence of iron-containing phases on the transformation of S2- is shown in Table 1.  and
and  were detected in the starting solution prepared by dissolving Na2S·9H2O, which may be contributed to the oxidation of S2- during the preparation.
were detected in the starting solution prepared by dissolving Na2S·9H2O, which may be contributed to the oxidation of S2- during the preparation.
Table 1 shows that the S2- concentration is reduced by varying degrees after digestion with adding different iron-containing phases, and the sulfur concentration in residues is increased. When adding iron, Fe3O4 or Fe2O3 powders, the concentrations of S2- in the digested solutions correspondingly decrease by 17.33%, 6.56% and 3.28% compared with the concentration of S2- in the blank digested solution. The S2- concentration in the blank digested solution decreases slightly along with a small increase of concentrations of  and
and  compared with the starting solution, which may be caused by the oxidation of S2- during the digestion. The result shows that S2- concentration decreases obviously on addition of the iron-containing phases in digestion process accompanying by an increase in
compared with the starting solution, which may be caused by the oxidation of S2- during the digestion. The result shows that S2- concentration decreases obviously on addition of the iron-containing phases in digestion process accompanying by an increase in  and
and  concentrations. Table 1 also reveals that the S2- concentration variation is related to the valence of iron, i.e., the iron with the smallest valence favors the most decreased S2- concentration in the digested solution.
concentrations. Table 1 also reveals that the S2- concentration variation is related to the valence of iron, i.e., the iron with the smallest valence favors the most decreased S2- concentration in the digested solution.
3.1.2 Effects of iron-containing phases on reaction behavior of 
The pyrite in bauxite will react with alkaline solution forming S2- and  firstly in the Bayer digestion process. The S2- ion can be oxidized into
firstly in the Bayer digestion process. The S2- ion can be oxidized into  and other high valence sulfur-bearing ions in sodium aluminate solution during the alumina production [19].
and other high valence sulfur-bearing ions in sodium aluminate solution during the alumina production [19].  was found to accelerate the equipment corrosion [22]. The effects of iron-containing phases on the transformation of
was found to accelerate the equipment corrosion [22]. The effects of iron-containing phases on the transformation of  in sodium aluminate solution were also studied. The results are shown in Table 2.
in sodium aluminate solution were also studied. The results are shown in Table 2.
As shown in Table 2, the concentration of  decreases significantly in sodium aluminate solution after digestion. Compared with the starting solution, about 75% of
decreases significantly in sodium aluminate solution after digestion. Compared with the starting solution, about 75% of  transforms to
transforms to  and S2- in the blank sample, possibly due to the disproportionation reaction of
and S2- in the blank sample, possibly due to the disproportionation reaction of  . The transformations of
. The transformations of  are enhanced by differing degrees on addition of the iron-containing phases. Besides,
are enhanced by differing degrees on addition of the iron-containing phases. Besides,  can completely convert into
can completely convert into  and S2- on addition of iron powder, suggesting that the transformation of
and S2- on addition of iron powder, suggesting that the transformation of  can be accelerated obviously by iron powder, and the possible reactions can be proposed as Eqs. (1) and (2) [23].
can be accelerated obviously by iron powder, and the possible reactions can be proposed as Eqs. (1) and (2) [23].
Fe + = FeS+
= FeS+ (1)
(1)
FeS+3OH-= +S2- (2)
+S2- (2)
In addition, the formed FeS may further react with OH- in sodium aluminate solution during the digestion process at elevated temperature (Eq. (2)), resulting in the increase of  and S2- concentrations in digested solution.
and S2- concentrations in digested solution.
3.1.3 Effects of iron-containing phases on transformation of  or
or 
In general, S2- can be oxidized to  and
and  by O2 or other oxidants [3,12] in the Bayer process. The effects of iron-containing phases on the transformation of
by O2 or other oxidants [3,12] in the Bayer process. The effects of iron-containing phases on the transformation of  and
and  were concerned in sodium aluminate solution, and the results are presented in Tables 3 and 4, respectively.
were concerned in sodium aluminate solution, and the results are presented in Tables 3 and 4, respectively.
