Trans. Nonferrous Met. Soc. China 27(2017) 2260-2271
Recovery of molybdenum and copper from porphyry ore via iso-flotability flotation
Qing-quan LIN1, Guo-hua GU1, Hui WANG2, You-cai LIU2, Chong-qing WANG2, Jian-gang FU2, Jun-yao ZHAO2, Luo-luo HUANG2
1. School of Minerals Processing and Bioengineering, Central South University, Changsha 410083, China;
2. School of Chemistry and Chemical Engineering, Central South University, Changsha 410083, China
Received 7 June 2016; accepted 25 October 2016
Abstract:
A copper-molybdenum iso-flotability flotation process has been developed to efficiently improve the recovery of molybdenite from Duobaoshan porphyry Cu-Mo ores. The effects of flotation approach, type of collector, feed particle size distribution, rougher pH value and reagent dosage on the recovery of molybdenite were evaluated systematically. The results suggest that compared with kerosene and diesel oil, transformer oil has stronger dispersion capability in water media and better flotation selectivity for molybdenite, providing a higher molybdenum recovery under low reagent dosage. Moreover, compared with bulk flotation approach, the iso-flotability flotation approach using transformer oil as a collector can obtain superior Mo recovery (90.77%) and grade (0.80%) in the cleaner concentrate, and increase the Mo recovery and grade by over 18% and 5% in the final Mo concentrate, respectively. The results of commercial flotation further indicate that the iso-flotability flotation approach is a rational and effective route to beneficiate the porphyry Cu-Mo ores.
Key words:
copper-molybdenum ore; iso-flotability flotation; oily collector; interfacial interaction; flotation separation;
1 Introduction
Currently, it is well known that about 99% of molybdenum production in the world is originated from molybdenite. Molybdenite occurs not only in a single molybdenum deposit, but also in the polymetallic deposits such as copper molybdenum sulphur deposit, and wolframium molybdenum bismuth deposit. Approximately 50% of the world’s molybdenum production comes from Cu-Mo ores as a by-product [1].
Until now, there are mainly three approaches used in the flotation recovery of molybdenum and copper from their porphyry sulfide ores. One is the bulk copper- molybdenum flotation followed by separation, which has been widely used in several large-scale mines such as Dexing copper mine (Jiangxi Province, China) [2]. This approach has an uncomplicated technological process and simple reagent scheme, but the separation operation of Mo/Cu sulfide minerals may encounter some difficulties such as high consumption of depressant and poor quality of concentrate product. The other is selective Cu flotation followed by bulk flotation or preferential Mo flotation followed by Cu flotation, which usually requires large reagent dosages and complex flowsheet [3]. LIU et al [4] reported that the preferential Mo flotation approach using a non-thiol collector was superior to bulk flotation process in improving the recovery of molybdenite from Dexing Cu-Mo ore. Their flotation indexes of pilot-scale testing were satisfactory, but the Na2S consumption of Mo/Cu separation still reached over 18 kg/t. The third approach is copper- molybdenum iso-flotability flotation followed by separation. In this case, a Cu-Mo rougher concentrate with a high recovery of molybdenite is first obtained and the recovery of copper from porphyry Cu-Mo ores is then implemented by two stages. The first stage is that using a high selective collector for molybdenite, molybdenum minerals in the porphyry Cu-Mo ores are concentrated as completely as possible; meanwhile, only a part of copper sulfide minerals with good floatability are also collected. This implies that the subsequent Mo/Cu flotation separation may be easily carried out by using low dosage of depressant against copper sulfides. Moreover, the second stage means that the rougher tailing of copper-molybdenum iso-flotability flotation (IFF) is treated by enhancing flotation of residual copper minerals, which involves thiol collectors with strong collection power to obtain a high overall recovery of copper.
Apparently, the emphasis of IFF approach lies in development of collectors with high selectivity for molybdenite. It is well known that molybdenite has a laminar crystalline structure consisting of a single sheet of molybdenum atoms sandwiched between two sheets of sulphur atoms. Strong covalent bonds act within S-Mo-S layers, but only weak van der Waals forces between adjacent S-S sheets. Molybdenite surfaces formed by rupture of weak van der Waals bonds are hydrophobic and consequently, naturally floatable [5,6]. Furthermore, several oily collectors such as kerosene, diesel and plant oil are typically introduced into the flotation system of molybdenite to enhance the mineral’s hydrophobicity [7-10]. In recent years, several new collectors such as emulsified kerosene, magnetized hydrocarbon oil and synthetic oil have also been developed and adopted for the flotation recovery of molybdenite [11-14]. However, these linear chain hydrocarbon oils commonly provide low recoveries of molybdenum in the case of treating with porphyry Cu-Mo ores. This phenomenon may be caused by various factors, including the inherent properties of molybdenite and other external factors, such as mineralogy of ore deposits, slime coatings, flotation reagents, grinding and liberation [15-18]. Although those factors have been studied intensely, the development of effective collectors as substitutes for fuel oils is always one of the research hotspots, because an appropriate collector plays an important role in improving the recovery of molybdenite from porphyry Cu-Mo ores. Furthermore, with respect to the oily collector, there exists a great challenge in creating a delicate balance between its adsorption intensity on the molybdenite surface, dispersing capability in the water phase and its selectivity for various minerals. Hence, a study on the interfacial interactions in the flotation system of molybdenite is of great significance, which can provide some insights into the selection of oily collectors.
