Trans. Nonferrous Met. Soc. China 28(2018) 2368-2374
Selective leaching and recovery of V as iron vanadate from industrially generated Mo-V residue
P. C. ROUT, G. K. MISHRA, D. MOHAPATRA, B. PADH, B. R. REDDY
Technology Centre, R&D Department, Rubamin Ltd., Halol-389350, India
Received 27 November 2017; accepted 10 May 2018
Abstract:
A commercial process was developed to treat a Ca-based Mo-V residue generated in Mo processing plant. Vanadium was selectively leached using acetic acid and recovered as iron vanadate by hydro process. Process conditions for selective V leaching and iron vanadate precipitation were investigated. V recovery efficiency of 90.3% was achieved with a V content of 26.5% and an Fe content of 29% in the iron vanadate cake suitable for ferrovanadium industry. The overall Mo recovery in the whole process was 98.6%. The obtained leach residue containing 14.3% Mo with negligible impurities can be used as a feed material for the Mo production process or ferromolybdenum industry. This simple and economical process generates two product streams from a single operation and has the potential to solve a long standing problem of handling such a mixed Mo-V residue.
Key words:
iron vanadate; leaching; acetic acid; precipitation; ferrovanadium;
1 Introduction
Vanadium is mainly used as an alloying element in the steel industry as ferrovanadium, accounting for about 85% of the total V production. Other major applications are in nonferrous alloys, mostly V-containing titanium alloys and Ni-based super-alloys for aerospace industry, as catalyst in the manufacture of sulphuric acid by the contact process, ceramics and batteries [1,2].
The major secondary sources of V are sludges generated during processing of bauxite ore by alumina industry, waste catalyst of H2SO4 manufacturing industry, steel making slag, oil and coal residues, spent catalysts, and uranium co-products. In addition to these, V is also present in deposits of heavy crude oil produced in countries like Canada, Venezuela, USA and its content varies widely from 1 to 1500 mg (V)/kg (crude) [3,4]. Hydro-treating of heavy crude oil using molybdenum- based catalyst for the removal of impurities such as V, Ni, Fe, S and N compounds by the oil producing industries worldwide, generates waste catalyst materials after prolonged usage. Such waste catalyst materials generally contain 8%-12% Mo, 0.5%-3% V, 1%-3% Ni/Co, 4%-8% S, 25%-30% Al2O3 and rest hydrocarbons depending on the crude oil origin and are considered to be hazardous due to the presence of toxic elements. At the same time, these are considered as valuable sources due to the presence of Mo, V and Ni/Co metal ions in high concentrations. These waste catalysts are commercially used by different industries to recover Mo as catalyst/chemical grade molybdenum compounds, sulphur as sodium sulphate and finally alumina to cement industry application. Various methods such as leaching, precipitation, ion exchange and solvent extraction (SX) are reported for the treatment of such waste catalysts [5-9], in which two solutions are generated: one is V-free but Mo-rich solution for further processing to Mo compounds and the other is the solution containing V and Mo.
Further treatment of the above Mo and V solution with lime generates mixed cake containing 2%-5% V, 10%-15% Mo, 12%-17% Ca and other impurities such as As, Co, Ni, P, SiO2, Al and Pb in variable quantities. At present, this type of residue is not saleable due to the presence of V as impurity for certain molybdenum applications. So, this type of material is stockpiled in the plant, thereby increasing the inventory cost. Processing of such a source of V is of considerable interest in the catalyst recycling industry to produce high-valued V and Mo products. However, due to unpredictable composition of such materials, technology and cost involved to treat such secondary resources are not well defined in the literatures [8,10].
The most conventional method to treat vanadium- bearing raw materials is the roasting at elevated temperature in the presence of alkali metal salts (usually NaCl, Na2CO3 and Na2SO4) followed by water leaching, purification and enrichment of leaching solutions and finally recovery of vanadium as the desired product [11-13]. However, the conventional process could be non-economical for low V content materials since it is fuel-intensive and requires high temperature. Also during water leaching, V and other elements such as Mo and W were leached into the aqueous medium, making the separation process complicated and less effective. Considering iron vanadate synthesis by wet chemistry processes, little information is reported and even then it is conflicting.
