Trans. Nonferrous Met. Soc. China 25(2015) 3484-3489
Extraction of lithium from salt lake brine by aluminum-based alloys
Yan-hong LI, Zhong-wei ZHAO, Xu-heng LIU, Xing-yu CHEN, Mao-li ZHONG
School of Metallurgy and Environment, Central South University, Changsha 410083, China
Received 8 May 2014; accepted 8 September 2015
Abstract:
Salt lake brine was reacted with activated aluminum-based alloys and lithium was precipitated. The effects of aluminum-based alloys on precipitating lithium were investigated and the reasonable alloy used to extract lithium from brine was obtained. The effects of the mole ratio of Al to Li and Ca content of Al-Ca alloy, the initial concentration of lithiumion ion in solution, reaction temperature and reaction time on the adsorption rate of lithium were studied, and the optimized process parameters were determined. The results show that the mole ratio of Al to Li and Ca content of Al-Ca alloy and reaction temperature have great influences on the precipitation rate of lithium. The precipitation rate of lithium reaches 94.6% under the optimal condition, indicating that Al-Ca alloy is suitable for the extraction of lithium from salt lake brine.
Key words:
salt lake brine; lithium extraction; aluminum matrix composite; Al-Ca alloy;
1 Introduction
Lithium, which is known as new energy metal in the 21st century, is more important in modern industry since lithium ion batteries are commercialized by Sony Corporation [1-3]. Along with the rapid development of new energy industry, the market demand of lithium increases sharply, which makes the exploitation of lithium resources more important. There are two kinds of lithium resources in nature, one is ore and another is brine containing lithium [4-6]. The lithium ore mainly contains granite pegmatite, lithium pyroxene, petalite lithium, lithium mica, and so on [7,8]. However, most lithium resources are contained in the salt lake brine. Until now, the amount of global lithium resources has been more than 100 million tons, and the content of lithium resources in salt lake brine is more than 80% [9-11]. There are abundant lithium resources in salt lake brine in China. The amount of salt lakes located in Tibetan Plateau are more than 80, and the mass of lithium resources in these lakes are more than 6 million tons.
The researches of lithium extraction technology from salt lake brine were carried out and a lot of methods, such as precipitation [12,13], solvent extraction [14-17], calcination leaching [18-20], electrolysis [21,22], carbonization [23,24], salting out method [25] and ion exchange adsorption [26-30], have been developed to extract lithium from salt lake brine. These methods promote the technology of extracting lithium from salt lake brine, and the precipitation with Al(OH)3 is considered to be one of the promising methods. Al(OH)3 can precipitate lithium from salt lake brine selectively, and then, LiCl solution can be obtained by leaching the roasted precipitate with water. However, this method has its deficiencies, such as low efficiency of precipitating lithium, large reagent consumption of NaOH and AlCl3, and high cost of production. The aluminum and lithium- containing composite can be precipitated through decomposing the water by Al-Li alloy [31]. Inspired by this, the precipitate containing aluminum and lithium is supposed to be obtained by splitting salt lake brine with aluminum-based composite. If this inference is feasible, it will be beneficial to simplifying the technology of extracting lithium from salt lake brine. At the same time, the amount of reagent and energy consumption will reduce, which is beneficial to decreasing the production cost. Based on this idea, a new method for the extraction of lithium by decomposing salt lake brine with aluminum-based alloy was put forward. The effects of operating parameters on the precipitation rate of lithium were investigated and the feasibility of this new method was verified.
2 Experimental
2.1 Precipitation of lithium
Aluminum-based alloy and NaCl were mixed together according to predesigned mass ratio and then ground by ball crusher for some time to get the activated powder. Aqueous LiCl solution was added into the flask and then placed in the water-bath reactor controlled at predesigned temperature. After this step, the activated powder was added into the flask to react with LiCl solution and the aluminum-lithium co-precipitate was obtained. The reaction mechanism of precipitating lithium is shown as
2LiCl+2Al+(x+6)H2O→2LiCl·2Al(OH)3·xH2O↓+3H2↑ (1)
2.2 Analytical methods
The concentration of Li was analyzed by atomic absorption spectroscopy (AAS, Persee of Beijing, China). The phase structure of the substance was characterized by X-ray diffraction analysis (XRD, Rint-2000, Rigaku) using Cu Kα radiation. The microstructures of the products were observed by scanning electron microscopy (JSM-6360LV).
