- Abstract:
- 1 Introduction▲
- 2 Experimental▲
- 3 Results and discussion▲
- 4 Conclusions▲
- References
- Figure
- Fig.1 XRD pattern of Ca-Bent sample
- Fig.2 SEM image of Ca-montmorillonite
- Fig.3 Experiment flow for sodium-modification
- Fig.4 Effects of ageing time on colloid value and dilation
- Fig.5 Effects of briquetting pressure on colloid value and dilation
- Fig.6 Effects of briquetting moisture on dilation and colloid value
- Fig.7 Effects of Na2CO3 dosage on colloid value and dilation
- Fig.8 XRD pattern of modified bentonite under optimum conditions
J. Cent. South Univ. Technol. (2010) 17: 1201-1206
DOI: 10.1007/s11771-010-0619-9![]()
Sodium-modification of Ca-based bentonite via semidry process
HUANG Yan-fang(黄艳芳), ZHANG Yuan-bo(张元波), HAN Gui-hong(韩桂洪), JIANG Tao(姜涛),
LI Guang-hui(李光辉), YANG Yong-bin(杨永斌), XU Bin(许斌), GUO Yu-feng(郭宇峰)
School of Resources Processing and Bioengineering, Central South University, Changsha 410083, China
? Central South University Press and Springer-Verlag Berlin Heidelberg 2010
Abstract:
In order to improve the quality of Hunyuan inferior Ca-based bentonite (Ca-Bent), semidry process was used to modify Ca-Bent into superior Na-based bentonite (Na-Bent). The factors affecting sodium-modification were investigated. The optimized experimental parameters are obtained as follows: Na2CO3 dosage 4.0%, ageing time 25 d, briquetting pressure 25 MPa and briquetting moisture 20%. Under the optimization conditions, the modified Na-Bent has a colloid value of 73.6 mL/(3g), dilation of 38 mL/g and water absorption in 2 h (2HWA) of 465%, respectively. The balling results indicate that the modified Na-Bent pellets have higher drop strength and compression strength than the Ca-Bent pellets.
Key words:
Ca-Bent; Na-Bent; sodium-modification; semidry process; pellet binder;
1 Introduction
Bentonite is widely used as binder in the production of iron ore pellets, which is able to improve the strengths of green and dry balls [1-4]. However, nearly 90% bentonite is residual in the finished pellets after roasted, leading to the decrease of total Fe (TFe) grade and poor metallurgical properties of them [5-9]. Therefore, reducing bentonite dosage in the pellets is significant to pellet production [10].
Although the reserves of bentonite in China are large, more than 90% bentonite is inferior Ca-based bentonite (Ca-Bent) and not used in pellet production completely. Hunyuan bentonite, more than 150 Mt, is one of large-type and typical Ca-Bent deposits in China. When it is used as pellet binder, the dosage of them is high to 3.0%-4.0%. Therefore, it should be firstly subjected to sodium- modification so as to be used in the iron ore pellets widely.
The conventional sodium-modification methods of Ca-Bent include two kinds: wet process and dry process [11-14]. Comparatively speaking, wet method is an effective sodium-modification of Ca-Bent. The quality of bentonite modified by wet method is good. However, this method is not usually applied to the Na-Bent commercial production presently because of the low drying efficiency and high energy consumption. On the other hand, the quality of modified products by dry process is still inferior to the iron ore pellets. Relatively, the semidry process overcomes the shortcomings of the wet method and dry process [15-18]. And the semidry process is popular in the industrial production.
In this work, based on the physicochemical properties and process mineralogy of the Hunyuan Ca-Bents, effects of different factors on the sodium- modification by means of semidry process were studied.
2 Experimental
2.1 Raw materials
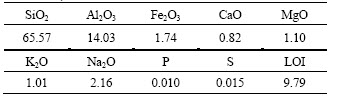
Main chemical compositions of the bentonite sample are given in Table 1. The chemical phases of silicon and calcium are analyzed and shown in Tables 2 and 3, respectively.
