
A new strain Acidithiobacillus albertensis BY-05 for bioleaching of metal sulfides ores
XIA Jin-lan(夏金兰), PENG An-an(彭安安), HE Huan(何 环),
YANG Yu(杨 宇), LIU Xue-duan(刘学端), QIU Guan-zhou(邱冠周)
Key laboratory of Biometallurgy of Ministry of Education, School of Minerals Processing and Bioengineering,
Central South University, Changsha 410083, China
Received 4 April 2006; accepted 23 July 2006
Abstract:
An acidophilic, rod-shaped Gram-negative sulfur oxidizing strain BY-05 was isolated from an acid mine drainage of copper ore in Baiyin area, Gansu Province, China. Ultrastructural studies show that the isolate has a tuft of polar flagella and possesses sulfur granules with clear membrane adhering to the cell innermembrane. Physiological study shows that this isolate grows autotrophically and aerobically by oxidizing S0 and reduced inorganic sulfur compounds (![]()
![]() S2- and ZnS) with the optimum growth at pH 3.5-4.0 and at the temperature range of 25-30 ℃. The 16S rRNA gene sequence (DQ 423683) of strain BY-05 has 100% sequence similarity to that of Acidithiobacillus albertensis (DSM 14366). So it is identified and named as A. albertensis BY-05. Bioleaching experiments with this new strain show that it can play an important role in recovery of metals from chalcopyrite and sphalerite.
S2- and ZnS) with the optimum growth at pH 3.5-4.0 and at the temperature range of 25-30 ℃. The 16S rRNA gene sequence (DQ 423683) of strain BY-05 has 100% sequence similarity to that of Acidithiobacillus albertensis (DSM 14366). So it is identified and named as A. albertensis BY-05. Bioleaching experiments with this new strain show that it can play an important role in recovery of metals from chalcopyrite and sphalerite.
Key words:
sulfur oxidation; Acidithiobacillus albertensis; Acidithiobacillus thiooxidans; isolation and characterization of bacteria; bioleaching;
1 Introduction
Since the middle of the 20th century, the researches of BRYNER and BECK[1] and TUOVINEN and KELLY[2] have made on the capacity of microorganisms on metal extraction from sulfide minerals recognized. There are more than 20 species of bioleaching organisms reported[3-4], which belong to 6 genera, ranging from obligate autotrophs, facultative autotrophs, mixotrophs to heterotrophs. Acidithiobacillus ferrooxidans can oxidize ferrous ions, elemental sulfur, thiosulfates and sulfides, which is considered to be the major and the widest researched leaching bacterium up to date. Leptospirillum ferrooxidans have higher affinity to ferrous ions than A. ferrooxidans[5-7]. A. thiooxidans [8-9] can well oxidize elemental sulfur and sulfide or polythionate generated in the process of bioleaching and can enhance the leaching ability of A. ferrooxidans or L.ferrooxidans, treated as the major assistant bacteria in the process, especially in sphalerite bioleaching with vast elemental sulfur generated. Mixed culture is proved to have higher leaching efficiency by lots of researches [10-15]. Biohydrometallurgy studies were focused on breeding of higher activity of leaching species and mechanism of bioleaching. Study on ecological niche and mechanism of mutualism of mixed culture in bioleaching process also attracts more attention recently. In this paper, a new strain for assisting bioleaching of metal sulfide ores was isolated from acid mine drainage of copper ore in Baiyin area, Gansu Province, China. It has similar characteristics with Acidithiobacillus albertensis[16] and A. thiooxidans. The 16S rRNA gene sequence (DQ 423683) of the isolate was analyzed.