3 Results and discussion
3.1 Selection of aluminum-based composite
Al-Ca alloy and Al-Fe alloy were selected to compare the performance of decomposing brine and precipitating lithium. The experiment conditions are as follows. The content of aluminum in the alloys is 70%, the mole ratio of Al to Li is 4:1, the reaction temperature is 70 °C, the initial concentration of lithium is 1 g/L and the reaction time is 3 h. The XRD patterns of decomposed products are shown in Fig. 1. It can be seen from Fig. 1 that, LiCl·Al(OH)3·xH2O is obtained by decomposing brine with Al-Ca alloy. However, the products obtained by decomposing brine with Al-Fe alloy are mainly Al and Al13Fe4, which indicates that Al-Fe alloy hardly reacts with brine. The morphologies of alloys and products are shown in Fig. 2. It can be seen from Fig. 2 that, the product obtained by decomposing brine with Al-Ca alloy shows dendritic crystal, indicating that Al-Ca alloy possesses an excellent reactivity with brine. The results of AAS show that the precipitation rate of lithium with Al-Ca alloy reaches 93.6%, but that with Al-Fe alloy is only 23.8% indicating that most of lithium still remains in solution. Therefore, Al-Ca alloy is used to decompose the brine in the subsequent experiments.
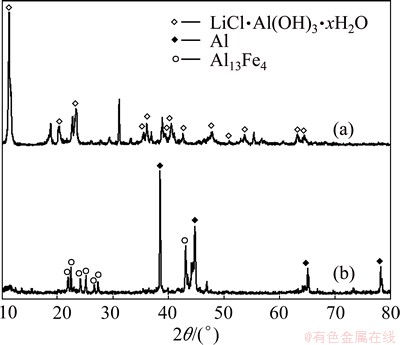
Fig. 1 XRD patterns of products obtained by decomposing brine with Al-Ca alloy (a) and Al-Fe alloy (b)
3.2 Effect of mole ratio of Al to Li on precipitation rate of lithium
In order to study the effect of mole ratio of Al to Li on the precipitation rate of lithium, the experiments were conducted under the conditions of the Ca content in Al-Ca alloy 30%, the initial lithium concentration 1 g/L, the reaction temperature 70 °C and the reaction time 3 h. The results are shown in Fig. 3. According to stoichiometric ratio, lithium ion in solution can be transferred into the precipitate when the mole ratio of Al to Li is 2:1, but the precipitation rate actually is only 72.1%. This may be due to the low lithium ion concentration. Because the lithium ion concentration is only 1 g/L, it is difficult for Al-Ca alloy to react with lithium ion in solution adequately. When the mole ratio of Al to Li increases to 3.5:1, the precipitation rate of lithium reaches 93.8%. When the mole ratio of Al to Li continues to increase, the increase of precipitation rate is not significant.
3.3 Effect of Ca content in Al-Ca alloy on precipitation rate of lithium
In order to investigate the effect of Ca content on the precipitation rate of lithium, the experiments were performed with the Ca content varying from 10% to 40%, and the results are shown in Fig. 4.

Fig. 2 SEM images of aluminum-based composites and decomposed products
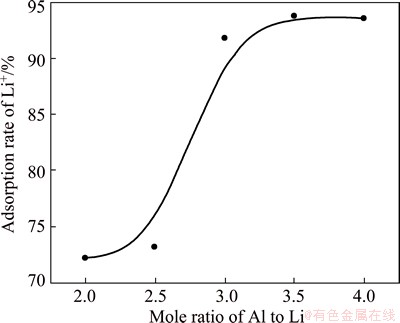
Fig. 3 Influence of mole ratio of Al to Li on precipitation rate of lithium
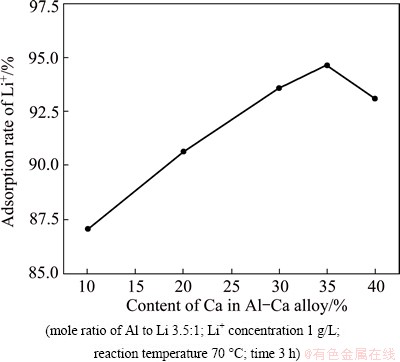
Fig. 4 Effect of Ca content in Al-Ca alloy on precipitation rate of lithium
It can be seen from Fig. 4 that the precipitation rate of lithium increases firstly, and then decreases with the increase of Ca content in Al-Ca alloy. Ca in alloy is helpful for the milling activation process. The increase of Ca content can minimize the particle size and inhibit the aggregation of powder, which makes Al-Ca alloy obtain large surface area and react with brine more adequately. Therefore, when the Ca content increases from 10% to 35%, the precipitation rate of lithium increases from 87.1% to 94.7%. However, the precipitation rate of lithium decreases when the Ca content is more than 35%. The increase of Ca content in alloy leads to the increase of the amount of Ca(OH)2, which is insoluble in water and coats on the surface of Al-Ca alloy to inhibit the reaction of alloy with brine. Therefore, the excessive Ca content in alloy is harmful to the precipitation rate of lithium.