Hence, the objective of the present study is to develop an economical and commercially viable process to treat a Ca-rich Mo-V residue to make both the products saleable. In the first step, attempts were made to treat such a material by selectively separating the V value to the aqueous phase and transforming the residual Mo cake to a saleable product. In the second step, the V-rich leach liquor was treated to synthesize iron vanadate for its further utilization in the making of ferrovanadium [10]. In the present study, acetic acid and ferric sulphate were used for selective V leaching and to synthesize iron vanadate, respectively. Different process parameters such as pH, temperature, time, pulp density (PD) and reagent concentration were studied for selective V leaching and iron vanadate precipitation. Product quality with respect to impurities, V content, process feasibility and applicability for ferrovanadium making were discussed. The whole process was piloted in 1 kg scale and finally, a flow sheet of the complete process along with material balance was presented.
2 Aqueous chemistry of vanadium
The precipitation of inorganic V compounds is considered to be difficult because V exhibits a complex variety of speciation and oxidation states in aqueous solution. Vanadium dissolved in aqueous solution mainly exists in three different oxidation states i.e. +3, +4 and +5 depending on solution pH, φ (potential) and V concentration [14]. In solutions containing millimolar and higher V5+ concentration, the major species in solution are various oligomers such as 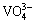 ,
, 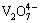 ,
, 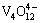 ,
, 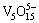 ,
, 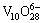 and
and 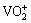 . Under acidic condition with pH values ranging from 3 to 6, the predominant oligomer is decavanadate,
. Under acidic condition with pH values ranging from 3 to 6, the predominant oligomer is decavanadate, 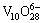 having a yellow-orange color; whereas below pH 3 the cationic monomer,
having a yellow-orange color; whereas below pH 3 the cationic monomer, 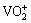 , is the major species. From pH 6 to 10, the major oligomeric species include
, is the major species. From pH 6 to 10, the major oligomeric species include 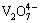 ,
, 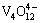 and
and 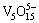 which are all colorless. Above pH 10, the anionic monomer,
which are all colorless. Above pH 10, the anionic monomer, 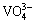 , is the favoured species [15].
, is the favoured species [15].
3 Experimental
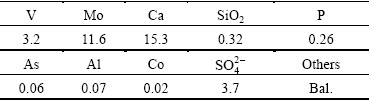
The raw material (Mo-V residue) used for this work was collected from a plant site situated in the western part of India. The chemical composition of the Mo-V residue was analyzed to be 11.6% Mo, 3.2% V, 15.3% Ca along with impurities such as SiO2, P, Al, As and Co (Table 1). Parts of Ca exist as free Ca(OH)2 and CaSO4. This material was used as such for all further experiments.
Table 1 Chemical composition of Mo-V residue (mass fraction, %)
All leaching and precipitation experiments were performed in a glass reactor (1 L) covered with a lid having three necks. The central neck was used to connect a condenser with continuous water flow to avoid evaporation loss. The other two necks were used for reagent addition, pH and temperature measurement when required. A magnetic stirrer fitted with a temperature controlling thermostat maintained at an accuracy of ±1 °C, was employed for the stirring of solutions in bench scale. For each run, 0.5 L leachant having pre-determined concentration was charged into the reaction flask and heated to the required temperature. The residue was added to the flask after the attainment of desired temperature. The contents were stirred at 300 r/min and the reaction time was counted after addition of the solid. Except the temperature variation experiments, all other experiments were carried out at room temperature of (27±1) °C.
Vanadium precipitation experiments were carried out by adding equal molar Fe3+ concentration with respect to vanadium present in the solution. The Fe3+ solution was added slowly through a burette. The pH of the suspension was maintained at a desired value by adding dilute H2SO4/NaOH. After complete addition of Fe3+ solution, the suspension solution was allowed for different time intervals to determine the iron vanadate conversion. All chemicals used were of analytical grade supplied by BDH/Merck, India.
Metal concentration in the aqueous phase was determined by ICP-OES (Spectro, Spectro Arcos) after suitable dilution. The iron vanadate precipitate was washed with deionized water to remove any residual sulphate and analyzed for its purity by dissolving in suitable aqueous solution followed by ICP-OES measurement.