2 Experimental 2.1 Materials and methods 2.1.1 Reagents
EZ-10 Spin Column Genomic DNA Minipreps Kit (Bio Basic Inc.), Taq DNA polymerase (Fermentas),E.Z.N.A? Gel Extraction Kit (Omega Bio-Tek, Inc.), pBS-T PCR Products Clone Kit (Tiangen Biotech Co. Ltd., Beijing), universal PCR primer pair 63f (5’-CAGGCCTAACACATGCAAGTC-3’)and 1387r (5’-GGGCGGWGTGTACAAGGC-3’)[17] were provided by Sunbiotech Co. Ltd., Beijing, China. Chalcopyrite (Cu 28.00%, Fe 32.00%, S 35.50%), pyrite (Fe 40.11%, S 9.02%) and sphalerite (Zn 61.76%, S 36.72%) used in this experiment were provided by Institute of Mineral Processing Engineering, School of Resources Processing and Bioengineering, Central South University, China.
2.1.2 Culture media
DSMZ Medium 71 (German Collection of Microorganisms and Cell Cultures/Deutsche Sammlung von Mikroorganismen und Zellkulturen GmbH: DSMZ) was selected for flasks enrichment:(NH4)2SO4 3.0 g, KH2PO4 3.0 g, MgSO4·7H2O 0.5 g, CaCl2·2H20 0.25 g, FeSO4·7H20 10 mg, distilled water 1 L, final pH 4.0. Isolate was incubated in 250 mL Erlenmeyer flasks containing 100 mL medium, at 30 ℃, and 175 r/min aerobic shacking conditions.
Starkey-Na2S2O3 solid culture medium, abbreviated as SK, was chosen for isolation of the organism[18]: Solution A: (NH4)2SO4 2.0 g, KH2PO4 3.0 g, MgSO4·7H2O 0.5 g, CaCl2·2H2O 0.25 g, FeSO4·7H2O 0.001 g, distilled water 500 mL, with 24 g/L powdered agar, pH 4.8; Solution B: Na2S2O3 10 g, distilled water 50 mL. Solutions were sterilized under low pressure, respectively. The two solutions were mixed for preparing plates when they were cooled to about 60 ℃. The organism was incubated at 30 ℃ in a thermostat incubator.
2.2 Enrichment and isolation
The strain BY-05 was isolated from acid mine drainage of copper ore in Baiyin area. 10 mL of the original sample was transferred into 90 mL of DSMZ Medium 71 for enrichment until the pH of the medium decreased to about 1.0, when it lasted about 7 d. After 3 generations of enrichment, the isolate was inoculated on Starkey-Na2S2O3 agar medium, and the pure culture was obtained after three successive subcultures.
2.3 Physicochemical characterization
1 mL of the 3 d-old inoculum (about 1×108 cells) was inoculated in 100 mL of DSMZ Medium 71. The cell concentration was then monitored by direct counting under microscope every 12 h. Liquid DSMZ medium 71 with 10 g/L alternative energy source (S0, Na2S2O3, K2S4O6, Na2S or ZnS) was used for testing the optimal metabolic substrate. Liquid medium with 0.5% glucose, yeast extract or peptone added was used for testing the heterotrophic source utilization, where CO2 in the air was absorbed by passing through 3 mol/L saturated KOH solution before it passed through the liquid medium. Alternative liquid medium with 1 g/L NH4H2PO4 or KNO3 instead of (NH4)2SO4 was used for testing the nitrogen source utilization. All these tests were performed by subculturing the isolate 3 times at 30 ℃ and during this process the cell concentration of the isolate was monitored[19]. Another alternative liquid DSMZ medium 71 with 1% elemental sulfur added was used for testing the optimum pH and temperature. They were repeated three times.
2.4 Morphology and subcellular structure observation
Morphological characteristics and motile behavior of the isolate were observed with optical microscope (Olympus CX-31). Surface and inner substructures of the cells harvested from the logarithm growth phase were examined with scanning electron microscope (SEM, JEOL JSM-6360 LV) and transmission electron microscope (TEM, JEOL JEM-1230) after cytochemical treatment and staining.
