Trans. Nonferrous Met. Soc. China 29(2019) 1090-1098
Oxygen pressure leaching-flotation joint process for Jinbaoshan platinum group minerals
Shuai RAO1,2,3, Zhi-qiang LIU1,2,3, Xian-yang QIU1,2,3, Dong-xing WANG1,2,3, Hong-yang CAO1,2,3, Wei LI1,2,3, Jin-zhang TAO1,2,3
1. Guangdong Research Institute of Rare Metals, Guangzhou 510650, China;
2. State Key Laboratory of Separation and Comprehensive Utilization of Rare Metals, Guangdong Academy of Sciences, Guangzhou 510650, China;
3. Guangdong Province Key Laboratory of Rare Earth Development and Application, Guangzhou 510650, China
Received 11 July 2018; accepted 19 November 2018
Abstract:
An oxygen pressure leaching-flotation joint process was proposed to treat Jinbaoshan platinum group minerals to produce a desired concentrate. The result demonstrates that leaching parameters which include particle size, stirring speed, liquid-solid ratio, and the dosage of calcium lignosulfonate, simultaneously affect the leaching rates of base metals and the recovery of platinum group metals (PGMs). The complete dissolution of base metals sulfides leads to a reduction in the amount of flotation carrier for enriching PGMs, decreasing the recovery of PGMs. The optimum leaching conditions are determined as follows: liquid-solid ratio of 10 mL/g, 73% occupancy of ore particle size below 0.043 mm, stirring speed of 400 r/min, and 0.6 g dosage of calcium lignosulfonate. Under optimal conditions, the leaching rates of Cu, Ni and Fe are 87.6%, 87.6% and 90.3%, respectively. The grade of PGMs enriched in the flotation concentrate is 420 g/t through the flotation technology.
Key words:
platinum group metals; oxygen pressure leaching; flotation; recovery;
1 Introduction
Platinum group metals (PGMs) are widely used in manufacturing catalysts, electronic devices, space materials, biomedical devices, and jewelry, due to their excellent performance and properties [1-3]. The main sources for the production of PGMs are platinum group minerals [4-7] or secondary resources [8-11]. The methods for extracting PGMs from these resources mainly depend on their grade and phases [12]. In general, high-grade platinum group concentrates, ranging from 200 to over 2000 g/t PGMs, can be used as metallurgy materials directly. For low-grade platinum group concentrates, PGMs are usually recovered as byproducts of other metals such as Cu or Ni.
Currently, extraction of PGMs from high-grade concentrates is conducted through a pyrometallurgy and hydrometallurgy combined process [13]. During the pyrometallurgical step, the concentrates are processed by smelting and converting to produce a white matte [14,15]. The white matte is then further treated in a reverse leaching operation whereby most of the accompanying minerals (copper, nickel, cobalt, and iron sulfides) are dissolved to leave behind an upgraded PGMs concentrate for PGMs refinery. Although the process has been applied successfully on a commercial scale, the flotation concentrates generally need to conform to many specifications to render ideal for smelting. Rock mineral content of the flotation concentrates should produce a fluid slag at the desired temperature and high contents of chromium and magnesium oxides are detrimental to the smelting process. At the same time, the material must contain enough sulfides to form a reasonable quantity of matte [16].
The Jinbaoshan platinum-palladium deposit is currently the largest independent PGMs deposit discovered in China [17,18]. There are eleven kinds of useful chemical components in the ore, including eight types of precious metal: platinum, palladium osmium, iridium, ruthenium, rhodium, gold, and silver; and three useful chemical elements: copper, nickel and cobalt. The reserves of platinum and palladium are 45.24 t, whereas the average grade of platinum and palladium is 1.45 g/t [19,20]. Additionally, the content of main gangue components (MgO and SiO2) exceeds 30%. The precious metal minerals in this deposit are very small in grain size, and more than 50% of the minerals are intimately associated with base metals oxides and gangues, meaning that, it is difficult to produce a desired PGMs concentrate through simple physical flotation [21]. The grade of concentrate obtained is also reasonably low, and requires further upgrade. Thus, the Jinbaoshan platinum-palladium deposit has not been utilized until now.
In this study, an efficient beneficiation-metallurgy combined process is proposed to treat the Jinbaoshan platinum-palladium deposit, including oxygen pressure leaching and physical flotation. The main aim of oxygen pressure leaching is to disconnect PGMs from base metals minerals and preliminarily enrich the PGMs. Flotation technology is then used to remove the main gangues to further improve the grade of PGMs. The beneficiation-metallurgy combined process will provide a new method for highly efficient utilization of the Jinbaoshan platinum-palladium deposit.