Table 1 Chemical compositions of Ca-Bent (mass fraction, %)

According to formula ![]()
![]() the alkali coefficient K of the sample is 0.34 (<1), which indicates that this bentonite is a typical Ca-Bent [14, 19]. The chemical phases of silicon show that 62.78% SiO2 exists in silicates, and 3.95% SiO2 exists as the dissociated form (Table 2). As far as calcium is concerned, 1.85% CaO exists in Ca-montmorillonite, and 12.66% CaO is in the insoluble silicates (Table 3).
the alkali coefficient K of the sample is 0.34 (<1), which indicates that this bentonite is a typical Ca-Bent [14, 19]. The chemical phases of silicon show that 62.78% SiO2 exists in silicates, and 3.95% SiO2 exists as the dissociated form (Table 2). As far as calcium is concerned, 1.85% CaO exists in Ca-montmorillonite, and 12.66% CaO is in the insoluble silicates (Table 3).
Table 2 Chemical phases of silicon (mass fraction, %)

Table 3 Chemical phases of calcium (mass fraction, %)

Refs.[16-17] revealed that the montmorillonite had the strongest intensities and bright peaks on (001) crystal face. In Fig.1, d(001) of montmorillonite is 1.553 9 nm, indicating that the bentonite sample is a kind of Ca-Bent. It is also shown in Figs.1 and 2 that, flaky and plate-like Ca-montmorillonite is the leading mineral in the sample, apart from a small quantity of dissociative quartz.
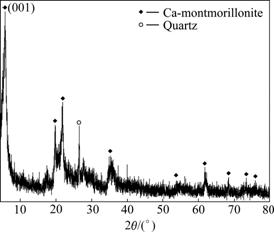
Fig.1 XRD pattern of Ca-Bent sample
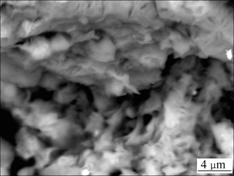
Fig.2 SEM image of Ca-montmorillonite
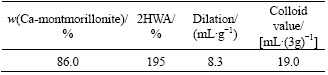
The basic physical indexes of the bentonite sample are listed in Table 4. The dilation, colloid value and the water absorption in 2 h (2HWA) were 8.3 mL/g, 19.0 mL/(3g) and 195%, respectively. According to the pellet production, this kind of Ca-Bent was not an effective binder for iron ore pellet so that they should be modified into Na-Bent before using.
Table 4 Basic physical properties of Ca-Bent
2.2 Modification principle and experimental methods
Montmorillonite, 2:1 type layered aluminosilicate, is the main mineral constituent of bentonite [11-12, 20]. Aluminium ions in the alumina octahedral sheet can be replaced by other ions, so do the silicon ions in silica tetrahedral sheet. The structure of montmorillonite will be negatively charged due to the isomorphous replacement. Therefore, montmorillonite lattice will adsorb exchangeable cations. Ca-Bent possesses exchangeable Ca2+ and Mg2+, which can be exchanged by Na+. The reaction mechanism is as follows:
Ca(Mg)-Bent+2Na+ =2Na-Bent+Ca(Mg)2+ (1)
In the industrial production, Na2CO3 is usually used as the Na-activation conversion agent [16]. In this work, analytically pure Na2CO3 is used as the sodium agent. The experimental process is presented in Fig.3 and described as follows: firstly, certain quality of Na2CO3 is dissolved into distilled water, then, mixed with Ca-Bent. Briquetting of the mixture is carried out using a press machine with a lab-scale mold with the diameter of 30 mm. After that, the briquettes are aged for a certain time at room temperature. The briquettes are dried at 105 ℃ and then ground to the granularity of less than 75 μm.