4 Results and discussion
4.1 Leaching of Mo-V residue
Considering the unpredictable composition of the raw material with respect to Mo, V and impurity concentrations, the selective leaching of vanadium is one of the critical and challenging step in this process. Typically, such residue contains 2%-5% V and 10%- 15% Mo. Based on our preliminary tests and literatures available [16,17], water soluble organic acid, i.e., acetic acid was selected for the selective leaching of V. The chemical reaction for vanadium leaching can be expressed by the following equation:
Ca(VO3)2+2CH3COOH=2HVO3+Ca(CH3COO)2 (l)
As per the above equation, stoichimetrically 1 mol of acetic acid is required for 1 mole of V for quantitative leaching. So, 1 g of V needs 1.17 g of acetic acid as per the above mechanism.
The effect of pH in the range of 3.0-7.0 on V and Mo leaching was studied keeping other experimental parameters constant such as stoichiometric acetic acid, temperature (27±1) °C, time 2 h and PD 20%. As shown in Fig. 1, V and Mo leaching efficiencies increased gradually from 76.6% to 95.3% and 0.9% to 7.3%, respectively with decrease in pH from 7.0 to 3.0. Any amount of Mo transferred to the V leach liquor is considered as a loss and should be minimized. Considering the fact that the Mo-rich residue is a sellable product, special attention was paid to its purity and composition. Taking into consideration Mo cake composition, purity and V selective leachability, pH 6.0 was considered as ideal for V leaching. At this pH, 90.6% V was leached with a loss of 1.3% Mo.
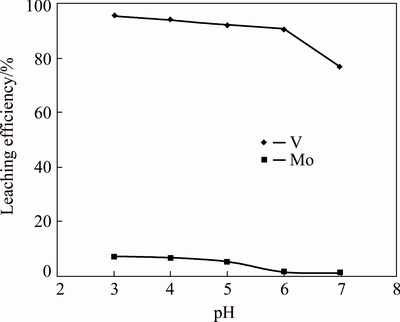
Fig. 1 Effect of pH on V and Mo leaching efficiencies (time 2 h, temperature 27 °C, PD 20%, stoichiometric acetic acid against V)
Table 2 shows the effects of time, temperature and PD on leaching efficiencies of V and Mo. Considering temperature effect on the V leaching efficiency, it was observed that, V leaching efficiency increased marginally from 90.6% to 93.8% by increasing leaching temperature from 27 to 80 °C, whereas Mo loss increased from 1.29% to 4.74%. Considering the fact that, any amount of Mo leached will increase impurity in V leach solution coupled with operational simplicity and energy efficiency, it was concluded that operating the leaching process at room temperature (27±1) °C is the ideal option. The effect of leaching time of 1-5 h on the V recovery and Mo loss showed that leaching efficiency of V was 82.8% at 1 h, and it increased up to 90.6% at 2 h. Further increase in reaction time up to 5 h resulted in insignificant increase in V leaching efficiency up to 92.2% along with increased Mo loss from 0.86% to 3.45%. PD is an important parameter in the leaching process that has significant impact on process economy. Results presented in Table 2 show that there is hardly any significant difference in leaching efficiencies of V and Mo within a PD range of 20%-40% keeping other parameters constant.
Table 2 Effects of temperature, time and PD on leaching efficiencies of V and Mo at pH 6 and stoichiometric acetic acid
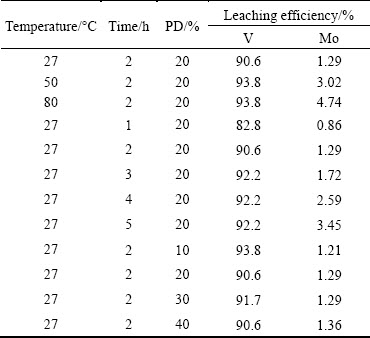
Considering process requirements and engineering aspects, the optimum V leaching conditions derived from the above experimental results were summarized as follows: leaching temperature RT (27±1 °C), pH 6.0, stoichiometric acetic acid against V, leaching time 2 h, PD 30%. Under the above optimum leaching conditions, the V leaching efficiency reached 91.7%, at the same time Mo loss was 1.29%. The leaching behavior of impurities such as As, P, SiO2 and Co followed a pattern similar to that of V. With the above condition, 84.6% P, 86.1% As, 89.6% SiO2 and 80% Co were leached out to the aqueous medium, giving a relatively clean solid residue rich in Mo. The chemical analysis of a typical Mo-cake is presented in Table 3. The Mo content in the cake is 14.3% which has multiple applications including as a feed material for the Mo processing plant. Alternatively, this type of cake can be used as a Mo source in ferromolybdenum production process.