3.4 Effect of initial lithium ion concentration on precipitation rate of lithium
The effect of initial lithium ion concentration on the precipitation rate of lithium was investigated under the conditions of the mole ratio of Al to Li 3.5:1, the Ca content 35%, the reaction temperature 70 °C and the reaction time 3 h. The results are shown in Fig. 5. It can be seen from Fig. 5 that the precipitation rate of lithium increases gradually with the increase of initial lithium ion concentration. The precipitation rate of lithium increases from 71.3% to 95.3% when the initial lithium ion concentration changes from 0.4 to 0.8 g/L. In order to obtain a precipitation rate of lithium above 90%, the initial lithium concentration should reach 0.8 g/L. Generally, the initial lithium ion concentration in brine is no more than 200 mg/L. Brine with high lithium concentration is obtained by evaporation, which is harmful to enhancing the production efficiency and reducing the production cost. The initial lithium concentration of 0.8 g/L is reasonable by overall consideration.
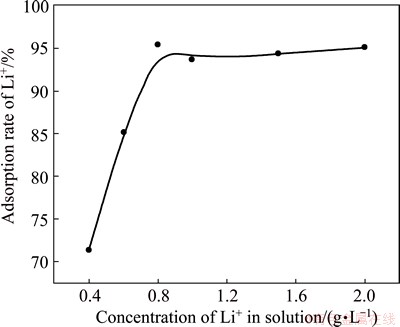
Fig. 5 Effect of initial Li+ concentration on precipitation rate of lithium
3.5 Effect of reaction temperature on precipitation rate of lithium
The effect of reaction temperature on the precipitation rate of lithium is shown in Fig. 6. It can be seen that the reaction temperature exhibits obvious influence on the precipitation rate of lithium. The precipitation rate of lithium keeps stable when the temperature is not over 70 °C. However, the precipitation rate of lithium decreases sharply once the temperature is over 70 °C. The precipitation rate of lithium decreases from 93.6% to 66% when the temperature decreases from 70 to 90 °C.
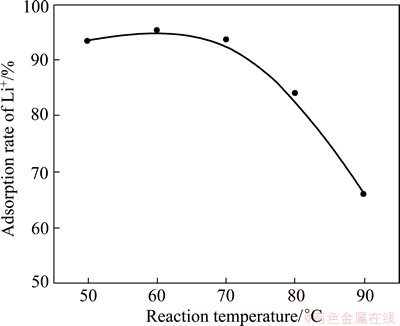
Fig. 6 Effect of reaction temperature on precipitation rate of lithium
The SEM images of precipitate obtained at different temperatures are shown in Fig. 7. It can be seen that, the higher the reaction temperatures, the smaller the particles. This is because the increase of temperature can enhance the reaction speed and make the reaction more completely. Therefore, the precipitation rate of lithium should increase with the increase of reaction temperature in theory, but the experimental results exhibit opposite trends. The combining power of LiCl to Al(OH)3 in LiCl·Al(OH)3·xH2O will be weakened when the temperature increases to a certain value, and then LiCl will fall off from LiCl·Al(OH)3·xH2O due to the thermal motion. When the temperature is over 70 °C, a part of LiCl·Al(OH)3·xH2O decomposes and LiCl dissolves into the solution again, which leads to the decreases of precipitation rate of lithium. This phenomenon is consistent with the results reported by LIU and ZHONG [32].