Table 3 indicates that  concentration has no remarkable variation and
concentration has no remarkable variation and  is present in solution for all experiments. The presence of
is present in solution for all experiments. The presence of  can be attributed to the oxidation of
can be attributed to the oxidation of  in sample preparation or the impurity of
in sample preparation or the impurity of  in Na2SO3. In comparison with the other samples, the lower
in Na2SO3. In comparison with the other samples, the lower  concentration with adding iron powder may be caused by the inhibition of
concentration with adding iron powder may be caused by the inhibition of  oxidation. The results in Table 4 demonstrate that iron-containing phases have no evident influence on the transformation of
oxidation. The results in Table 4 demonstrate that iron-containing phases have no evident influence on the transformation of  in sodium aluminate solution. Moreover, the sulfur contents in residues approximate to zero, and neither
in sodium aluminate solution. Moreover, the sulfur contents in residues approximate to zero, and neither  nor
nor  appears to react with iron-containing phases during the Bayer digestion process of diasporic bauxite, which is in agreement with previous research [18].
appears to react with iron-containing phases during the Bayer digestion process of diasporic bauxite, which is in agreement with previous research [18].
Table 1 Effects of iron-containing phases on reaction behavior of S2- in sodium aluminate solution
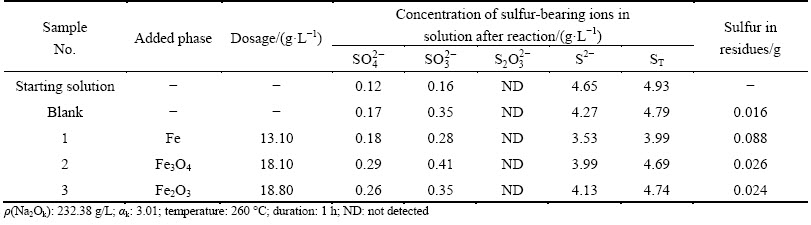
Table 2 Effects of iron-containing phases on transformation of  in sodium aluminate solution
in sodium aluminate solution

Table 3 Effects of iron-containing phases on transformation of  in sodium aluminate solution
in sodium aluminate solution

Table 4 Effects of iron-containing phases on the transformation of  in sodium aluminate solution
in sodium aluminate solution

3.1.4 Phase analysis of residues obtained from reaction of iron-containing phases with S2- or 
As discussed above, both S2- and  in sodium aluminate solution can react with the iron-containing phases. For better understanding the reaction mechanism, the XRD analysis of residues generated by the reaction of iron-containing phases with S2- and
in sodium aluminate solution can react with the iron-containing phases. For better understanding the reaction mechanism, the XRD analysis of residues generated by the reaction of iron-containing phases with S2- and  was conducted, and the results are displayed in Fig. 2.
was conducted, and the results are displayed in Fig. 2.

Fig. 2 XRD patterns of residues produced by reaction of iron-containing phases with S2- (a) and  (b)
(b)
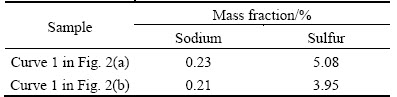
No new phases are detected using XRD analysis in the residues obtained by adding Fe2O3 or Fe3O4, which suggests that either Fe2O3 or Fe3O4 has little influence on the transformation of S2- or  in sodium aluminate solution, being consistent with the results in Tables 1 and 2. Whereas, evident characteristic peaks of Fe3O4 and faint characteristic peak (at about 2θ=16.5°) appear in the XRD patterns of the residues obtained by adding iron powder. The possible phase at 2θ=16.5° may be FeS2 or NaFeS2·2H2O [8], which can be formed by the ferrous or ferric compound reacting with S2- in sodium aluminate solution, respectively. The contents of sodium and sulfur were detected (in Table 5) to identify the phases in residues. In view of the low sodium contents and high sulfur contents in residues, the formation of FeS2 at 2θ=16.5° can be verified.
in sodium aluminate solution, being consistent with the results in Tables 1 and 2. Whereas, evident characteristic peaks of Fe3O4 and faint characteristic peak (at about 2θ=16.5°) appear in the XRD patterns of the residues obtained by adding iron powder. The possible phase at 2θ=16.5° may be FeS2 or NaFeS2·2H2O [8], which can be formed by the ferrous or ferric compound reacting with S2- in sodium aluminate solution, respectively. The contents of sodium and sulfur were detected (in Table 5) to identify the phases in residues. In view of the low sodium contents and high sulfur contents in residues, the formation of FeS2 at 2θ=16.5° can be verified.