Table 3 Chemical analysis of leach residue after V leaching (mass fraction, %)

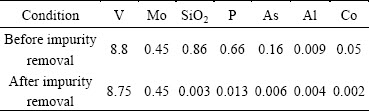
The chemical analysis of the V-rich leach liquor is shown in Table 4. As shown in Table 4, the concentration of V in the leach liquor is 8.8 g/L having considerable level of impurities such as 0.45 g/L Mo, 0.86 g/L SiO2, 0.66 g/L P, 0.16 g/L As and 0.05 g/L Co. The solution was further purified before vanadium recovery stage in which the impurities such as SiO2, P, As and Co were removed from the leach liquor by the standard procedure reported in Ref. [18]. During this purification process, V loss was negligible and most of Mo transferred to the V leach liquor. The purified leach liquor concentration is presented in Table 4. Considering the yellow-orange color of the leach liquor, pH 6.0, and φ of 0.5 V, it was assumed that V exists as pentavalent (V5+) state in the leach liquor [14].
Table 4 Vanadium leach liquor composition before and after impurity removal ( g/L)
4.2 Vanadium precipitation as iron vanadate
The recovery of V from the leached solution can be done in a variety of ways. The choice of processing route is based upon the form in which V is intended to be recovered considering commercial aspects. Industrially, ferrovanadium is produced by smelting a mixture of vanadium oxide, aluminium, iron/iron oxide and lime. The compound iron vanadate simply represents a joint carrier of oxides of iron and vanadium (Fe2O3·V2O5) [13]. From this viewpoint, it appears that it will be an attractive proposition to produce such specific compounds directly from the leach liquor that is suitable for ferrovanadium preparation. As the synthesis of iron vanadate was carried out by hydro-thermal process, the content of V and the impurity profile in the cake are more important than its chemical phase. However, precipitation of V in the form of iron ortho-vanadate (FeVO4) is preferred since this intermediate represents essentially the career of more V compared with other precipitated compounds.
4.2.1 Initial experiments on iron vanadate precipitation
Initial experiments for iron vanadate precipitation were carried out by adding calculated quantity of ferric salt as slurry into the V-rich leach liquor followed by pH control by adding H2SO4 or NaOH. The slurry was added in a single lot. Under the best possible condition, the precipitation efficiency of V reached only 78.5% with a V content of 16.8% in the iron vanadate cake even though 100% excess ferric salt was added for V precipitation. Theoretically, V content in anhydrous FeVO4 is 29.8% having an Fe/V molar ratio of 1:1. The reason for this low efficiency and lower V content in the iron vanadate cake was probably due to the hydrolysis of ferric salt as Fe(OH)3, thereby making Fe3+ unavailable for V precipitation.
Considering the above inefficiencies in the process, experiments were designed to get an iron vanadate having V content as close as to the theoretical figure by modifying the reagent addition patterns such as Fe3+ in solution form, Fe3+ concentration, addition time and pH control.