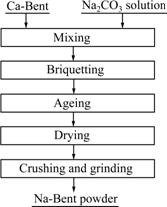
Fig.3 Experiment flow for sodium-modification
Among the physical indexes of bentonite, Refs.[16, 18] showed that colloid value and dilation were the important indexes to appraise the bentonite quality. Colloid value is the index to evaluate the hydration properties of bentonite. The measurement for colloid value is described as follows: put 3 g bentonite into a stoppered measuring glass which is d 25 mm, 100 mL; fill with some distilled water till the scale of 90 mL, and shake it carefully for 5 min to make it to form an even suspending liquid. After that, add 1 g magnesia as dispersant into it, and then fill it till the scale of 100 mL with water. Leave it still for 24 h after shaking for 1 min. Read the percent of sediment to the whole height and that is the colloid index of the sample. Dilatation which is also called as degree of expansion is related to the dispersibility of bentonite in water. It can be measured by the method: Weigh up 1 g bentonite sample which is thoroughly dried. Drop it slowly, evenly and discontinuously to a 100 mL glasstest tube with a diameter of 25 mm which has 75 mL distilled water in it; put in 25 mL hydrochloric acid, which concentration is 1 mol/L. Shake it for 3 min and leave it still for 24 h. Read the scale at the interface of the sediment which is the volume of bentonite in the graduated measuring glass after expansion. Therefore, the two indexes are also used to evaluate the effect of the modified bentonite in this work.
3 Results and discussion
3.1 Effects of different parameters on quality of Na- Bent
The effects of primary parameters, including ageing time, briquetting pressure, briquetting moisture and dosage of Na2CO3 on the sodium-modification of Ca-Bent were studied in this experiment.
3.1.1 Effects of ageing time
The effects of ageing time on the colloid index and dilation of modified bentonite were researched under the conditions: dosage of Na2CO3 3%, briquetting pressure 25 MPa and briquetting moisture 20%. The testing results are plotted in Fig.4.
As shown in Fig.4, the colloid value and dilation of modified bentonite are obviously increased with the increase of ageing time until the ageing time is 25 d. When ageing time is 5 d, the colloid value and dilation of modified bentonite are respectively 35 mL/(3g) and 17 mL/g, while the colloid value and dilation respectively increase to 62 mL/(3g) and 29.8 mL/g when ageing time prolongs to 25 d. According to reaction (1), Ca2+ and Mg2+ can be exchanged by Na+ in Na2CO3 solution. With the increase of ageing time, more and more Na ions enter into the crystal lattice of Ca-Bent. At the same time, the interlayer spacing becomes wider and Na+ solution can easily enter into the interlayer space. The reaction (1) will positively carry out with prolonging ageing time, until reaches equilibrium. When reaction (1) reaches equilibrium, Na+ in bentonite will not become more and the quality of modified bentonite will be stable. In Fig.4, it can be concluded that the ion-exchange reaction is almost balanced when ageing time is 25 d.
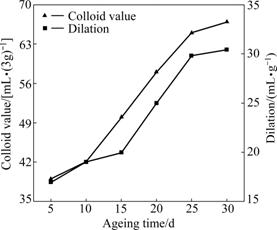
Fig.4 Effects of ageing time on colloid value and dilation
3.1.2 Effects of briquetting pressure
Fig.5 illustrates the effects of briquetting pressure on the dilation rate and colloid index of modified bentonite. The experimental conditions are fixed as follows: dosage of Na2CO3 3%, ageing time 25 d and briquetting moisture 20%.