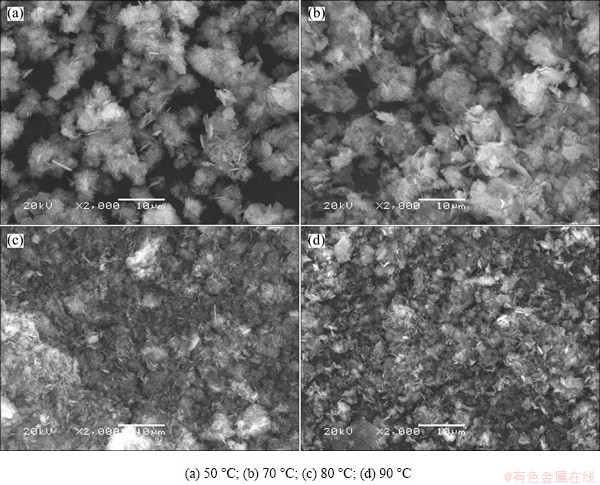
Fig. 7 SEM images of precipitate produced at different temperatures
3.6 Effect of reaction time on precipitation rate of lithium
The effect of reaction time on the precipitation rate of lithium was studied with the time varying from 0.5 to 3 h under the conditions of the mole ratio of Al to Li of 3.5:1, Ca content of 35%, initial lithium concentration of 0.8 g/L and reaction temperature of 70 °C. The reaction of Al-Ca alloy with brine is very quick and the results are shown in Fig. 8. It can be seen that the precipitation rate of lithium reaches 94.6% after reaction for 1 h. The precipitation rate of lithium does not change with the increase of the reaction time, which indicates that the reaction time of 1 h is enough for the precipitation of lithium from brine.
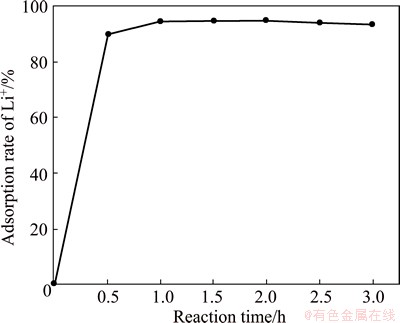
Fig. 8 Effect of reaction time on precipitation rate of lithium
4 Conclusions
1) The effect of Al-Ca alloy and Al-Fe alloy on the precipitation of lithium was compared. The results show that Al-Ca alloy has excellent reactivity with brine and LiCl·Al(OH)3·xH2O is obtained, while Al-Fe alloy reacts with brine hardly .
2) In the process of precipitating lithium by decomposing brine with Al-Ca alloy, the mole ratio of Al to Li, Ca content in Al-Ca alloy and reaction temperature have very significant influence on the precipitation rate of lithium. Under the conditions of mole ratio of Al to Li of 3.5:1, Ca content of 35%, initial Li+ concentration of 0.8 g/L, reaction temperature of 70 °C and reaction time of 1 h, the precipitation rate of lithium from brine reaches 94.6%.
References
[1] LIN Chun-jing, XU Si-chuan, LI Zhao, LI Bin, CHANG Guo-feng, LIU Jin-ling. Thermal analysis of large-capacity LiFePO4 power batteries for electric vehicles [J]. Journal of Power Sources, 2015, 294(30): 633-642.
[2] WANG Wei, JIANG Bo, XIONG Wei-yi, WANG Zhen, JIAO Shu-qiang. A nanoparticle Mg-doped Li4Ti5O12 for high rate lithium- ion batteries [J]. Electrochimica Acta, 2013, 114(30): 198-204.
[3] LIU Xue-wu, TANG Jie, QIN Xu-song, DENG Yuan-fu, CHEN Guo-hua. Supercritical-hydrothermal accelerated solid state reaction route for synthesis of LiMn2O4 cathode material for high-power Li-ion batteries [J]. Transactions of Nonferrous Metals Society of China, 2014, 24(5): 1414-1424.
[4] DAI Zi-xi. Status and development trend of the world lithium resources [J]. China Nonferrous Metallurgy, 2008, 4: 17-20. (in Chinese)
[5] ZHOU Ping, TANG Jin-rong, ZHANG Tao. Supply and demand prospect of global lithium resources and some suggestions [J]. Geological Bulletin of China, 2014, 33(10): 1532-1538. (in Chinese)
[6] STEPHEN E K, PAUL W G, PABLO A M, GREGORY A K, MARK P E, TIMOTHY J W. Global lithium resources: Relative importance of pegmatite, brine and other deposits [J]. Ore Geology Reviews, 2012, 48: 55-69.