Table 5 Contents of sodium and sulfur in residues
According to the previous studies [24-26], iron can convert to  and Fe3O4 in alkaline solution at elevated temperatures.
and Fe3O4 in alkaline solution at elevated temperatures.  may react with S2- to form FeS2 in sodium aluminate solution during the temperature decrease process after digestion, as discussed by LI et al [8], leading to decreased S2- concentration (Table 1). On addition of Fe2O3 and Fe3O4 powders, little
may react with S2- to form FeS2 in sodium aluminate solution during the temperature decrease process after digestion, as discussed by LI et al [8], leading to decreased S2- concentration (Table 1). On addition of Fe2O3 and Fe3O4 powders, little  is formed in the solution. Therefore, iron powder promotes the transformation of S2- into residues more greatly than Fe2O3 or Fe3O4 powders. It is comprehensible that iron powders can enhance the conversion of
is formed in the solution. Therefore, iron powder promotes the transformation of S2- into residues more greatly than Fe2O3 or Fe3O4 powders. It is comprehensible that iron powders can enhance the conversion of  to
to  and S2-, and S2- then partly incorporates in FeS2, reducing
and S2-, and S2- then partly incorporates in FeS2, reducing  concentration and increasing
concentration and increasing  concentration (in Table 2).
concentration (in Table 2).
3.2 Effects of duration and iron powder dosage on transformation of S2- and 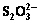
The influences of duration and iron powder dosage on the transformation of S2- and  at different temperatures are presented in Figs. 3-5, respectively.
at different temperatures are presented in Figs. 3-5, respectively.
Figure 3(a) indicates that the main sulfur-bearing ion is S2- in solution, coexisting a small amount of  and
and  . The concentrations of ST,
. The concentrations of ST,  ,
, 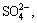
 and S2- in solution remain almost unchanged at 80 °C as a function of duration, suggesting that S2- reacts weakly with iron powder in sodium aluminate solution at low temperatures. However, when the reaction temperature is increased to 260 °C (Fig. 3(b)), the concentration of S2- decreases slightly with the duration prolonging. The S2- concentration decreases from 4.05 to 3.52 g/L in 10 min, and then reaches 3.27 g/L at 90 min. The
and S2- in solution remain almost unchanged at 80 °C as a function of duration, suggesting that S2- reacts weakly with iron powder in sodium aluminate solution at low temperatures. However, when the reaction temperature is increased to 260 °C (Fig. 3(b)), the concentration of S2- decreases slightly with the duration prolonging. The S2- concentration decreases from 4.05 to 3.52 g/L in 10 min, and then reaches 3.27 g/L at 90 min. The  concentration increases slightly and
concentration increases slightly and  concentration remains almost constant, i.e.,
concentration remains almost constant, i.e.,  is difficult to convert to
is difficult to convert to  .
.
 concentration decreases and
concentration decreases and  concentration increases with duration at either 120 or 260 °C (Fig. 4). Increasing temperature favors the
concentration increases with duration at either 120 or 260 °C (Fig. 4). Increasing temperature favors the  conversion, e.g.,
conversion, e.g.,  concentration reduces to zero in 10 min at 260 °C along with marked increase in
concentration reduces to zero in 10 min at 260 °C along with marked increase in  concentration and slight raising of S2- concentration instead of being proportional to
concentration and slight raising of S2- concentration instead of being proportional to  concentration, where the ST concentration reduces from 5.28 to 4.17 g/L. The obvious reduction of ST concentration and the inconspicuous variation of S2- concentration are caused by the formation of FeS2.
concentration, where the ST concentration reduces from 5.28 to 4.17 g/L. The obvious reduction of ST concentration and the inconspicuous variation of S2- concentration are caused by the formation of FeS2.