2 Experimental
2.1 Materials
The material investigated was taken from the Jinbaoshan platinum-palladium deposit and obtained through preliminary flotation. The contents of Pt, Pd, and other main elements are presented in Table 1. As seen, the material contains 17.5 g/t Pt and 27.3 g/t Pd. In addition, the contents of Mg and Si are relatively high, indicating that direct extraction of PGMs from the raw concentrate using a conventional smelting process is very difficult.
The main phases of the Jinbaoshan platinum group minerals were detected by X-ray diffraction (XRD). The results, illustrated in Fig. 1, demonstrate that there are several diffraction peaks of pyrite, chalcopyrite, talc and violarite, whereas no diffraction peaks of other components are observed due to their low contents.
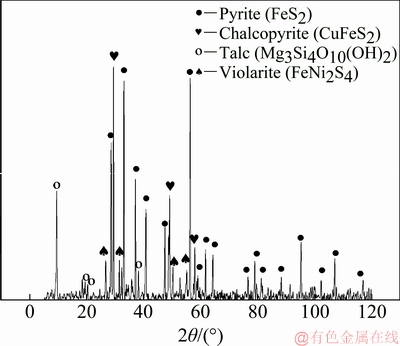
Fig. 1 XRD pattern of Jinbaoshan platinum group minerals
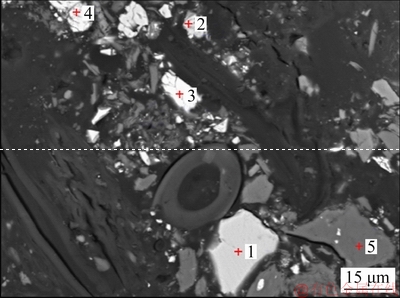
Fig. 2 Backscattered electron image of Jinbaoshan platinum group minerals
A backscattered electron image of the Jinbaoshan platinum group minerals and chemical composition of the selected particle are presented in Fig. 2 and Table 2, respectively. It can be seen from Fig. 2 that the main phase in spots 1 and 2 is pyrite (FeS2), owing to high Fe and S contents. Spot 3 has a large proportion of S, Cu and Fe, which can be identified as chalcopyrite. Similarly, the main phase in spot 4 is violarite. Spot 5 is composed of Si, Mg, and O, confirming the presence of talc.
To further confirm the phase compositions of the main metals, Jinbaoshan platinum group minerals were examined by mineral liberation analyzer (MLA). The result, shown in Table 3, indicates that the contents of chalcopyrite and violarite in the mineral are 25.70% and 3.71%, respectively. The occurrence states of iron are more complex, including pyrite, pyrrhotite and magnetite. It can be seen from Table 4 that PGMs present mainly as telluride, arsenide and antimonide, or as native elements.
2.2 Methods
2.2.1 Oxygen pressure leaching
Pressure oxidation leaching experiments were performed in a 2 L vertical titanium-lined autoclave from a Zhaoyuan autoclave factory in China, equipped with a typical mixing propeller. Leaching solutions were prepared using reagent grade sulfuric acid, calcium lignosulfonate, and deionized water. For each test, a certain amount of the ore and leaching solution were poured into the reactor, and the slurry was then heated to the desired temperature. At the set temperature, the oxygen was introduced to the autoclave and its partial pressure was adjusted to the desired value. After completing the experiment, oxygen flow was turned off and the autoclaves were rapidly cooled with water. Next, the slurry was filtered, washed, dried and weighed for XRD, scanning electron microscopy-energy dispersive spectroscopy (SEM-EDS), and inductively coupled plasma-atomic emission spectroscopy (ICP-AES) analyses.
Table 1 Chemical composition of Jinbaoshan platinum group minerals

Table 2 Chemical compositions of selected particle shown as Fig. 2 (mass fraction, %)
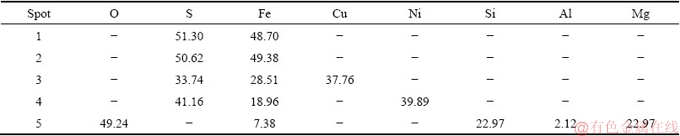
Table 3 Main metals phases and their contents in platinum group minerals (mass fraction, %)

Table 4 Main PGMs phases and their contents in platinum group minerals (mass fraction, %)

2.2.2 Flotation
The leaching residue obtained from the oxygen pressure leaching process was transferred into the air-inset hanged trough cell (XFGC II, Prospecting Machinery Plant, Jilin, China) to enrich the PGMs. Flotation took place at natural pH=6.5 with 130 g/t potassium ethyl xanthate as the collector, then 250 g/t copper sulfate was added as the activator. The flotation circuit for the leaching residue consisted of a rougher stage and two cleaner stages, in which closed-circuit cleaners were used. After completing the flotation experiment, the foam product and residue in the cell were filtered, dried, and weighed for further analyses.