2.5 Amplification, sequencing and phylogenetic analysis of 16S rRNA gene 2.5.1 Genomic DNA extraction
Cells of strain BY-05 were harvested after being incubated in liquid DSMZ medium 71 with 10 g/L S0 for 5 d. After filtrated and centrifuged at 3 000 r/min for 5 min to remove residual solid elemental sulfur in the culture, cells were collected by centrifuging at 10 000 r/min for another 15 min and washed several times by Tris·HCl- EDTA(TE) (pH8.0) buffer. Genomic DNA was extracted by EZ-10 Spin Column Genomic DNA Minipreps Kit according to the operation instruction of the Kit.
2.5.2 Amplification and sequencing of 16S rRNA gene
Amplification of 16S rRNA gene of the isolate was carried out in PCR cycler (T-Gradient Thermoblock, Biometra) using universal primers 63f (5’- CAGGCCTAACACATGCAAGTC-3’) and 1387r (5’- GGGCGGWGTGTACAAGGC-3’)[17]. Amplification reactions were performed in a total volume of 50 μL. The reaction mixture contained 5 μL of 10×PCR buffer, 5 μL of 25 mmol/L MgCl2, 5 μL of 2 mmol/L dNTPs mixture, 2 μL of primers 63f(62.5 μmol/L)and 1387r (62.5 μmol/L) respectively, 1 μL of Taq polymerase (5U/μL) and 2 μL of template DNA. Thermo cycling procedure was as follow: An initial keeping at 94 ℃ for 5 min, followed by 33 cycles at 94 ℃ for 45 s, 55 ℃ for 45 s, and 72 ℃ for 90 s, then an extension to the last cycle at 72 ℃ for 10 min.
The PCR product was purified using E.Z.N.A? Gel Extraction Kit. The product was integrated into pBS-T vector using the pBS-T PCR Products Clone Kit (Tiangen Biotech, Beijing). 3 μL of ligation mixture was used to transform high efficiency competent cells of Escherichia coli DH 5α and plated on Luria-Bertani (LB) medium containing ampicillin, 5-bromo-4-chloro-3- ndolyl-β-D-galactopyranoside (X-Gal) and isopropyl β-D-thiogalactopyranoside(IPTG). White colonies including the positive recon were picked out and cultured, then sent to Sunbiotech Co. Ltd for sequencing.
2.5.3 Phylogenetic analysis
The 16S rRNA gene sequence of strain BY-05 was comparatively analyzed with the nucleic acid database of Genbank. The similarity analysis between the strain and the alignments of typical sulfur oxidation bacteria was done using Clustal X (1.8). Phylogenetic trees were constructed based on the similarity analysis.
2.6 Leaching experiment with mixing culture of isolate BY-05 and A. ferrooxidans
The pure isolate strain BY-05, A. ferrooxidans BY-0502 (DQ427104) and their mixed cultures were used to leach sulfide minerals chalcopyrite, pyrite and sphalerite. Experiments were carried out in 250 mL Erlenmeyer flasks containing 100 mL of leaching solution plus 10 g/L of <75 μm minerals powder at pH 3.5. The cell concentration of the inoculum was controlled at 1×107 CFU/mL, and the experiments were conducted at 30 ℃ and under 175 r/min of shaking condition. 1 mL of samples were removed every 4 days and diluted to 2 5mL for determining the soluble metal ion concentrations of Cu(Ⅱ), Fe(Ⅱ, Ⅲ)and Zn(Ⅱ) by atomic absorption spectrophotometer (Hitachi Z-8000). The lost water in the medium was supplemented with sterilized deionized water after sampling each time.
3 Results 3.1 Isolation of strain
During the enrichment, it was found that after 3 d incubating of the isolate BY-05 in DSMZ liquid medium with 1% sulfur, the particle size of elemental sulfur granules floating on the top of liquid medium decreased and majority of them sank to the bottom of flask. And after 5 d of incubation, the lowest pH of the medium decreased to about 0.5 and concentration of cells rose up to 108-109 CFU/mL. Growth on the SK solid agar medium showed white colonies, which were round (approximately 0.5-2 mm in diameter), smooth, convex, and had complete edges. The colonies appeared with bright edges at beginning and finally became limpid on prolonged cultivation after 12 d, and the culture medium around colonies changed to ivory-white halos gradually until the whole plate turned to opacity instead of translucence (Fig.1).