The iron powder dosage has a significant influence on reducing the S2- concentration until 8.75 g/L (mole ratio of Fe to S being ~1), further increase in iron powder dosage does not result in further reduction of S2- concentration (Fig. 5(a)). The variation of  concentration shows a similar trend with the effect of iron powder dosage, the critical dosage is about 4.38 g/L (mole ratio of Fe to S being ~0.5) (Fig. 5(b)). The variation tendencies of other sulfur-bearing ions with increasing the iron powder dosage in Figs. 5(a) and (b), including
concentration shows a similar trend with the effect of iron powder dosage, the critical dosage is about 4.38 g/L (mole ratio of Fe to S being ~0.5) (Fig. 5(b)). The variation tendencies of other sulfur-bearing ions with increasing the iron powder dosage in Figs. 5(a) and (b), including  ,
,  and S2-, are coincident with the results in Figs. 3(b) and 4(b), respectively.
and S2-, are coincident with the results in Figs. 3(b) and 4(b), respectively.

Fig. 3 Effects of duration on reaction behavior of S2- in sodium aluminate solution at 80 °C (a) and 260 °C (b)

Fig. 4 Effects of duration on reaction behavior of  in sodium aluminate solution at 120 °C (a) and 260 °C (b)
in sodium aluminate solution at 120 °C (a) and 260 °C (b)
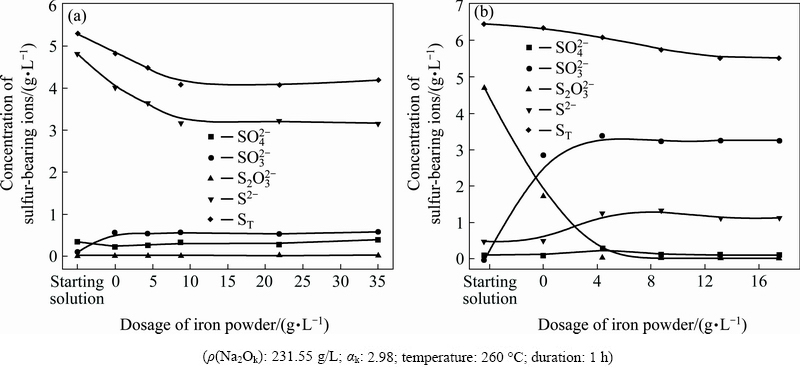
Fig. 5 Effects of iron powder dosage on reaction of S2- (a) and  (b) in sodium aluminate solution
(b) in sodium aluminate solution
3.3 Effects of αk and Na2Ok concentration on transformation of S2- and 
As αk and Na2Ok concentration are important characteristics of the sodium aluminate solution and vary in the Bayer process, their influences on the transformation of S2- and  were determined experimentally. The compositions of starting sodium aluminate solutions are listed in Table 6, and the results of the effect of αk and Na2Ok concentration are presented in Figs. 6 and 7, respectively.
were determined experimentally. The compositions of starting sodium aluminate solutions are listed in Table 6, and the results of the effect of αk and Na2Ok concentration are presented in Figs. 6 and 7, respectively.
Table 6 Compositions of starting sodium aluminate solutions


Fig. 6 Effects of αk on reaction of S2- (a) and  (b) in sodium aluminate solution
(b) in sodium aluminate solution
The S2- concentration reduces with increasing αk, while the ST in solution initially decreases and then remains stable (Fig. 6(a)). Figure 6(b) shows that the  concentration also decreases with increasing αk, and that
concentration also decreases with increasing αk, and that  can even completely convert to
can even completely convert to  and S2- in solution at αk greater than 2.07.
and S2- in solution at αk greater than 2.07.

Fig. 7 Effects of Na2Ok concentration on reaction of S2- (a) and  (b) in sodium aluminate solution
(b) in sodium aluminate solution
It is also demonstrated that higher Na2Ok concentration can expedite the reaction of S2- with iron powder (Fig. 7(a)) with the Na2Ok concentration ranging from 170 to 300 g/L,  can be completely transformed and its concentration declines to zero (Fig. 7(b)).
can be completely transformed and its concentration declines to zero (Fig. 7(b)).