Recently, we have found that transformer oil can be used as the suitable bridging reagent for the recovery of molybdenite from ultrafine waste tailings, because it has appropriate length of carbon chain, kinematic viscosity and cyclical structure [19]. Therefore, in the present work, the interfacial interactions between minerals, collectors and water were discussed by using transformer oil, kerosene and diesel oil as collectors of molybdenite. And the IFF approach using transformer oil as a collector was then investigated to recover molybdenum and copper from the porphyry Cu-Mo ore through bench-scale and pilot-scale tests.
2 Experimental
2.1 Materials and reagents
The porphyry Cu-Mo ore samples were taken from the Duobaoshan copper mine of Heilongjiang Province in China. Process mineralogy of ore samples suggests that the copper bearing minerals include bornite, chalcopyrite, covellite and cuprite. Chalcopyrite, as the most important form of copper sulfide minerals, distributes in the gangue minerals as the microgranular star-shaped formation. Molybdenum metal mainly exists as molybdenite, which commonly disseminates in the crack of gangue minerals with an occurrence of automorphic-hypautomorphic and flake aggregation. The gold occurs in forms of native gold and electrum, mainly embedded in bornite and chalcopyrite. The iron exists as pyrite and magnetite. Moreover, the nonmetallic minerals mainly consist of quartz, plagioclase, sericite, chlorite, calcite, apatite and zircon. The chemical composition analysis results of ore samples are given in Table 1, and the phase analysis results of copper and molybdenum minerals are presented in Table 2.
Table 1 Compositions of ore samples (mass fraction, %)
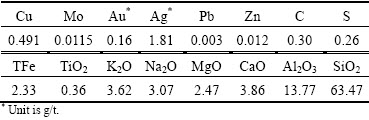
Table 2 Copper and molybdenum phase analysis result of ore samples

As seen from Tables 1 and 2, copper, molybdenum, gold and silver are main metal elements meriting flotation recovery from the porphyry ores. Furthermore, copper and molybdenum minerals are primary sulfide ores and their oxidation ratios are less than 9%.
Test reagents such as calcium oxide, sodium sulfide and sodium hexametaphosphate (SHMP) were analytically pure. Kerosene, 0# diesel oil, transformer oil, pine oil, sodium butyl xanthate (SBX), and ammonium dibutyl dithiophosphate (ABDTP) were industrial products. The tap water was used for all bench-scale flotation tests.
2.2 Experimental methods
In a bench-scale test, the raw ore (500 g, crushed to <2 mm during sampling) was ground to 68% passing 74 μm in a closed stainless steel d240 mm × 90 mm XMQ ball mill with adding effective amounts of calcium oxide at a pulp density of 55% in mass fraction. After wet grinding, the pulp was transferred to a XFD-1.5 L flotation cell (Changchun, China) and effective amounts of collector and frother were added to the slurry while agitating at about 1500 r/min. After the slurry was further conditioned for 3 min, air was fed and the froth flotation was continued for 5 min during which a rougher concentrate was collected for the subsequent flotation. Then, the enhancing flotation of residual copper minerals from the IFF tailings and cleaner flotation of molybdenum and copper minerals were performed in XFD-1.0 L and XFD-0.5 L flotation cells, respectively. Finally, both froth concentrates and final tailings were filtered, dried and weighed for further analysis and mass balance calculation. In order to convince the accuracy of flotation experimental results, the calculated grade of feed would be compared with the head assay. If the calculated Mo and Cu grades of feed were not in the range of (0.0115±0.0005)% and (0.491±0.004)%, respectively, the concentrates and tailings were re-assayed and even the flotation tests were redone. The flowchart of bench-scale and pilot-scale tests using Cu-Mo iso-flotability flotation approach is described in Fig. 1.
By contrast, the bench-scale test of bulk flotation approach was also conducted as the flowsheet proposed by LIU et al [2]. In the rougher flotation, SBX and kerosene were used as collectors of copper and molybdenum sulfide minerals. Then, the Mo/Cu flotation separation of cleaner concentrates was carried out by using kerosene as molybdenum collector and adopting the closed circuit flowsheet of one-stage rougher, one-stage scavenger and four-stage cleaner.