2.2.3 Characterization and analyses
Leaching residue and flotation concentrate samples were measured by a Rigaku-TTRIII XRD with Cu Kα radiation, and the potential phases presented in the sample were identified by MDI Jade 6.5 software. The morphology of the sample and the chemical composition of the selected particles were characterized by SEM-EDS analysis (SEM, JEOL Ltd., JSM-6360LV). The contents of PGMs contained in the mineral, leaching residue, flotation concentrate and tailing were detected by the fire-assay method. The contents of base metals including Cu, Ni and Fe were detected by ICP-AES analysis (IRIS Intrepid II, XRS).
3 Results and discussion
The chemical composition of the Jinbaoshan platinum group minerals demonstrates that direct extraction of PGMs from the raw concentrate is difficult owing to low PGMs grade and high contents of Mg and Si. It is necessary to further improve PGMs grade for producing a desired PGMs concentrate before conducting the conventional smelting process. Considering that a proportion of PGMs minerals are intimately associated with base metals minerals and gangues, oxygen pressure leaching is proposed to destroy the association and create a superior flotation carrier for enriching PGMs minerals. Oxidation pressure leaching not only focuses on the leaching rates of base metals such as Cu, Ni, and Fe, but also attaches importance to the flotation efficiency of PGMs minerals during subsequent flotation. In previous research [22], basic leaching conditions were optimized as follows: oxygen partial pressure of 0.7 MPa, leaching temperature of 150 °C, sulfuric acid concentration of 2 mol/L, and leaching time of 3 h. In this study, the effects of particle size, amount of calcium lignosulfonate, stirring speed, and liquid-solid ratio, on the leaching rates of base metals and the recovery of PGMs were investigated in detail.
3.1 Effect of particle size
The effects of particle size on the leaching rates of base metals and the recovery of PGMs are illustrated in Fig. 3. As observed from Fig. 3(a), a decrease in particle size enhances the leaching rates of Cu, Ni, and Fe significantly. When the particle size of the ore is below 0.043 mm, the leaching rates of Cu, Ni and Fe increase to 94.62%, 74.78% and 96.10%, respectively. The ore with finer particle size increases the contact area between the ore and the leaching agent, intensifying the leaching process. It can be seen from Fig. 3(b) that a decrease in particle size improves PGMs grade but results in a reduction in PGMs recovery. The ore with finer particle size is beneficial to decreasing the leaching residue yield. Overall, the grade of PGMs in the flotation concentrate is improved. However, the complete dissolution of base metals sulfides is adverse for subsequent flotation technology. The main reason for this result is that base metals sulfides can be used as the flotation carrier for enriching PGMs minerals.
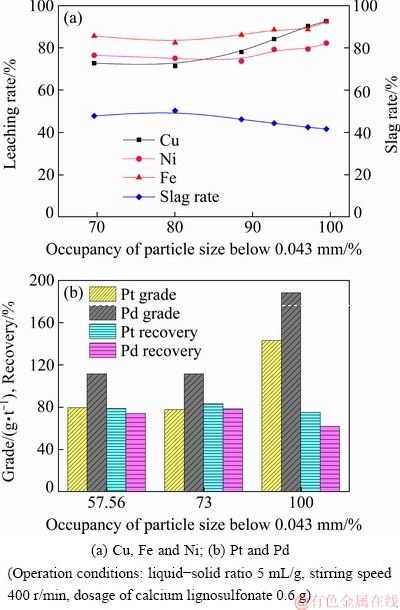
Fig. 3 Effects of particle size on leaching rates of base metals, grade and recovery of PGMs
3.2 Effect of stirring speed
Figure 4 illustrates the effects of stirring speed on the leaching rates of base metals and the recovery of PGMs. It can be seen from Fig. 4(a) that increasing stirring speed to 500 r/min improves the leaching rates of Cu, Ni, and Fe dramatically. At 500 r/min, approximately 100% of the Cu and Ni contained in the initial concentrate are dissolved, and the leaching rate of Fe is over 80%. A further increase of stirring speed has no obvious effect on the leaching process. Increasing stirring speed accelerates mass transfer and eliminates the effect of external diffusion. Additionally, the strong swirl generated by rapid agitation also has a large impact on the surface property of the leaching residue, affecting flotation efficiency. As visible in Fig. 4(b), the complete dissolution of chalcopyrite and violarite leads to a reduction in the amount of flotation carrier for enriching PMGs minerals, which hinders PGMs recovery. Therefore, 400 r/min is considered a suitable stirring speed.