Fig.1 Colony morphology of strain BY-05
3.2 Cell morphology and ultrastructure
The cell morphology and motility of strain BY-05 observed under optical microscope showed that it was long rod-shaped, Gram-negative and motile, which swung in one plane or occasionally in radial, most occurring singly and others in pairs or in short chains during exponential phase in liquid medium. It was also observed that particles of minerals or elemental sulfur often attached to the polar of the cell, or sometimes the joined parts of paired or short chained cells. It was interesting to find that when the cells grew in the medium with 1% Na2S2O3, the majority of the cells during mid-logarithm phase expanded their bodies and moved dully, with bright central and dense black edge observed, which is obviously different from that in any other sulfur-containing medium (data not given). Observation under TEM (Fig.2) showed that the cells had long tufts of polar flagella that might refer to the adhering to surface of the substrates[20]. Fig.3 shows the morphology and subcellular structure of the cells cultivated in typical substrates of elemental sulfur or thiosulfate sodium in liquid media. It showed that the cells were thicker and shorter, about (0.4-0.6) μm×(1-2) μm (width×length) grown in elemental sulfur medium (Figs.3(a) and (c)), while they were thinner and longer, about (0.3-0.4) μm×(2-4) μm grown in thiosulfate medium (Figs.3(b) and (d)). The change in morphology reflected the adaptation of the bacterium to the cultural nutrition. It was also shown in Figs.3(c) and (d) that inside the cells there were highly refractile granules which possessed a separate membrane parting from the cytoplasm, and they may consist of elemental sulfur and played the role of energy storage according to BRYANT et al[16].
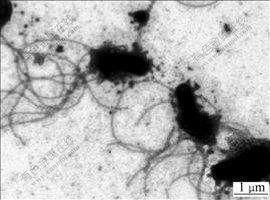
Fig.2 TEM morphology of flagella of strain BY-05

Fig.3 Comparison in morphology and subcellular microstructures of BY-05 cultivated respectively in DSMZ 71-S0 medium (a) and (c) and DSMZ 71- Na2S2O3 medium (b) and (d) by SEM and TEM
3.3 Physicochemical properties
3.3.1 Optimal growth conditions
The growth status of the isolate at different temperatures on elemental sulfur substrate culture medium is shown in Fig.4. It was indicated that the isolate was mesophilic and was able to survive in quite wide temperature range. The optimal temperature was at about 30 ℃, and the cells could survive for a period of time at 40 ℃ but no longer over 45 ℃. The growth characteristics of the organism at different initial pH ranging from 0 to 7 are shown in Fig.5. Good growth was observed over a range of initial pH from 0.5 to 6.0, with optimal growth at 3.5 to 4.0, and the cells could adapt well to the low acidity range of 0.5-1.5.
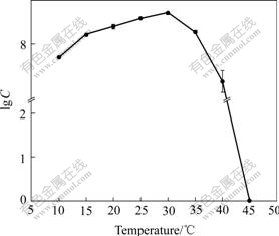
Fig.4 Effect of temperature on growth of strain BY-05

Fig.5 Effect of pH values on growth of strain BY-05
3.3.2 Utilization of typical substrates
Metabolic substrates test (data not shown) showed that the new isolate could grow well in the substrates of elemental sulfur, Na2S2O3, K2S4O6, Na2S and ZnS, with the highest growth activity in the substrate of elemental sulfur, but it could not oxidize ferrous iron and pyrite. On the other hand, the isolate grew aerobically and auto- trophically with carbon dioxide and ![]() as the carbon and nitrogen sources, respectively, and the growth of the cells ceased and the concentration of cells declined when any of the two substrates was lack in the medium.
as the carbon and nitrogen sources, respectively, and the growth of the cells ceased and the concentration of cells declined when any of the two substrates was lack in the medium. ![]() could not be utilized as nitrogen source. It could not, however, grow in substrate of glucose, yeast extract, peptone or other alternative organic energy source.
could not be utilized as nitrogen source. It could not, however, grow in substrate of glucose, yeast extract, peptone or other alternative organic energy source.