2.3 Calculation of interfacial interaction free energy
Molybdenite is naturally hydrophobic and the oily collectors used in flotation process are neutrally charged. Hence, the electrostatic interaction between interfaces is relatively weak and can be assumed negligible. Consequently, according to the extended Derjaguin- Landau-Verwey-Overbeek (EDLVO) theory, the total free energy of the interaction between two kinds of materials (components) includes the Lifshitz-van der Waals (LW) interaction free energy and the Lewis acid-base (AB) interaction free energy [20-24]. These interfacial interactions involve three forces, namely, van der Waals force, hydrophobic attractive force and hydration exclusive force. So, the interfacial interaction free energy can be calculated using the LW-AB approach, which is expressed by the following equation [20,21]:

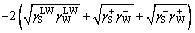 (1)
(1)
where is LW-AB interaction free energy between the solid surface and the liquid film surface or water (mJ/m2), subscript “S” is solid surface and subscript “W” means liquid film surface or water;
is LW-AB interaction free energy between the solid surface and the liquid film surface or water (mJ/m2), subscript “S” is solid surface and subscript “W” means liquid film surface or water; is Lifshitz-van der Waals (LW) interaction free energy of the two components (mJ/m2);
is Lifshitz-van der Waals (LW) interaction free energy of the two components (mJ/m2);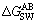 is Lewis acid base (AB) interaction free energy of the two components (mJ/m2); γ is surface energy (mJ/m2), the superscripts “LW”, “+” and “-” refer to Lifshitz-van der Waals, Lewis acid component and Lewis base component, respectively.
is Lewis acid base (AB) interaction free energy of the two components (mJ/m2); γ is surface energy (mJ/m2), the superscripts “LW”, “+” and “-” refer to Lifshitz-van der Waals, Lewis acid component and Lewis base component, respectively.
If water is taken as the third component, the interaction free energy of components 1 and 2 via component 3 can be calculated by the following equation [22]:
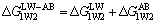

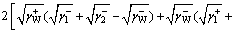
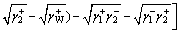 (2)
(2)
where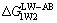 is LW-AB interaction free energy of two components 1 and 2 in aqueous phase (mJ/m2), the subscripts “W”, “1” and “2” mean water, component 1 and component 2, respectively;
is LW-AB interaction free energy of two components 1 and 2 in aqueous phase (mJ/m2), the subscripts “W”, “1” and “2” mean water, component 1 and component 2, respectively; and
and are LW interaction free energy and AB interaction free energy of the two components in aqueous phase (mJ/m2), respectively.
are LW interaction free energy and AB interaction free energy of the two components in aqueous phase (mJ/m2), respectively.
3 Results and discussion
3.1 Interfacial interaction free energy and flotation properties of oily collectors
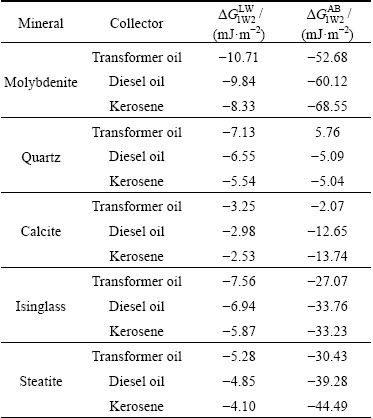
The interfacial interaction free energy of a series of interfaces occurring between minerals, water, collectors and bubble in the flotation system of molybdenite was calculated by WANG et al [25] using Eq. (1) or Eq. (2). The calculated results of interaction free energy between the oily collector, water and minerals are listed in Tables 3 and 4, respectively.