[7] SONG Peng-sheng, XIANG Ren-jie. Utilization and exploitation of lithium resources in salt lakes and some suggestions concerning development of Li industries in China [J]. Mineral Deposits, 2014, 33(5): 977-992. (in Chinese)
[8] WANG Zhen-zhen, ZHANG Fu-liang, HU Yong-da, LEI Xiao-li. The global status of lithium resource and suggestion on its development and utilization in China [J]. China Mining Magazine, 2014, 23(S1): s1-s5. (in Chinese)
[9] GAO Feng, ZHENG Mian-ping, NIE Zhen, LIU Jian-hua, SONG Peng-sheng. Brine lithium resource in the salt lake and advances in its exploitation [J]. Acta Geoscientica Sinica, 2011, 32(4): 483-492. (in Chinese)
[10] SONG Peng-sheng, LI Wu, SUN Bo, NIE Zhen, BU Ling-zhong, WANG Yun-sheng. Recent development on comprehensive utilization of salt lake resources [J]. Chinese Journal of Inorganic Chemistry, 2011, 27(5): 801-815. (in Chinese)
[11] JI Kang-ping. Development and utilization of lithium resources [J]. Inorganic Chemicals Industry, 2005, 37(5): 7-9. (in Chinese)
[12] XU Hui, XU Liang, CHEN Bai-zhen, SHI Xi-chang, YANG Xin. Separating technique for magnesium and lithium from high Mg/Li ratio salt lake brine [J]. Journal of Central South University (Science and Technology), 2009, 40(1): 36-40. (in Chinese)
[13] ZHONG Hui, ZHOU Yan-fang, YIN Hui-an. Progress in technology for extracting lithium from Li-bearing brine resources [J]. Multipurpose Utilization of Mineral Resources, 2003, 1: 23-28. (in Chinese)
[14] YANG Li-xin, WU Sai-xiang, LIU Xiao-li, HE Jing, CHEN Wen-guang. Lithium and magnesium separation from salt lake brine by tributyl phosphate under action of co-extraction reagent ClO4- [J]. Chemical Journal of Chinese Universities, 2013, 34(1): 55-60. (in Chinese)
[15] ZHANG Yan-hui, LI Li-juan, LI Jin-feng, JI Lian-min, XU Qing-hua, CHEN Guang-hua. Study on mechanism of extraction of lithium from salt lake brine by tributylphosphate [J]. Inorganic Chemicals Industry, 2012, 44(3): 12-17. (in Chinese)
[16] SUN Shu-ying, YE Fan, SONG Xing-fu, LI Yun-zhao, WANG Jin, YU Jian-guo. Extraction of lithium from salt lake brine and mechanism research [J]. Chinese Journal of Inorganic Chemistry, 2011, 27(3): 439-444. (in Chinese)
[17] SUN Xi-liang, CHEN Bai-zhen, XU Hui, SHI Xi-chang. Extraction of lithium from bittern [J]. Journal of Central South University (Science and Technology), 2007, 38(2): 262-266. (in Chinese)
[18] NIE Zhen, BU Ling-zhong, WANG Yun-sheng, SONG Peng-sheng, ZHENG Mian-ping. Industrial technology for separation of lithium from magnesium rich salt lake brines [J]. Inorganic Chemicals Industry, 2013, 45(5): 1-4. (in Chinese)
[19] ZHU Zeng-hu, ZHU Chao-liang, WEN Xian-ming, ZHU Ge-qin, LING Bao-ping. Progress in production process of lithium carbonate [J]. Journal of Salt Lake Research, 2008, 16(3): 64-72. (in Chinese)
[20] CUI Xiao-qin, CHENG Fang-qin, ZHANG Ai-hua, LI Yong-gang. Study on precipitation separating technique for magnesium and lithium from salt lake brine [J]. Inorganic Chemicals Industry, 2012, 44(7): 33-35. (in Chinese)
[21] SOMRANI A, HAMZAOUI A H, PONTIE M. Study on lithium separation from salt lake brines by nanofiltration (NF) and low pressure reverse osmosis (LPRO) [J]. Desalination, 2013, 317(15): 184-192.
[22] MA Pei-hua, DENG Xiao-chuan, WEN Xian-min. Method for separating magnesium and enriching lithium from salt lake brine by NF: China, CN 1542147 [P]. 2004-11-3.