4.2.2 Optimization of iron vanadate precipitation
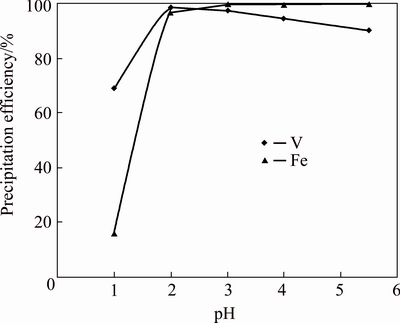
Fig. 2 Effect of pH on precipitation efficiency of iron vanadate (Fe/V molar ratio 1:1, temperature 50 °C, time 3 h)
Considering the solubility minimum of Fe3+ and V5+ ions in the aqueous medium, a pH range of 1.0 to 5.5 was chosen for iron vanadate precipitation keeping other parameters constant such as, Fe/V molar ratio 1:1, reaction temperature 50 °C and reaction time 3 h. The precipitation efficiencies of Fe and V against pH are presented in Fig. 2. Over all, V precipitation efficiency reached the maximum at pH 2.0, below and above that pH the efficiency decreased. However, Fe precipitation efficiency increased up to pH 3.0, with insignificant improvement up to pH 5.5. At pH 1.0, the efficiencies of Fe and V precipitated were 16.3% and 68.8%, respectively. This corresponds to absolute 1.5 g Fe and 5.9 g V going to the precipitate with an Fe/V molar ratio of 0.24:1. This was closely matched with the chemical analysis of the cake that contained 32.5% V and 8.3% Fe representing an Fe/V mole ratio of 0.23:1. Since the Fe/V molar ratio is less than that at pH 1.0, this implied the precipitation of some other V compounds other than iron vanadate and there may be the existence of more than one species of V in the precipitate. Insoluble V compounds such as sodium polyvanadate (xNa2O·yV2O5·nH2O), Fe2V4O13 and V2O5·H2O would be the possible V compounds precipitated at pH 1.0 [19].
Between pH 2.0 and 5.5, the V and Fe precipitation mechanism looks different as compared to that at pH 1.0. Considering the filtrate analysis, 98.2% V and 97.4% Fe were precipitated at pH 2.0. V precipitation efficiency decreased steadily when pH increased from 2.0 to 5.5 and reached 90.2% at pH 5.5, whereas Fe precipitation efficiency was more than 99.8% in the pH range of 3.0-5.5. This corresponds to an Fe/V molar ratio of 0.99:1 at pH 2.0 and further increased up to 1.11:1 at pH 5.5. Considering the content of V in the cake and the molar ratio of Fe/V, pH 2.0 was found to be ideal for iron vanadate precipitation. The precipitation of Fe3+ as Fe(OH)3 when it comes in contact with the leach liquor in the favorable pH region could be the reason for the decreased V precipitation efficiency at higher pH. Therefore, less amount of Fe3+ is available in the solution to make complex with V, thereby decreasing the V precipitation efficiency. Other possible mechanism could be that the precipitate formed at lower pH is gradually transformed into iron oxide/hydroxide with the increase of pH, as a result releasing V back into the solution.
The effects of time on iron vanadate precipitation at pH 2.0 and 5.5 are presented in Figs. 3(a) and (b), respectively, keeping other parameters constant such as Fe/V molar ratio of 1:1 and temperature of 50 °C. When vanadium precipitation was carried out at pH 2.0, both Fe and V followed identical reaction kinetics with similar precipitation efficiency throughout the studied time period. For example, after 0.5 h of reaction 75.3% V and 79.6% Fe were precipitated, and they increased up to 93.4% for V and 95.5% for Fe after 1 h of reaction. The kinetics slowed down afterwards and reached precipitation efficiencies of 98.2% for V and 97.4% for Fe after 3 h of reaction time. The results showed that V was precipitated as an equimolar compound with iron. This was further validated by the chemical analysis of the cake that showed 26.5% V and 29.1% Fe, suggesting an Fe/V molar ratio close to 1:1, confirming an iron ortho-vanadate species.