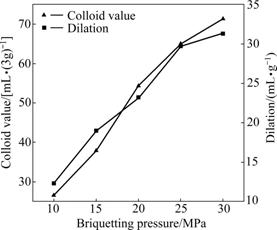
Fig.5 Effects of briquetting pressure on colloid value and dilation
It can be seen from Fig.5 that the briquetting pressure has apparent effects on the dilation and colloid index of modified bentonite. At the briquetting pressure of 15 MPa, the colloid value and dilation respectively are only 38 mL/(3g) and 19 mL/g. While the briquetting pressure is increased, the dilation and colloid index are continuously improved. With the increase of briquetting pressure, the bentonite particles become closer and closer to one another, which is beneficial to the ion-exchange reaction between Na+ and Ca2+ (Mg2+). Therefore, more Na+ ions enter into the Ca-montmorillonite crystal and take the place of Ca2+ (Mg2+), resulting in the improvement of the quality of modified bentonite. However, if the briquetting pressure is overlarged, the lumps are hard to separate from mould, which is not in favor of the industry production.
3.1.3 Effects of briquetting moisture
The effects of briquetting moisture were investigated under the conditions: dosage of Na2CO3 3%, briquetting pressure 25 MPa, and ageing time 25 d. The results are given in Fig.6.
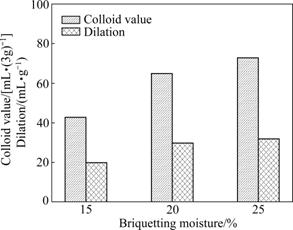
Fig.6 Effects of briquetting moisture on dilation and colloid value
As observed from Fig.6, briquetting moisture has perceivable impact on the colloid index and dilation. With rising briquetting moisture, channels between the montmorillonite crystal grains are increased, so the dispersion of Na+ between the Ca-montmorillonite crystal grains becomes easy. Therefore, the ion-exchange reaction between Na+ and Ca2+ also becomes easy and the quality of bentonite is improved. However, while the briquetting moisture is in excess of 20%, the briquettes will be not easy to escape the mould during the process of briquetting. The suitable briquetting moisture is not higher than 20%.
3.1.4 Effects of Na2CO3 dosage
Fig.7 shows the effects of Na2CO3 dosage on the dilation and colloid value of modified bentonite. The experimental conditions include briquetting pressure 25 MPa, briquetting moisture 20% and ageing time 25 d.
As shown in Fig.7, the colloid index and dilation are first increased and then decreased with ascending Na2CO3 dosage. When Na2CO3 dosage is 4%, the colloid value and the dilation nearly arrive to the maximum, 73.6 mL/(3g) and 33 mL/g, respectively. With the proceeding of reaction (1), the layer space becomes larger and the dispersibility of montmorillonite is improved. Therefore, the colloid value and dilation increase. If Na2CO3 dosage exceeds the right value, part of the excessive free Na+ ions are absorbed on the surface of the montmorillonite crystal grains and a hydrated shell forms, preventing the outer moisture from entering the crystal layers. Moreover, the equilibrium of ion-exchange reaction between Na+ and Ca2+ will be destroyed owing to free Na+ having high ionization rate and activity. The interlamellar spacing between crystal grains is compressed, so part of the interlayer water is extruded out, which is adverse to the sodium reaction. Therefore, the suitable dosage of Na2CO3 should be 4%.
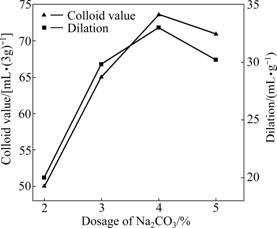
Fig.7 Effects of Na2CO3 dosage on colloid value and dilation
3.2 Physico-chemical properties of modified bentonite
From the above investigations, the optimization conditions of sodium-modification for Hunyuan Ca-Bent by semidry process are obtained as follows: the dosage of Na2CO3 4.0%, ageing time 25 d, briquetting pressure 25 MPa, and briquetting moisture 20%. Under the conditions, the Ca-Bent is modified, and the chemical compositions and physical properties of it are respectively shown in Tables 5 and 6.
Table 5 Chemical compositions of modified bentonite (mass fraction, %)
As given in Table 5, the content of Na2O is obviously increased to 2.16%. By contrast, the content of CaO is decreased to 0.82%. d(001) of modified bentonite is 1.203 8 nm [20] and 2θ is 7.338? (see Fig.8), indicating that the Ca-Bent has been successfully modified into a typical Na-Bent under the optimum conditions.