The growth curve and pH change of the organisms cultivated in liquid medium DSMZ-71 with 1%S0 and 1%Na2S2O3 are shown in Fig.6 and Fig.7, respectively. It was indicated that the cells grown in the medium with 1%S0 had shorter lag phase, higher growth rate in the exponential growth phase, and higher maximal cell concentration and lower pH that could attain at the stationary phase than in the medium with 1%Na2S2O3, indicating that elemental S0 is more fitful for growth of the cells than thiosulfate.
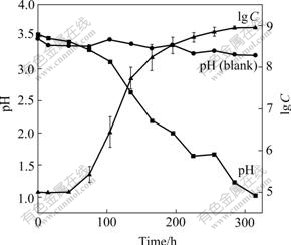
Fig.6 Growth curve and change in pH values during growth of strain BY-05 cultivated in S0 medium
It was found that during the growth in the medium with 1%Na2S2O3 some white deposit appeared in the solution and pH value of the medium increased up to 4.0 at first, then decreased to about 1.3. Thiosulfate may be first oxidized to ![]() according to the equation 2Na2S2O3+O2+2H+→Na2S4O6+2NaOH, which was catalyzed by thiosulfate?acceptor oxidoreductase in the periplasm of the bacterium[21]. This equation can just explain the increase in pH in the beginning stage. As exhausting of
according to the equation 2Na2S2O3+O2+2H+→Na2S4O6+2NaOH, which was catalyzed by thiosulfate?acceptor oxidoreductase in the periplasm of the bacterium[21]. This equation can just explain the increase in pH in the beginning stage. As exhausting of ![]()
![]() may be oxidized by tetrathionate oxidoreductase to S0, and at last to the end product
may be oxidized by tetrathionate oxidoreductase to S0, and at last to the end product ![]() [22]. But these occurrences did not appear during elemental sulfur metabolism.
[22]. But these occurrences did not appear during elemental sulfur metabolism.
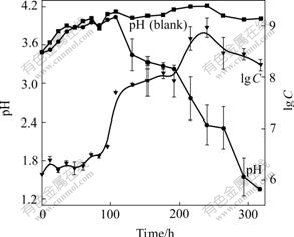
Fig.7 Growth curve and pH change during growth of strain BY-05 cultivated in Na2S2O3 medium
3.4 Analysis of 16S rRNA gene sequence
1349bp of 16S rRNA gene of the strain BY-05 was obtained by sequencing and the accession number in Genbank was DQ423683. The phylogenetic tree shown in Fig.8 summarized the phylogenetic relationship among the typical sulfur oxidizing bacteria species. It revealed that the strain BY-05 was closely related to acidophilic sulfur oxidizing bacteria A. thiooxidans ATCC 19377 and A. albertensis DSM 14366 with 99.8% and 100.0% similarity, respectively.
3.5 Leaching experiment of sole or mixed bacteria
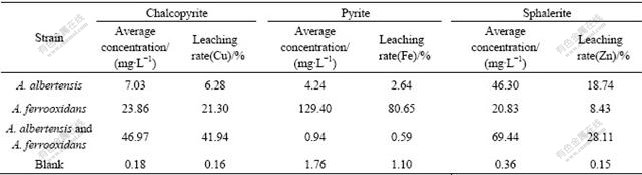
The bioleaching rates of metals copper, iron, and zinc after 25 d incubation of sole strain BY-05, A.ferrooxidans strain BY-0502 (DQ427104) or the mixed culture of these two strains are listed in Table 1. The results indicated that the extraction rates were very low in the acidic media without bacteria. The typical bioleaching bacterium A. ferrooxidans worked efficiently on metal sulfides especially pyrite with leaching rate up to 80%. The strain BY-05 could not oxidize pyrite, but had somewhat effects on chalcopyrite leaching and worked well on sphalerite. The mixed cultures consisting of both strains (1?1) could accelerate the leaching rate of chalcopyrite and sphalerite, but not better than the pure culture on pyrite. Though the cell concentration increased up to (3-4)×108/mL after 25 d growth, leach- ing rate of pyrite by the mixed culture was even lower than that by any one of the two strains, respectively.