In summary, higher αk or concentrated Na2Ok, corresponding to greater free sodium hydroxide concentration, is conducive to the reaction of S2- and  with iron powder due to increased formation of
with iron powder due to increased formation of  [26]. In addition, the variations of
[26]. In addition, the variations of  and
and  concentrations with αk or Na2Ok concentration exhibit the similar tendency to those with the increasing duration discussed in section 3.2.
concentrations with αk or Na2Ok concentration exhibit the similar tendency to those with the increasing duration discussed in section 3.2.
4 Conclusions
1) Fe promotes the transformation of S2- into residues, nevertheless, Fe2O3 and Fe3O4 have minimal effects on the reaction of S2- at 260 °C. The iron- containing phases including Fe, Fe2O3 and Fe3O4, can promote  transformation to
transformation to  and S2-, but have little influences on the transformation of
and S2-, but have little influences on the transformation of  and
and  in sodium aluminate solution.
in sodium aluminate solution.
2) The  formed by iron powder at elevated temperatures may react with S2- in sodium aluminate to generate FeS2 after digestion, resulting in the S2- concentration decrease in solution.
formed by iron powder at elevated temperatures may react with S2- in sodium aluminate to generate FeS2 after digestion, resulting in the S2- concentration decrease in solution.
3) Increasing the temperature, duration, dosage of iron powder, αk and Na2Ok concentration accelerates the transformation of S2- and  in solution in the presence of iron powder.
in solution in the presence of iron powder.
References
[1] FU Shi-wei. Prospection analysis of development of high-sulfur bauxite of Guizhou [J]. Mineral Exploration, 2011(2): 159-164. (in Chinese)
[2] YIN Jian-guo, XIA Wen-tang, HAN Ming-rong. Resource utilization of high-sulfur bauxite of low-median grade in Chongqing, China [C]//Light Metals. San Diego, California: Wiley, 2011: 19-22.
[3] PENG Xin, JIN Li-ye. Development and application of bauxite containing high sulfur [J]. Light Metals, 2010(11): 14-17. (in Chinese)
[4] HU Xiao-lian, CHEN Wen-mi, XIE Qiao-ling. Sulfur phase and sulfur removal in high sulfur-containing bauxite [J]. Transactions of Nonferrous Metals Society of China, 2011, 21(7): 1641-1647.
[5] WANG Xiao-min, ZHANG Ting-an,  Guo-zhi, BAO Li. Selection of flotation desulfurization collector for high-sulfur bauxite [J]. Journal of Northeastern University (Natural Science), 2010, 31(4): 555-558. (in Chinese)
Guo-zhi, BAO Li. Selection of flotation desulfurization collector for high-sulfur bauxite [J]. Journal of Northeastern University (Natural Science), 2010, 31(4): 555-558. (in Chinese)
[6] ZHOU Ji-kui, LI Hua-xia. Experimental research on bacterial oxidation of pyrite in high sulfur bauxite [J]. Metal Mine, 2011(12): 67-69. (in Chinese)
[7] LEWIS A E. Review of metal sulphide precipitation [J]. Hydrometallurgy, 2010, 104(2): 222-234.
[8] LI Xiao-bin, LI Chong-yang, PENG Zhi-hong, LIU Gui-hua, ZHOU Qiu-sheng, QI Tian-gui. Interaction of sulfur with iron-containing substances in sodium aluminate solutions [J]. Transactions of Nonferrous Metals Society of China, 2015, 25(2): 608-614.
[9] HE Run-de, TIAN Zhong-liang. Discussion on reasonable cost of sulphur removal with barium aluminate from industrial sodium aluminate solution [J]. Journal of Guizhou University of Technology (Natural Science Edition), 2000, 29(6): 54-58. (in Chinese)
[10] HU Xiao-lian, CHEN Wen-mi. Desulfurization from sodium aluminate solution by wet oxidation [J]. Journal of Central South University (Science and Technology), 2011, 42(10): 2911-2916. (in Chinese)
[11] LIU Zhan-wei, LI Wang-xing, MA Wen-hui, YIN Zhong-lin, WU Guo-bao. Conversion of sulfur by wet oxidation in the Bayer process [J]. Metallurgical and Materials Transactions B, 2015, 46(4): 1702-1708.