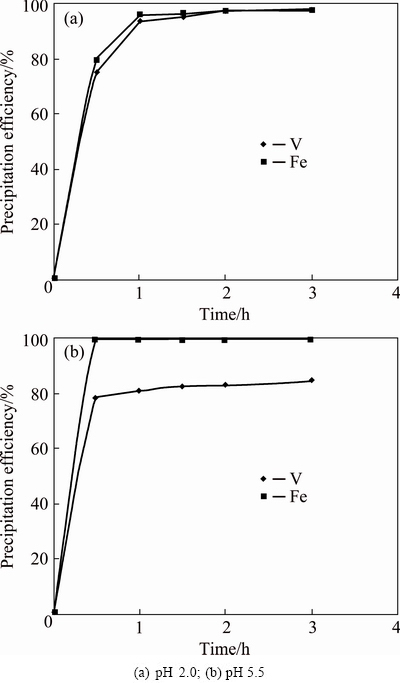
Fig. 3 Effect of time on precipitation of iron vanadate at Fe/V molar ratio of 1:1, 50 °C and different pH
When V precipitation was carried out at pH 5.5, the results showed different precipitation mechanisms compared to precipitation at pH 2.0. After reaction time of 0.5 h, 99.6% Fe was precipitated against a V precipitation efficiency of only 77.9%. Considering the absolute V precipitated, the theoretical quantity of Fe to be precipitated to produce iron ortho-vanadate precipitate should be less than that of the Fe precipitated in this condition. So, it was confirmed that some part of Fe might be precipitated as iron hydroxide along with iron vanadate. Between 0.5 and 3 h, there was hardly any Fe available for V precipitation since more than 99.6% Fe had already been precipitated within 0.5 h. On the other hand, V precipitation continued slowly but steadily from 77.9% to 84% between 0.5 and 3 h. This phenomenon can be best explained by the fact that freshly prepared iron hydroxide is a good adsorbent for many metals. This is supported by reported literature that V can be removed from solution by iron hydroxide adsorbents by inner-sphere surface complexation mechanism [20]. Overall, V precipitation in the presence of Fe followed two different mechanisms. At lower pH, V was precipitated by a chemical reaction with Fe; whereas at higher pH, V precipitation followed both chemical and surface adsorption mechanisms.
The effect of reaction temperature from 27 to 80 °C on V precipitation was studied at Fe/V molar ratio 1:1, time 3 h and pH 2.0. The precipitation efficiencies of Fe and V against temperature are presented in Fig. 4. According to the results, V precipitation efficiency almost remained constant at about 98% irrespective of precipitation temperature. However, Fe precipitation efficiency was 97.0% at 27 °C, 97.4% at 50 °C and 83.9% at 80 °C. The decrease in Fe precipitation efficiency at 80 °C is probably due to the higher solubility of iron vanadate at higher temperature. Soluble V could be re-precipitated as V2O5 leaving behind Fe in the solution due to its favorable pH and temperature conditions [21].
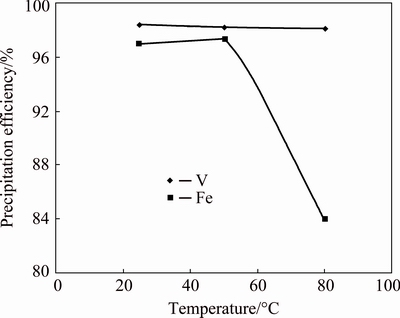
Fig. 4 Effect of temperature on precipitation of iron vanadate at Fe/V molar ratio 1:1, pH 2.0 and 3 h
Under optimum process conditions, substantial quantity of iron vanadate cake was generated. The cake contained 26.5% V and 29% Fe with negligible amount of impurities which have application for ferrovanadium production. A process flow diagram along with mass balance for Mo and V is presented in Fig. 5. On an input of 1 kg raw material basis that contained absolute 32 g V and 116 g Mo, the amount of Mo remaining in Mo-cake was 114.4 g representing 98.6% Mo recovery. After leaching and impurity removal, 3.3 L leach liquor was obtained bearing 8.9 g/L V (Abs V: 29.4 g) which was subjected to iron vanadate precipitation. To this leach liquor, 177.5 g ferric sulphate containing 32.2 g Fe (Fe/V molar ratio 1:1) was added according to the pre-discussed pattern. The quantity of iron vanadate cake obtained was 109 g having 26.5% V and 29% Fe. The absolute amount of V transferred to iron vanadate cake was 28.9 g, indicating an overall recovery of 90.3%. Considering the value rendered from Mo and V by processing such a residue, it was concluded that the process is commercially feasible and economically viable.