Fig.8 Phylogenetic tree derived from 16S rRNA gene sequence of BY-05 strain
Table 1 Comparison of leaching rate by sole or mixed A. albertensis and A. ferrooxidans after 25 d flask shaking
4 Discussion
The genera Acidithiobacillus[23], member of the Gram-negative γ-Proteobacteria, contains four species A. albertensis, A. ferroxidans, A. thiooxidans and A. caldus. A. albertensis was first isolated from an acid soil in Alberta Province, Canada in 1983, then validated a new species according to the description of BRYANT et al[24] in 1988 by IJSB. The mesophilic A. ferrooxidans and A. thiooxidans were the classical bioleaching bacteria, which were also used in treatment of sewage sludge and desulphurization of coal by their sulfur and/or ferrous iron oxidizing ability. Moderately thermophilic A. caldus[25] attracted much attention recently for the apparently arsenic resistance[26] and potential of enhancing bioleaching rate at 45-50 ℃. Study on A. albertensis seems to be very lack though taxonomists suggest that it may be widely geographical distributed[27]. A. albertensis was very similar to most A. thiooxidans strains in morphological and physiological characteristics. The sulfur granules were observed in most of the cells during the stationary growth phase. Tuft of polar flagella and condensed glycocalyx that may help adhering to the substrate surface were observed when being treated by negative-staining and cytochemistry preparation. A. albertensis was validated a genomically distinct species with the GC content of DNA between 61%-62%[16], relatively higher than that of A. thiooxidans that typically has the GC content between 50%-52%.
Phylogenetic analysis of 16S rRNA gene sequence showed the isolate BY-05 falls into the γ-Proteobacteria with very close relation to both A. albertensis (with 100.0% similarity) and A. thiooxidans (with 99.8% similarity). The comparison in the main characteristics concluded in Table 2 indicates the strain BY-05 is a strain of Acidithiobacillus genus. More detailed analysis on Table 2 shows that strain BY-05 is more similar to A. albertensis DSM 14366 than any other Acidithiobacillus species. The isolate BY-05 has polar tufts of flagella and in vivo sulfur particles that are similar to that of A. albertensis, but different from that of A. thiooxidans. The optimum pH and temperature of strain BY-05 were closer to those of A. albertensis than to those of A. thiooxidans, though the final pH 0.5 attained was more similar to that of A. thiooxidans. So, it is reasonable to name the isolate BY-05 as A. albertensis BY-05. Strong sulfur oxidizing activity of this strain revealed its enormous potential on bioleaching application.
Table 2 Main characteristics of strain BY-05 and four type species of Acidithiobacillus genus

Most bacteria of Acidithiobacillus genus were widely studied for bioleaching, biodegradation and coal desulfurization. For several reasons, there were not, however, enough research reports about A. albertensis and its applications. A. albertensis is closely relative to A. thiooxidans. Oxidizing activity of the new strain A. albertensis BY-05 on several metal sulfides was first tested in this experiment. Results indicated that A. albertensis had similar leaching capacity to A. thiooxidans. As sulfur oxidizing bacterium, A. albertensis played important role in bioleaching. Metal recovery improved apparently when A. albertensis BY-05 was added with the same amount of A. ferrooxidans for bioleaching copper ions from chalcopyrite. The sole strain A. albertensis BY-05 could also play an important role in recovery of zinc irons from sphalerite. However, metal extraction from pyrite was extremely inhibited when A. ferrooxidans and A. albertensis BY-05 were mixed. This phenomenon may be related to some special cross reactions during the metabolism process of these two strains. Research on these aspects would make huge significance for proper preparation and cultivation mode of mixed bioleaching culture.