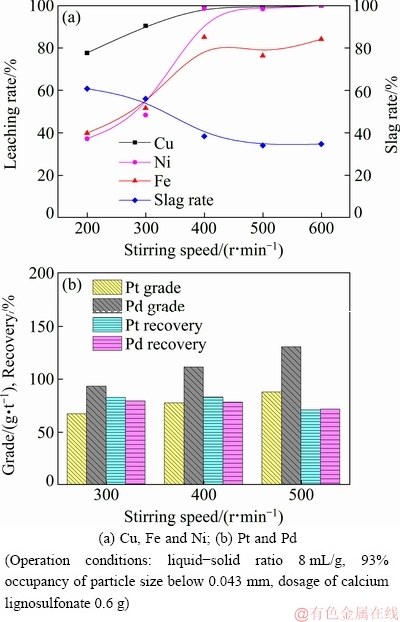
Fig. 4 Effects of stirring speed on leaching rates of base metals, grade and recovery of PGMs
3.3 Effect of amount of calcium lignosulfonate
Comprehensive research has found that S2- is transformed into S precipitate during the acid pressure oxidation leaching process [23,24]. To avoid the secondary capsulation of elemental sulfur, calcium lignosulfonate is added to the leaching solution. As a dispersant, calcium lignosulfonate is used to break up the elemental sulfur and reduce the coating effect by altering the adsorption of sulfur on the surface of mineral particles. As a result, it can improve leaching reactions. The effect of the amount of calcium lignosulfonate on the leaching rates of Cu, Ni, and Fe is shown in Fig. 5(a). The addition of calcium lignin sulfonate, ranging from 0 to 0.6 g, exerts a positive effect on the leaching process. However, a further increase of calcium lignosulfonate dosage is adverse for the leaching process. A larger calcium lignosulfonate dosage will promote the formation of CaSO4 precipitate and hinder the contact between the mineral surface and leaching agent, leading to decreased leaching rates of base metals. It can be observed in Fig. 5(b) that the highest PGMs recovery is obtained with 0.6 g calcium lignosulfonate added to the leaching solution. The result suggests that elemental sulfur can also be viewed as the flotation carrier for enriching PGMs minerals. A good dispersibility of elemental sulfur precipitate can simultaneously improve the leaching process and flotation efficiency.
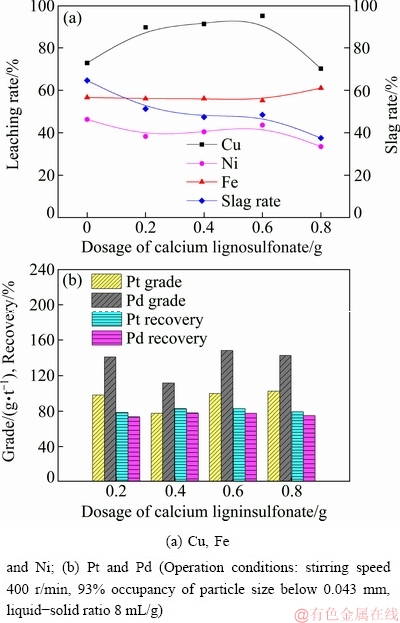
Fig. 5 Effects of dosage of calcium lignosulfonate on leaching rates of base metals, grade and recovery of PGMs
3.4 Effect of liquid-solid ratio
The effect of liquid-solid ratio on the leaching rates of base metals and the recovery of PMGs was examined from 4 to 10 mL/g. As illustrated in Fig. 6(a), the leaching rates of Cu, Ni, and Fe exhibit a decreasing tendency when liquid-solid ratio increases from 4 to 10 mL/g. The possible reason for this is that the forming elemental sulfur precipitate is easily wrapped on the mineral surface under conditions of high liquid-solid ratio. Moreover, it is easier to form soluble sulfate ions with more raw material added into the leaching solution due to the large consumption of leaching agent. It can be seen from Fig. 6(b) that increasing the liquid-solid ratio simultaneously improves the grade and recovery of PGMs. Therefore, liquid-solid ratio of 10 mL/g is considered an optimal condition.