5 Conclusions
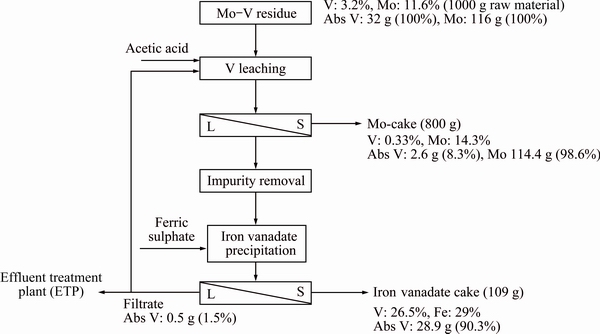
Fig. 5 Process flow diagram for recovery of V and Mo from Mo-V residue
1) Vanadium was selectively leached leaving 98.7% of Mo in the cake, which can be further utilized as a raw material for Mo production process or direct sale to Fe-Mo producing companies.
2) Precipitation efficiency of V was improved from 78.5% to 98.2% by modifying the reagent addition pattern which is the key step during precipitation. As a result, purity of V in iron vanadate was increased from 16.8% to 26.5% suitable for ferrovanadium applications with an overall V recovery of 90.3%.
3) Mo recovery was 98.6% in the leach residue which contained 14.3% Mo with negligible quantity of impurities having multiple applications.
4) The novelty of the present process is the handling of Mo-V residue generated from the post processing of high V-bearing waste catalyst to produce iron ortho- vanadate and at the same time generating a saleable Mo by-product, thereby making the process cost effective.
5) A flow diagram on 1 kg input basis with mass balance for the above process was suggested.
Acknowledgments
The authors are thankful to the management of Rubamin Limited, Vadodara (India) for granting permission to publish this research work.
References
[1] SINGH A K. Indian minerals yearbook 2015 (Vol. II): Reviews on metals and alloys [M]. 54th ed. Nagpur: Indian Bureau of Mines, 2017.
[2] TAYLOR P R, SHUEY S A, VIDAL E E, GOMEZ J C. Extractive metallurgy of vanadium containing titaniferous magnetite ores: A review [J]. Minerals and Metallurgical Processing, 2006, 23(2): 80-86.
[3] THEAKSTON F. Inorganic pollutants: Vanadium [M]. 2nd ed. Copenhagen: WHO Regional Publications, 2000: 170-172.
[4] GAO Y Y, SHEN B X, LI J C. Distribution of Ni and V in Venezuela crude oil [J]. Petroleum Science & Technology, 2013, 31(5): 509-515.
[5] CHEN Xiang-yang, LAN Xin-zhe, ZHANG Qiu-li, MA Hong-zhou, ZHOU Jun. Leaching vanadium by high concentration sulfuric acid from stone coal [J]. Transactions of Nonferrous Metals Society of China, 2010, 20: 123-126.
[6] LIU Hui-bin, DU Hao, WANG Da-wei, ZHANG Yi. Kinetics analysis of decomposition of vanadium slag by KOH sub-molten salt method [J]. Transactions of Nonferrous Metals Society of China, 2013, 23: 1489-1500.
[7] CHEN Bian-fang, HUANG Sheng, LIU Biao, GE Qi, XIE Shu-shan, WANG Ming-yu, WANG Xue-wen. Thermodynamic analysis for separation of vanadium and chromium in V(IV)-Cr(III)-H2O system [J]. Transactions of Nonferrous Metals Society of China, 2018, 28: 567-573.
[8] NGUYEN H T, LEE M S. Recovery of molybdenum and vanadium from acidic leaching solution of spent catalysts by solvent extraction [J]. Journal of the Korean Institute of Resources Recycling, 2013, 22: 3-11.
[9] NGUYEN T H, LEE M S. Recovery of molybdenum and vanadium with high purity from sulfuric acid leach solution of spent hydrodesulfurization catalysts by ion exchange [J]. Hydrometallurgy, 2014, 147-148: 142-147.
[10] PATTNAIK S P, MUKHERJEE T K, GUPTA C K. Ferrovanadium from a secondary source of vanadium [J]. Metallurgical Transactions B, 1983, 14: 133-135.
[11] BURWELL B. Extractive metallurgy of vanadium [J]. Journal of Metals, 1961, 13(8): 562-566.