5 Conclusions
1) A new acidophilic sulfur oxidizing bacillus was isolated from acid mine drainage of copper ore in Gansu Province, China. According to the physiological features, biochemical properties and gene sequence analysis, the new isolate falls into the species A. albertensis. We name it A. albertensis BY-05.
2) Fundamental leaching experiment reveals that A. albertensis BY-05 accelerates the oxidation rate of the sulfide minerals, enhances chalcopyrite leaching capacity of other ferrous oxidizing bacteria such as A. ferrooxidans. Great recovery rate can be obtained by sole A. albertensis BY-05 in sphalerite leaching, but interestingly, leaching activity of A. ferrooxidans on pyrite can be inhibited by this strain mixed.
3) It can be worthy to focus further attention on the sulfur oxidizing mechanism of the strain A. albertensis BY-05, as well as the effect on metabolism mechanism when the mixed bacteria are incubated. The isolate provides a new important resource for study on sulfur biooxidation, and will impose significant effect on development of mixed beneficial cultures for bioleaching of metal sulfides ores.
References
[1] BRYNER L C, BECK J V. Microorganisms in leaching sulfides minerals [J]. Ind Eng Chem, 1954, 46: 2587-2592.
[2] TUOVINEN O H, KELLY D P. Use of microrganisms for recovery metals [J]. Int Metall Rev, 1974,19: 21-31.
[3] ZHANG Zai-hai. Screening, Breeding of Bacteria Species with High Performance for Leaching Copper Sulfide Ores, and their Bioleaching Mechanisms [D]. Changsha: Central South University, 2002. (in Chinese)
[4] YANG Xian-wan, QIU Ding-fan. Hydrometallurgy [M]. Beijing: Metallurgy Industry Press, 2001. (in Chinese)
[5] GUAY R, SILVER M. Thiobacillus acdophilus sp. nov.; isolation and some physiological characteristics [J]. Can J Microbiol, 1975, 21: 281-288.
[6] TSUCHIYA H M, TRIVEDI N C, SCHULER M L. Microbial mutualism in ore leaching [J]. Biotechnol Bioeng, 1974, 16: 991-995.
[7] BOONA M, BRASSER H J, HANSFORD G S, HEIJNEN J J. Comparison of the oxidation kinetics of different pyrites in the presence of Thiobacillus ferrooxidans or Leptospirillum ferrooxidans [J]. Hydrometallurgy, 1999, 53: 57-72.
[8] LIZAMA H M, SUZUKI I. Bacterial leaching of a sulfide ore by Thiobacillus ferrooxidans and Thiobacillus thioxidans (1): Shake flask studies [J]. Biotechnol Bioeng, 1988, 32: 110-116.
[9] LORS C, TIFFREAU C, LABOUDIGUE A. Effects of bacterial activities on the release of heavy metals from contaminated dredged sediments [J]. Chemosphere, 2004, 56: 619-630.
[10] DONATI E, CURUTCHET G, POGLIANI C, TEDESCO P H. Bioleaching of covellite using pure and mixed cultures of Thiobacillus ferroxidans and Thiobacillus thiooxidans [J]. Process Biochem, 1996, 31(2): 129-134.
[11] FALCO L, POGLIANI C. A comparison of bioleaching of covellite using pure cultures of Acidithiobacillus ferrooxidans and Acidithiobacillus thiooxidans or a mixed culture of Leptospirillum ferrooxidans and Acidithiobacillus thiooxidans [J]. Hydrometallurgy, 2003, 71: 31-36.
[12] SHI S Y, FANG Z X. Bioleaching of marmatite flotation concentrate by Acidithiobacillus ferrooxidans and Leptospirillum ferrooxidans [J]. Trans Nonferrous Metal Soc China, 2004, 14(3): 569-575.