[23] PENG Qiu-hua. Production of battery-grade lithium carbonate from carbonate-type lithium concentrate by deep carbonization method [J]. Inorganic Chemicals Industry, 2014, 46(1): 38-42. (in Chinese)
[24] ZHOU Qi-li,WANG Mo-fei. Preparation of high purity lithium carbonate by carbonization method [J]. Inorganic Chemicals Industry, 2012, 44(7): 36-38. (in Chinese)
[25] HUANG Wei-nong, WANG Xue-kui, SUN Zhi-nan, SHA Zuo-liang, NIE Zhen, WANG Yan-fei. The preliminary study on the equilibrium solubility and crystallization laws of lithium carbonate based on the composition of Zhabuye salt lake brine [J]. Journal of Salt and Chemical Industry, 2009, 38(2): 8-12. (in Chinese)
[26] LI Li, LIU Fang, WU Feng, CHEN Ren-jie. Progress of research on the manganese oxide ion-sieve for extracting lithium [J]. Journal of Inorganic Materials, 2012, 27(10): 1009-1016. (in Chinese)
[27] ZHANG Li-fen, CHEN Bai-zhen, SHI Xi-chang, MA Li-wen, CHEN Ya. Synthesis and adsorption property of H2TiO3 type adsorbent [J]. The Chinese Journal of Nonferrous Metals, 2010, 20(9): 1849-1854. (in Chinese)
[28] SHI Xi-chang, ZHOU Ding-fang, ZHANG Zhi-bing, YU Liang-liang, Xu Hui, CHEN Bai-zhen, YANG Xi-yun. Synthesis and properties of Li1.6Mn1.6O4 and its adsorption application [J]. Hydrometallurgy, 2011, 110(1-4): 99-106.
[29] XIAO Jia-li, SUN Shu-ying, SONG Xing-fu, LI Ping, YU Jian-guo. Lithium ion recovery from brine using granulated polyacrylamide– MnO2 ion-sieve [J]. Chemical Engineering Journal, 2015, 279(1): 659-666.
[30] ZANDEVAKILI S, RANJBAR M, EHTESHAMZADEH M. Recovery of lithium from Urmia Lake by a nanostructure MnO2 ion sieve [J]. Hydrometallurgy, 2014, 149: 148-152.
[31] CHEN Xing-yu. Research of hydrogen generation by rapid splitting water with Al-based materials [D]. Changsha: Central South University, 2010: 114-116. (in Chinese)
[32] LIU Gao, ZHONG Hui. Study on the adsorption of Li+ by precipitation of Al(OH)3 [J]. Industrial Minerals & Processing, 2010, 5: 17-20. (in Chinese).
采用铝基材料从盐湖卤水中沉淀锂
李艳红,赵中伟,刘旭恒,陈星宇,钟茂礼
中南大学 冶金与环境学院,长沙 410083
摘 要:采用活化后的铝基材料分解卤水,同步实现卤水中锂的沉淀。研究不同的铝基材料吸附沉淀锂的效果,筛选出适合于提锂的铝基材料,并研究Al/Li摩尔比、Al-Ca合金中的Ca含量、溶液初始锂离子浓度、反应温度和时间等因素对锂沉淀率的影响,确定最优工艺参数。结果表明:Al/Li摩尔比、Ca含量和反应温度对锂沉淀率的影响较大;在优化后的工艺条件下,卤水中锂的沉淀率达到94.6%,Al-Ca合金的提锂效果良好。
关键词:盐湖卤水;提锂;铝基材料;Al-Ca合金
(Edited by Mu-lan QIN)
Foundation item: Project (U1407137) supported by the National Natural Science Foundation of China
Corresponding author: Xu-heng LIU; Tel: +86-731-88830476; Fax: +86-731-88710171; E-mail: liuxuheng@csu.edu.cn
DOI: 10.1016/S1003-6326(15)64032-8
Abstract: Salt lake brine was reacted with activated aluminum-based alloys and lithium was precipitated. The effects of aluminum-based alloys on precipitating lithium were investigated and the reasonable alloy used to extract lithium from brine was obtained. The effects of the mole ratio of Al to Li and Ca content of Al-Ca alloy, the initial concentration of lithiumion ion in solution, reaction temperature and reaction time on the adsorption rate of lithium were studied, and the optimized process parameters were determined. The results show that the mole ratio of Al to Li and Ca content of Al-Ca alloy and reaction temperature have great influences on the precipitation rate of lithium. The precipitation rate of lithium reaches 94.6% under the optimal condition, indicating that Al-Ca alloy is suitable for the extraction of lithium from salt lake brine.