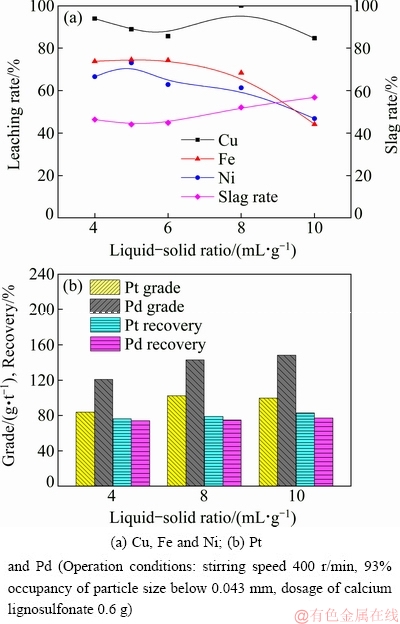
Fig. 6 Effects of liquid-solid ratio on leaching rates of base metals, grade and recovery of PGMs
3.5 Characterization of leaching residue
Based on above analyses, the formation of elemental sulfur and the presence of base metals sulfides are found to be favorable for the subsequent flotation technology. Optimal leaching conditions are determined as follows: liquid-solid ratio of 10 mL/g, 73% occupancy of ore particle size below 0.043 mm, stirring speed of 400 r/min and 0.6 g dosage of calcium lignosulfonate. Under these conditions, leaching experiments were conducted three times and the results are presented in Table 5. The leaching rates of Cu, Ni, and Fe are 87.6%, 87.6%, and 90.3%, respectively with Pt and Pd grade enriched to 43.9 g/t and 67.7 g/t, respectively. The content of Si in the leaching residue also increases to 26.10%. The main phases of the leaching residue were detected by XRD. The result, shown in Fig. 7, indicates that the main mineralogical compositions of the leaching residue are talc, elemental sulfur, and pyrite.
Table 5 Chemical compositions of leaching residue and leaching rates of main elements under optimal leaching conditions
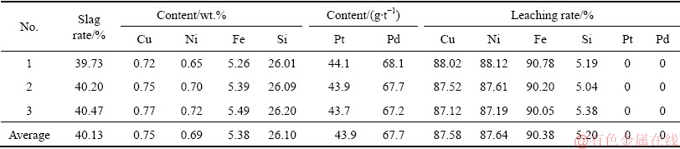
Table 6 Chemical compositions of flotation concentrate and flotation tailing obtained under optimal flotation conditions
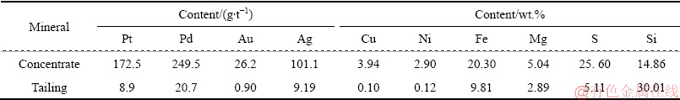
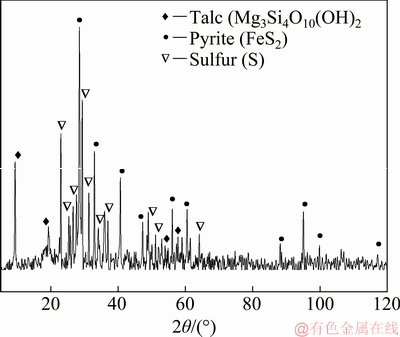
Fig. 7 XRD pattern of leaching residue obtained under optimal leaching conditions
3.6 Characterization of flotation concentrate and flotation tailing
After completing oxygen pressure leaching, the leaching residue was further treated by physical flotation. The best results were reached at pH=6.5 in the presence of 130 g/t potassium ethyl xanthate as the collector, and 250 g/t copper sulfate as the activator. The chemical compositions of the flotation concentrate and the flotation tailing are listed in Table 6. As shown, the grade of PMGs enriched in the flotation concentrate is above 420 g/t, which can be used as the metallurgy material for direct extraction of PGMs through the conventional smelting process. The XRD patterns of the flotation concentrate and flotation tailing, shown in Fig. 8, demonstrate that the main phases of the flotation concentrate are pyrite and sulfur, whereas the main mineral components are talc and silica in the flotation tailing.
A backscattered electron image of the flotation concentrate and chemical composition of the selected particle are shown in Fig. 9 and Table 7, respectively.
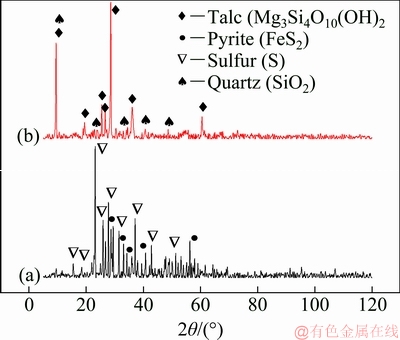
Fig. 8 XRD patterns of flotation concentrate (a) and flotation tailing (b) obtained under optimal flotation conditions
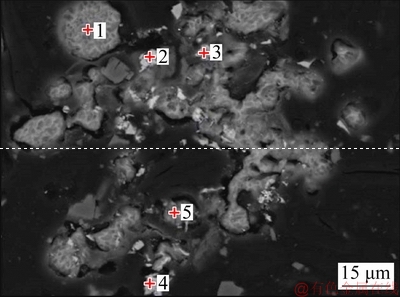
Fig. 9 Backscattered electron image of flotation concentrate
The main phase in spot 1 is elemental sulfur. Spots 2 and 4 can be identified as chalcopyrite, owing to high S, Cu, and Fe contents. Similarly, the main phases of spots 3 and 5 are pyrite and violarite, respectively. These results further confirm that elemental sulfur and base metals sulfides are effective flotation carriers for enriching PGMs minerals.