[13] FOUCHER S, D'HUGUES P, GALLE-CAVALLONI P, MORIN D. Continuous bioleaching of chalcopyrite using a novel extremely thermophilic mixed culture [J]. Int J Min Processing, 2002, 66(1): 107-119.
[14] STOTT M B, SUTTON D C. Comparative leaching of chalcopyrite by selected Acidophilic Bacteria and Archaea [J]. Geomicrobiol J, 2003, 20(3): 215-230.
[15] DEVECIA H, AKCILB A, ALPA I. Bioleaching of complex zinc sulphides using mesophilic and thermophilic bacteria: comparative importance of pH and iron [J]. Hydrometallurgy, 2004, 73: 293-303.
[16] BRYANT, R D, MCGROARTY K M, COSTERTON J W, LAISHLEY E J. Isolation and characterization of a new acidophilic Thiobacillus species (T.albertis) [J]. Can J Microbiol, 1983, 29: 1159-1170.
[17] MARCHESI J R, SATO T, WEIGHTMAN A J, MARTIN T A, FRY J C, HIOM S J, WADE W G. Design and evaluation of useful bacterium-specific PCR primers that amplify genes coding for bacterial 16S rRNA [J]. Appl Environ Microbiol, 1998, 64(2): 795-799.
[18] JIN Mo-song, YAN Wang-min, WANG Zu-nong. Build-up of conjugative transfer system for Thiobacillus thiooxidans [J]. Chinese J Biotechnol, 1992, 8: 1-3. (in Chinese)
[19] DONG Xiu-zhu, CAI Miao-ying. Manual of Systematic Identification for Common Bacteria [M]. Beijing: Science Press, 2001. (in Chinese)
[20] OHMURA N, TSUGITA K, KOIZUMI J I, SAIKI H. Sulfur-binding protein of flagella of Thiobacillus ferrooxidans [J]. J Bacteriol, 1996, 178(19): 5776-5780.
[21] KAMIMURA K, HIGASHINO E, KANAO T, SUGIO T. Effects of inhibitors and NaCl on the oxidation of reduced inorganic sulfur compounds by a marine acidophilic, sulfur-oxidizing bacterium, Acidithiobacillus thiooxidans strain SH [J]. Extremophiles, 2005, 9: 45-51.
[22] HALLBERG K B, DOPSON M, B?RJE LINDSTR?M E. Reduced sulfur compound oxidation by Thiobacillus caldus [J]. J Bacteriol, 1996, 178(1): 6-11.
[23] KELLY D P, WOOD A P. Reclassification of some species of Thiobacillus to the newly designated genera Acidithiobacillus gen.now. Halothiobacillus gen. nov. and Thermithiobacillus gen. nov. International [J]. J Sys Evol Microbiol, 2000, 50: 511-516.
[24] International Union of Microbiolgical Societies. Validation of the publication of new names and new combinations previously effectively published outside the IJSB [J]. Int J Syst Bacteriol, 1988, 38: 220-222.
[25] HALLBERG K B, LINDSTR?M E B. Characterization of Thiobacillus caldus sp. Nov. a moderately thermophilic acidophile [J]. Microbiology, 1994, 140: 3451-3456.
[26] DOPSON M, LINDSTR?M E B, HALLBERG K B. Chromosomally encoded arsenical resistance of the moderately thermophilic acidophile Acidithiobacillus caldus [J]. Extremophiles, 2001, 5: 247-255.
[27] KELLY D P, HARRISON A P. Genus Thiobacillus. Bergey’S Manual of Systematic Bacteriology (Vol.3) [M]. Baltimore: Williams and Wilkins, 1989.
Foundation item: Project(50321402) supported by the National Natural Science Foundation of China; Project(2004CB619204) supported by the National Basic Research Program of China
Corresponding author: XIA Jin-lan; Tel: +86-731-8836944; E-mail: jlxia@mail.csu.edu.cn