[12] LIU Zhan-wei, LI Wang-xing, MA Wen-hui, YIN Zhong-lin, WU Guo-bao. Comparison of deep desulfurization methods in alumina production process [J]. Journal of Central South University, 2015, 22(10): 3745-3750.
[13] KUZNETSOV S I, GRACHEV V V, TYURIN N G. Interaction of iron and sulfur in alkaline aluminate solutions [J]. Russian Journal of Applied Chemistry, 1975, 48(4): 748-750.
[14] LI Xiao-bin, LI Chong-yang, QI Tian-gui, ZHOU Qiu-sheng, LIU Gui-hua, PENG Zhi-hong. Reaction behavior of pyrite during Bayer digestion at high temperature [J]. The Chinese Journal of Nonferrous Metals, 2013, 23(3): 829-835. (in Chinese)
[15] XIE Qiao-ling, CHEN Wen-mi. Corrosion behavior of 16Mn low alloy steel in sulfide-containing Bayer solutions [J]. Corrosion Science, 2014, 86: 252-260.
[16] XIE Q L, CHEN W M, YANG Q. Influence of sulfur ions on corrosion of 16Mn low-alloy steel in sulfide-containing Bayer solutions [J]. Corrosion, 2014, 70(8): 842-849.
[17] XIE Qiao-ling, CHEN Wen-mi. Effect of S2- on corrosion behavior of low alloy steel in sodium aluminate solution [J]. The Chinese Journal of Nonferrous Metals, 2013, 23(12): 3462-3469. (in Chinese)
[18] WENSLEY D A, CHARLTON R S. Corrosion studies in kraft white liquor potentiostatic polarization of mild steel in alkaline solutions containing sulfur species [J]. Corrosion, 1980, 36(8): 385-389.
[19] ABIKENOVA G K, KOVZALENKO V A, AMBARNIKOVA G A, IBRAGIMOVA A T. Investigation of the effect and behavior of sulfur compounds on the technological cycle of alumina production [J]. Russian Journal of Non-Ferrous Metals, 2008, 49(2): 91-96.
[20] WATTS H, UTLEY W. Volumetric analysis of sodium aluminate solutions [J]. Analytical Chemistry, 1953, 25(6): 864-867.
[21] CHEN Wen-mi, HU Qin. Research on the analysis of low-valence sulphion in sodium aluminate solution [J]. Light Metals, 2012(10): 17-20. (in Chinese)
[22] CHEN Wan-kun, PENG Guan-cai. The intensifying digestion of diasporic bauxite [M]. Beijing: Metallurgical Industry Press, 1997: 112-116. (in Chinese)
[23] VASQUEZ MOLL D V, SALVAREZZA R C, VIDELA H A, ARVIA A J. A comparative pitting corrosion study of mild steel in different alkaline solutions containing salts with sulphur-containing anions [J]. Corrosion Science, 1984, 24(9): 751-767.
[24] TREMAINE P R, LEBLANC J C. The solubility of magnetite and the hydrolysis and oxidation of Fe2+ in water to 300 °C [J]. Journal of Solution Chemistry, 1980, 9(6): 415-442.
[25] UCHIDA S, KASHIWAGI H, SATO T, OKUWAKI A. Formation of iron oxides by the oxidation of iron in Fe-MOH-H2O and Fe-MOH-H2O-O2 systems (M=Li, Na, K) [J]. Journal of Materials Science, 1996, 31(14): 3827-3830.
[26] LI Xiao-bin, LIU Nan, QI Tian-gui, WANG Yi-lin, ZHOU Qiu-sheng, PENG Zhi-hong, LIU Gui-hua. Conversion of ferric oxide to magnetite by hydrothermal reduction in Bayer digestion process [J]. Transactions of Nonferrous Metals Society of China, 2015, 25(12): 3467-3474.