[12] SHLEWIT H, ALIBRAHIM M. Extraction of sulfur and vanadium from petroleum coke by means of salt-roasting treatment [J]. Fuel, 2006, 85(5-6): 878-880.
[13] MOSKALYK R R, ALFANTAZI A M. Processing of vanadium: A review [J]. Minerals Engineering, 2003, 16(9): 793-805.
[14] GUPTA C K, KRISHNAMURTHY N. Extractive metallurgy of vanadium [M]. Amsterdam: Elsevier, 1992.
[15] CRANS D C, TRACEY A S. The chemistry of vanadium in aqueous and nonaqueous solution (ACS symposium series) [M]. Washington: American Chemical Society, 1998.
[16] LUO L, MIYAZAKI T, SHIBAYAMA A, YEN W T, FUJITA T. Separation of vanadium and tungsten from a sodium tungstate solution [J]. Canadian Metallurgical Quarterly, 2003, 42(4): 411-420.
[17] MENASHI J, RAPPAS A S, DOUGLAS D A. Vanadium recovery from scrap alloys: US patent, 4298582 [P]. 1981-11-03.
[18] LASSNER E, SCHUBERT W D. Tungsten properties, chemistry, technology of the element, alloys, and chemical compounds [M]. New York: Plenum Publishers, 1999.
[19] CHEN Liang, LIU Feng-qiang, LI Da-biao. Precipitation of crystallized hydrated iron (III) vanadate from industrial vanadium leaching solution [J]. Hydrometallurgy, 2011, 105: 229-233.
[20] PEACOCK C L, SHERMAN D M. Vanadium (V) adsorption onto goethite (α-FeOOH) at pH 1.5 to 12: A surface complexation model based on ab initio molecular geometries and EXAFS spectroscopy [J]. Geochimica et Cosmochimica Acta, 2004, 68(8): 1723-1733.
[21] GAO Qiang, SHI Pei-yang, LIU Cheng-jun, JIANG Mao-fa. Experimental study on liquid precipitation of iron vanadate [J]. Journal of Northeastern University (Natural Science), 2015, 36(1): 33-37 (in Chinese).
从工业钼钒渣中选择性浸出钒并以钒酸铁形式回收钒
P. C. ROUT, G. K. MISHRA, D. MOHAPATRA, B. PADH, B. R. REDDY
Technology Centre, R&D Department, Rubamin Ltd., Halol-389350, India
摘 要:开发一种处理钼加工厂产出的钙基钼钒渣的工业化生产工艺。用乙酸选择性浸出钒,采用湿法工艺以钒酸铁形式回收钒。研究选择性浸出钒和沉淀钒酸铁的工艺条件。钒回收率达到90.3%,钒酸铁滤饼中钒含量为26.5%,Fe含量为29%,适合于钒铁工业。全流程钼的总回收率为98.6%。所得的浸出渣含钼14.3%,杂质含量可忽略不计,可用作钼生产或者钼铁工业的原料。这个简单而经济的工艺在单一操作中产生两个产品流,有可能解决长期存在的处理这种混合钼钒残渣的问题。
关键词:钒酸铁;浸出;乙酸;沉淀;钒铁
(Edited by Wei-ping CHEN)
Corresponding author: B. R. REDDY; Tel: +91-9687605535; E-mail: ramachandra.reddy@rubamin.com, drbrcreddy_iict@yahoo.com
DOI: 10.1016/S1003-6326(18)64882-4
Abstract: A commercial process was developed to treat a Ca-based Mo-V residue generated in Mo processing plant. Vanadium was selectively leached using acetic acid and recovered as iron vanadate by hydro process. Process conditions for selective V leaching and iron vanadate precipitation were investigated. V recovery efficiency of 90.3% was achieved with a V content of 26.5% and an Fe content of 29% in the iron vanadate cake suitable for ferrovanadium industry. The overall Mo recovery in the whole process was 98.6%. The obtained leach residue containing 14.3% Mo with negligible impurities can be used as a feed material for the Mo production process or ferromolybdenum industry. This simple and economical process generates two product streams from a single operation and has the potential to solve a long standing problem of handling such a mixed Mo-V residue.