Backscattered electron images of the flotation tailing in different regions are presented in Fig. 10. Flotation tailing is mainly composed of quartz and talc. A small proportion of violarite remains in the flotation tailing owing to the fine particles encapsulated in quartz. Additionally, some PGMs minerals with tiny particle size (less than 2 μm) are also observed in the flotation tailing. The flotation method struggles to recover these PGMs minerals due to their fine granularity, exerting a negative effect on PGMs recovery.
3.7 Evaluation of different technological routes for Jinbaoshan platinum group minerals
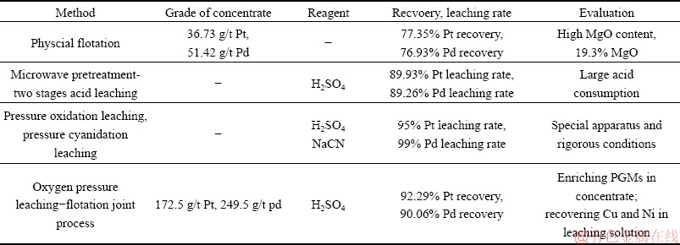
An evaluation of different technological routes for the Jinbaoshan platinum group minerals is presented in Table 8 [25]. It can be seen that the recovery of PGMs obtained through single flotation is very low owing to complex mineral structure and fine grained dissemination. Moreover, a high MgO content is adverse for the subsequent smelting process. Direct leaching of PGMs through microwave pretreatment two-stage acid leaching or pressure cyanidation leaching leads to a high acid consumption due to the dissolution of gangues. In addition, pressure cyanidation leaching requires special apparatus and rigorous conditions, making commercial application more difficult. Therefore, it is reasonable to select oxygen pressure leaching- flotation joint process for treating the Jinbaoshan platinum group minerals. This process enables the production of flotation concentrate with high PGMs grade and low MgO content, which is suitable for the conventional smelting process. The leaching solution including Cu and Ni can then be further treated to recover Cu and Ni effectively.
Table 7 Chemical composition of selected particle (mass fraction, %)
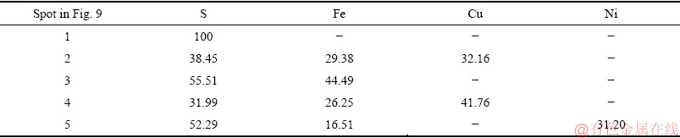
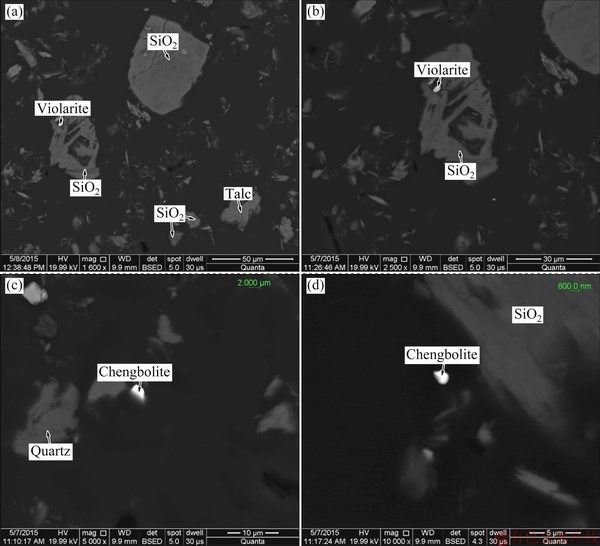
Fig. 10 Backscattered electron images of flotation tailing in different regions with different magnifications
Table 8 Various technological routes for treating Jinbaoshan platinum group minerals
4 Conclusions
(1) Leaching parameters simultaneously affect the leaching rates of base metals during oxidation pressure leaching, and the recovery of PGMs during the subsequent flotation technology.
(2) The complete dissolution of base metals sulfides leads to a reduction in the amount of flotation carrier for enriching PGMs minerals, thus decreasing PGMs recovery.
(3) Under optimal conditions, PMGs grade enriched in the flotation concentrate is over eight times higher than that of the raw concentrate.
References
[1] RAO C R M, REDDI G S. Platinum group metals (PGM): occurrence, use and recent trends in their determination [J]. Trends in Analytical Chemistry, 2000, 19(9): 565-586.
[2] MUDD B G M. Sustainability reporting and the platinum group metals: A global mining industry leader? [J]. Platinum Metals Review, 2012, 56(1): 2-19.