Table 6 Physical properties of modified bentonite

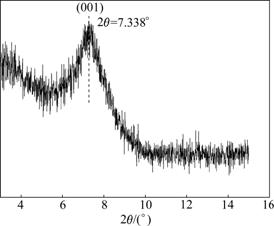
Fig.8 XRD pattern of modified bentonite under optimum conditions
Using the modified Na-Bent and the sample Ca-Bent as binders, the balling tests are carried out under conditions that the bentonite dosage is 1.5%, the moisture content of green balls is 8.5%, and balling time is 10 min. The balling results are presented in Table 7.
Table 7 Comparison of balling results of Ca-Bent and modified Na-Bent
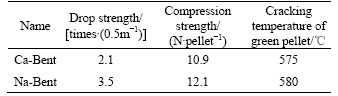
In Table 7, comparatively speaking, the compression strength and thermal cracking temperature of the Na-Bent pellets are approximate to those of the Ca-Bent pellets. However, the drop strength of Na-Bent pellets is clearly improved compared to the compression strength, greater than that of Ca-Bent pellets.
4 Conclusions
(1) More than 90% bentonite is inferior Ca-Bent and it is necessary to modify inferior Ca-Bent into superior Na-Bent. The bentonite used in this paper is a typical kind of Ca-Bent. As iron ore pellet binder, the 2HWA, dilation and colloid value of this Ca-Bent are only 195%, 8.3 mL/g and 19.0 mL/(3g), respectively. Presently, they are not adequately utilized in pellet production.
(2) Semidry process is effective to modify Hunyuan Ca-Bent into Na-Bent. Optimized conditions are obtained as follows: Na2CO3 dosage of 4.0%, ageing time of 25 d, briquetting pressure of 25 MPa and briquetting moisture of 20%. After modified under optimal conditions, the 2HWA, dilation and colloid value of the modified bentonite are respectively increased to 465%, 38.0 mL/g, 73.6 mL/(3g). The alkali coefficient K of it is 1.07 and d(001) of modified bentonite is about 1.20 nm, which indicates that the modified bentonite is a kind of Na-benonite.
(3) The balling tests show that the modified Na-Bent pellets have higher drop strength than the sample Ca-Bent pellets. Hunyuan Ca-Bent can be used as effective iron ore pellet binder through the sodium-modification by semi-dry process.
References
[1] QIU Guan-zhou, JIANG Tao, LI Hong-xu, WANG Dian-zuo. Functions and molecular structure of organic binders for iron ore pelletization [J]. Colloids and Surfaces A, 2003, 224: 11-22.
[2] QIU Guan-zhou, JIANG Tao, HUANG Zhu-cheng, ZHU De-qing, FAN Xiao-hui. Characterization of preparing cold bonded pellets for direct reduction using an organic binder [J]. ISIJ International, 2003, 43(1): 20-25.
[3] YANG Yong-bin, HUANG Gui-xiang, JIANG Tao, HUANG Zhu-cheng, LUO Yong, HUANG Ya-lei. Application of organic binder as substitutes for bentonite in pellet preparation [J]. Journal of Central South University: Science and Technology, 2007, 38(5): 850-856. (in Chinese)
[4] QIU Guan-zhou, JIANG Tao, FA Ke-qing, ZHU De-qing, WANG Dian-zuo. Interfacial characterizations of iron ore concentrates affected by binders [J]. Powder Technology, 2004, 139(1): 1-6.
[5] ZHANG Yong-xiang, TIAN Fa-chao, ZHANG Ke-cheng, ZHAO Heng. Pelletizing test of adding various complex binders [J]. Sintering and Pelletizing, 2004, 29(5): 9-11. (in Chinese)
[6] KAWATRA S K, RIPKE S J. Developing and understanding the bentonite fiber bonding mechanism [J]. Minerals Engineering, 2001, 14(6): 647-659.