含铁物质对含硫离子在铝酸钠溶液中转化的影响
李小斌,牛 飞,刘桂华,齐天贵,周秋生,彭志宏
中南大学 冶金与环境学院,长沙 410083
摘 要:用拜耳法处理高硫铝土矿时,矿石中的硫化物会与含铁物质在铝酸钠溶液中反应,进而导致严重的设备腐蚀和氧化铝产品降级。本文作者研究含铁物质对含硫离子(S2-, ,
, 和
和 )在铝酸钠溶液中转化的影响。研究结果表明:铁粉、Fe2O3和Fe3O4均难以与
)在铝酸钠溶液中转化的影响。研究结果表明:铁粉、Fe2O3和Fe3O4均难以与 和
和 反应,而且所有含铁物质,特别是铁粉,均能促进
反应,而且所有含铁物质,特别是铁粉,均能促进 转化为
转化为 和S2-;在高温条件下铁粉与铝酸钠溶液反应生成
和S2-;在高温条件下铁粉与铝酸钠溶液反应生成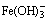 ,进而可与S2-反应生成FeS2,但Fe2O3和Fe3O4对S2-的反应影响很小;升高温度、延长反应时间、增加铁粉添加量、提高溶液中Na2Ok与Al2O3的摩尔比和苛碱浓度均有利于
,进而可与S2-反应生成FeS2,但Fe2O3和Fe3O4对S2-的反应影响很小;升高温度、延长反应时间、增加铁粉添加量、提高溶液中Na2Ok与Al2O3的摩尔比和苛碱浓度均有利于 向
向 和S2-转化。本研究结果有助于在拜耳法处理高硫铝土矿过程中开发减缓设备腐蚀和降低碱耗的技术。
和S2-转化。本研究结果有助于在拜耳法处理高硫铝土矿过程中开发减缓设备腐蚀和降低碱耗的技术。
关键词:高硫铝土矿;铝酸钠溶液;含硫离子;含铁物质;转化
(Edited by Wei-ping CHEN)
Foundation item: Project (51604309) supported by the National Natural Science Foundation of China; Project (201509048) supported by the Environmental Protection’s Special Scientific Research for Chinese Public Welfare Industry; Project (2015CX001) supported by the Innovation-driven Plan in Central South University, China
Corresponding author: Tian-gui QI; Tel/Fax: +86-731-88830453; E-mail: qitiangui@csu.edu.cn
DOI: 10.1016/S1003-6326(17)60105-5
Abstract: Sulfides in the high-sulfur bauxite lead to serious steel equipment corrosion and alumina product degradation via the Bayer process, owing to the reactions of sulfur and iron-containing phases in the sodium aluminate solution. The effects of iron-containing phases on the transformation of sulfur-bearing ions (S2-,  ,
,  and
and  ) in sodium aluminate solution were investigated. Fe, Fe2O3 and Fe3O4 barely react with
) in sodium aluminate solution were investigated. Fe, Fe2O3 and Fe3O4 barely react with  and
and  , but all of them, particularly Fe, can promote the conversion of
, but all of them, particularly Fe, can promote the conversion of  to
to  and S2- in sodium aluminate solution. Fe can convert to
and S2- in sodium aluminate solution. Fe can convert to 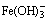 in solution at elevated temperatures, and further react with S2- to form FeS2, but Fe2O3 and Fe3O4 have little influence on the reaction behavior of S2- in sodium aluminate solution. Increasing temperature, duration, dosage of Fe, mole ratio of Na2Ok to Al2O3 and caustic soda concentration are beneficial to the transformation of
in solution at elevated temperatures, and further react with S2- to form FeS2, but Fe2O3 and Fe3O4 have little influence on the reaction behavior of S2- in sodium aluminate solution. Increasing temperature, duration, dosage of Fe, mole ratio of Na2Ok to Al2O3 and caustic soda concentration are beneficial to the transformation of  to
to  and S2-. The results may contribute to the development of technologies for alleviating the equipment corrosion and reducing caustic consumption during the high-sulfur bauxite treatment by the Bayer process.
and S2-. The results may contribute to the development of technologies for alleviating the equipment corrosion and reducing caustic consumption during the high-sulfur bauxite treatment by the Bayer process.