[3] MUDD G M. Key trends in the resource sustainability of platinum group elements [J]. Ore Geology Reviews, 2012, 46(2): 106-117.
[4] MWASE J M, PETERSEN J, EKSTEEN J J. A conceptual flowsheet for heap leaching of platinum group metals (PGMs) from a low-grade ore concentrate [J]. Hydrometallurgy, 2011, 111(1): 129-135.
[5] HUANG Kun, CHEN Jing, CHEN Yi-ran, ZHAO Jia-chung, LI Qi-wei, YANG Qiu-xue, ZHANG Yong. Enrichment of platinum group metals (PGMs) by two-stage selective pressure leaching cementation from low-grade Pt-Pd sulfide concentrates [J]. Metallurgical & Materials Transactions B, 2006, 37(5): 697-701.
[6] MWASE J M, PETERSEN J, EKSTTEN J J. A conceptual flowsheet for heap leaching of platinum group metals (PGMs) from a low-grade ore concentrate [J]. Hydrometallurgy, 2012, 111-112: 129-135.
[7] MPINGA C N, EKSTTEN J J, ALDRICH C, DYER L. Atmospheric leach process of high-chromitite PGM-bearing oxidized mineralized ore through a single-stage and two-stage techniques [J]. Minerals Engineering, 2018, 125: 165-175.
[8] KIM B S, LEE J C, JEONG J K. Current status on the pyrometallurgical process for recovering precious and valuable metals from waste electrical and electronic equipment (WEEE) scrap [J]. Tempo, 2009, 57: 74-75.
[9] NOGUERIA C A, PAIVA A P, OLIVERIA P C, COSTA M C, COSTA A. Oxidative leaching process with cupric ion in hydrochloric acid media for recovery of Pd and Rh from spent catalytic converters [J]. Journal of Hazardous Materials, 2014, 278: 82-90.
[10] JHA M K, LEE J C, KIM M S, JEONG J K, KIM B S, KUMAR V. Hydrometallurgical recovery/recycling of platinum by the leaching of spent catalysts: A review [J]. Hydrometallurgy, 2013, 133(2): 23-32.
[11] SAFARZADEH M S, HORTON M, RYTHOVEN A D V. Review of recovery of platinum group metals from copper leach residues and other resources [J]. Mineral Processing & Extractive Metallurgy Review, 2018, 39(1): 1-17.
[12] MPINGA C N, EKSTEEN J J, ALDRICH C, DYER L. Direct leach approaches to platinum group metal (PGM) ores and concentrates: A review [J]. Minerals Engineering, 2015, 78: 93-113.
[13] YANG Tian-zu. Precious metal metallurgy and product deep processing [M]. Changsha: Central South University Press, 2005. (in Chinese)
[14] BEZUIDENHOUT J J, EKSTEEN J J, BRADSHAW S M. Computational fluid dynamic modelling of an electric furnace used in the smelting of PGM containing concentrates [J]. Minerals Engineering, 2009, 22(11): 995-1006.
[15] JONES R T, KOTZE I J. DC arc smelting of difficult PGM-containing feed materials [J]. Minerals Engineering, 2004, 16(2): 165-173.
[16] LIDDEL K, NEWTON T, ADAMS M, MULLER B. Energy consumption for Kell hydrometallurgical refining versus conventional pyrometallurgical smelting and refining of PGM concentrates [J]. Journal of the Southern African Institute of Mining & Metallurgy, 2011, 111(2): 127-132.
[17] LU Yi-guan, ZHAO Kai, XIONG Yi-qu, LI Po, DU Da-yang, YUAN Ming-wei. Elements geochemistry of Jinbaoshan Pt-Pd deposit western Yunnan, China [J]. Acta Petrologica Sinica, 2014, 30(9): 2681-2694.
[18] LU Yi-guan, YANG Li-qiang, ZHAO Kai, XIONG Yi-qu, LI Po, Du Da-yang, YUAN Ming-wei. Origin of the Jinbaoshan Pt-Pd deposit, western Yunnan: Implications for the relationship of crustal contamination and mineralization [J]. Acta Geologica Sinica, 2014, 88(s2): 302-303.
[19] TAO Yan, ZHU Dan, GAO Zhen-min, LUO Tai-yi, YAO Lin-bo, ZHANG Huan. An additional study on the Pd-occurrence states in the Jinbaoshan Pt-Pd deposit [J]. Acta Mineralogica Sinica, 2007, 27(16): 262-264.
[20] SONG Huan-bin, HE Ming-qin, ZHANG Shang-zhong, YI Feng-hua. Chemical composition of the ore and occurrence state of the elements in Jinbaoshan platinum-palladium deposit [J]. Acta Geochimica, 2008, 27(1): 104-108.