[7] KAWATRA S K, RIPKE S J. Laboratory studies for improving green-ball strength in bentonite-bonded magnetite concentrate pellets [J]. International Journal of Mineral Processing, 2003, 72(1/4): 429-441.
[8] KAWATRA S K, RIPKE S J. Effects of bentonite fiber formation in iron ore pelletization [J]. International Journal of Mineral Processing, 2002, 65(3/4): 141-149.
[9] LI Hong-xu, WANG Dian-zuo, HU Yue-hua, QIU Guan-zhou, JIANG Tao. The mechanism of improving pellet strength by carboxyl methlated amylum [J]. Journal of Central South Technology University: Science and Technology, 2001, 32(4): 351-354. (in Chinese)
[10] LIU Peng-jun, ZHANG Yu-zhu, CAO Zhao-zhen, LI Zhen-guo, YIN Hai-sheng. Preparation of Na-bentonite from Ca-bentonite and its application in pellet [J]. Multipurpose Utilization of Mineral Resources, 2006(4): 3-6. (in Chinese)
[11] ZHOU Jia-rong, YI Fa-cheng, HOU Li. Study status on solidum-modified method of Ca-bentonite [J]. China Mining Magazine, 2007, 16(3): 84-87. (in Chinese)
[12] YI Fa-cheng, CHEN Ting-fang, LIU Sui-hai. Technology and application of organic bentonite [J]. Chinese Mineral Industry, 1998, 7(1): 70-73. (in Chinese)
[13] CHEN Zhi-yong, LI Yu-ling, ZHANG Zhi-yang, JIANG Ke-ge. Preparation and structural performance analysis of organic bentonite [J]. Non-metallic Mines, 2002, 25(5): 45-47. (in Chinese)
[14] LI Bao-yi, YANG Dian-fan, WANG Yu-jie. A study on the modified-property of bentonite [J]. Jilin Geology, 2005, 24(2): 44-49. (in Chinese)
[15] CAO Yao-hua, YANG Shao-wen, GAO Zhao-guo, LI Qi. Modification research of Ca-bentonite as drilling Na-bentonit [J]. Conservation and Utilization of Mineral Resources, 2007(1): 20-22. (in Chinese)
[16] JIANG Gui-lan, ZHANG Pei-ping. Processing and application of bentonite [M]. Beijing: Chemical Industry Press, 2005: 162. (in Chinese)
[17] LI Qi-lin. Studies on preparation mechanism and application of alkaline Ca-bent [D]. Nanning: Guangxi University, 2007: 1-10. (in Chinese)
[18] YAN Jing-hui, LI Jing-mei, HUI Buo-ran. Study on the technics of preparing high-efficient active white clay by the method of half–dry [J]. Journal of Changchun University of Science and Technology, 2003, 26(4): 37-39. (in Chinese)
[19] QIN Pei-cheng. Productive practice of sodium-modification of Ca-bentonite [J]. China Non-Metallic Mining Industry Herald, 2003, 37(6): 28-30. (in Chinese)
[20] ?NAL M, SARIKAYA Y. Preparation and characterization of acid-activated bentonite powders [J]. Powder Technology, 2007, 172: 14-18.
Foundation item: Project(50725416) supported by the National Science Fund for Distinguished Young Scholars; Project(50804059) supported by the National Natural Science Foundation of China; Project(2008BAB32B06) supported by the Key Project in the National Science and Technology Pillar Program during the 11th Five-Year Plan Period; Project(200805331080) supported by the Specialized Research Fund for the Doctoral Program of Higher Education
Received date: 2009-12-12; Accepted date: 2010-03-08
Corresponding author: ZHANG Yuan-bo, PhD; Tel: +86-731-88877214; E-mail: zybcsu@126.com
- Sodium-modification of Ca-based bentonite via semidry process