[21] LIU Shi-jie, YANG Mao-cai, WANG Yun-hua, ZHANG Guan-lu, LU Yue-hua, WANG Yong-lu, WU Xiao-feng. A new process engineering for comprehensive exploitation of the Jinbaoshan Pt-Pd ore resources, Yunnan Province, China [J]. Precious Metals, 2012, 33(4): 1-8. (in Chinese)
[22] LIU Zhi-qiang, WANG Wu, CAO Hong-yang, ZHOU Xiang-qian, ZHANG Kui-fang, QIU Xian-yang. Oxygen pressure leaching of low-grade Pt-Pd concentrate [J]. The Chinese Journal of Nonferrous Metals, 2016, 26(1): 223-232. (in Chinese)
[23] MU Wang-zhong, ZHANG Ting-an, LIU Yan, GU Yan, DOU Zhi-he, LV Guo-zhi, BAO Li, ZHANG Wei-guang. E-pH diagram of ZnS-H2O system during high pressure leaching of zinc sulfide [J]. Transactions of Nonferrous Metals Society of China, 2010, 20(10): 2012-2019.
[24] PADILLA R, VEGA D, RUIZ M C. Pressure leaching of sulfidized chalcopyrite in sulfuric acid-oxygen media [J]. Hydrometallurgy, 2007, 86(1): 80-88.
[25] CHEN Jing, HUANG Kun, CHEN Yi-ran. Several techniques for handling flotation sulfide concentrates of Jinbaoshan platinum mine [J]. Chinese Journal of Rare Metals, 2006, 30(3): 401-406. (in Chinese).
金宝山铂族矿物氧压浸出-浮选联合工艺
饶 帅1,2,3,刘志强1,2,3,邱显扬1,2,3,王东兴1,2,3,曹洪杨1,2,3,李 伟1,2,3,陶进长1,2,3
1. 广东省稀有金属研究所,广州 510650;
2. 广东省科学院 稀有金属分离与综合利用国家重点实验室,广州 510650;
3. 广东省稀土开发及应用研究重点实验室,广州 510650
摘 要:为了生产适合冶炼的铂族精矿,提出一种氧压浸出-浮选联合工艺处理金宝山铂族矿物。结果表明:氧压浸出条件(矿物粒度、搅拌速度、液固比以及木质素磺酸钙用量)对浸出过程中贱金属(铜、铁和镍)浸出率以及浮选工艺中铂族金属(铂和钯)的回收率产生明显影响。浸出过程中贱金属硫化矿物的完全溶解导致铂族金属浮选降低了浮选载体的数量,降低浮选过程中铂族金属回收率。综合考虑贱金属浸出率和铂族金属回收率,确立如下最佳工艺条件:精矿粒度-0.043 mm占有率为73%,搅拌速度为400 r/min,液固比为 10 mL/g,木质磺酸钙用量为0.6 g。最佳工艺条件下,铜、镍和铁的浸出率分别为87.6%、87.6%和90.3%。采用浮选工艺处理浸出渣获得浮选精矿铂族金属品位为420 g/t。
关键词:铂族金属;氧压浸出;浮选;回收率
(Edited by Xiang-qun LI)
Foundation item: Projects (51804083, 51204060) supported by the National Natural Science Foundation of China; Project (2017B090907026) supported by Science and Technology Planning Project of Guangdong Province, China; Projects (2018GDASCX-0938, 2018GDASCX-0939) supported by Guangdong Academy of Science Doctor Special Program, China
Corresponding author: Zhi-qiang LIU; Tel: +86-20-37238536; E-mail: 1031494987@qq.com
DOI: 10.1016/S1003-6326(19)65017-X
Abstract: An oxygen pressure leaching-flotation joint process was proposed to treat Jinbaoshan platinum group minerals to produce a desired concentrate. The result demonstrates that leaching parameters which include particle size, stirring speed, liquid-solid ratio, and the dosage of calcium lignosulfonate, simultaneously affect the leaching rates of base metals and the recovery of platinum group metals (PGMs). The complete dissolution of base metals sulfides leads to a reduction in the amount of flotation carrier for enriching PGMs, decreasing the recovery of PGMs. The optimum leaching conditions are determined as follows: liquid-solid ratio of 10 mL/g, 73% occupancy of ore particle size below 0.043 mm, stirring speed of 400 r/min, and 0.6 g dosage of calcium lignosulfonate. Under optimal conditions, the leaching rates of Cu, Ni and Fe are 87.6%, 87.6% and 90.3%, respectively. The grade of PGMs enriched in the flotation concentrate is 420 g/t through the flotation technology.