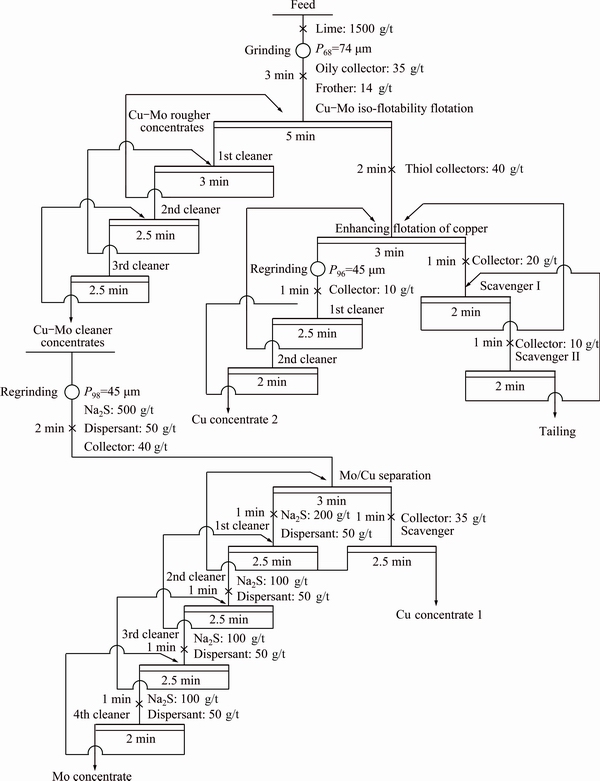
Fig. 1 Flowchart of molybdenum-copper iso-flotability flotation followed by separation and enhancing flotation of copper minerals
Table 3 Interfacial interaction free energy between oily collector and water in flotation system of molybdenite

As given in Table 3, the values of Lifshitz-van der Waals (LW) and Lewis acid base (AB) interaction free energy are negative, indicating that both van der Waals forces and hydrophobic attractive forces can cause the oily collector to disperse in the water media. Moreover, the LW interaction free energy is more negative than the AB free energy. Hence, the van der Waals force, caused by the LW interaction, is the dominant force between oily collector and water. From Table 3, it can also be seen that the dispersion capability of three oily collectors in the water solution follows the order: transformer oil > diesel oil > kerosene.
Table 4 exhibits the interfacial interaction free energy between various minerals and oily collectors in the flotation system of molybdenite. It is indicated that the van der Waals force between the molybdenite and the collector is attractive in the water media, but it is not the main force acting between the two surfaces. By contrast, the hydrophobic attractive force resulted from the AB interaction plays a more significant role. Taking the intensity of hydrophobic attraction into consideration, the absorption intensity of three collectors on the molybdenite surface falls in the rank: kerosene > diesel oil > transformer oil. Molybdenite has a good natural floatability, so whether the absorption intensity of oily collectors on the molybdenite surface can be used as a criterion for evaluating their flotation properties remains to be determined by flotation tests. Moreover, both van der Walls and hydrophobic attractive forces occur between collectors and gangue minerals in aqueous media. In particular, there exists large hydrophobic attraction between isinglass, steatite and collectors, which can cause an adverse impact on the flotation separation when the molybdenite occurs in conjunction with these minerals. Table 4 also indicates that among three oily collectors, transformer oil has the best selectivity for molybdenite over gangue minerals. And the flotation selectivity of three oily collectors for molybdenite decreases in the order: transformer oil > diesel oil > kerosene. In addition, it should be specially noted that the AB interaction free energy between quartz surface and transformer oil in the water media is positive, indicating that a hydrated repulsive force is able to appear between the two surfaces.
Table 4 Interfacial interaction free energy between minerals and oily collectors in flotation system of molybdenite
Furthermore, to study the relationships between the flotation behavior of molybdenite and the collector’s performance, we selected kerosene, diesel oil and transformer oil as collectors for flotation recovery of molybdenite from the Duobaoshan porphyry Cu-Mo ore. Under the conditions of feed particle size distribution of 68% passing 74 μm, calcium oxide amount of 1500 g/t and pine oil dosage of 18 g/t, the flotation tests were carried out as the flowchart given in Fig. 1. The results are shown in Fig. 2.

Fig. 2 Effects of types of collector on Mo recoveries and grades in Cu-Mo rougher concentrates
As shown in Fig. 2, compared with kerosene and diesel oil, transformer oil has superior collection performance with the highest Mo recovery and excellent Mo grade in the Cu-Mo rougher concentrate. It is indicated that transformer oil is more suitable to collect molybdenite from the porphyry ore, due to its good dispersion capability in water and high flotation selectivity for various minerals. This is consistent with the results reported in our previous work [25]. Moreover, the Mo recovery of above 92% can be obtained by the flotation tests using 32 g/t transformer oil, but the Mo recovery is less than 89% when large amounts of kerosene (120 g/t) and diesel oil (50 g/t) are used respectively. This phenomenon may be explained by the fact that cycloalkane of transformer oil has stronger dispersion capability and better selectivity for molybdenite than n-alkanes of linear chain oils [26]. Therefore, transformer oil was selected as an effective collector of molybdenite to perform the sequent flotation tests.
3.2 Condition flotation tests
3.2.1 Effects of feed particle size distribution
Based on preliminary exploratory tests, the copper- molybdenum IFF process was determined and several condition flotation tests were carried out. Under the rougher flotation conditions of calcium oxide amount of 1500 g/t, transformer oil of 30 g/t and pine oil of 18 g/t, the effects of the feed particle size distribution on the recoveries and grades of molybdenum and copper in the Cu-Mo rougher concentrates were studied. The results are shown in Fig. 3.

Fig. 3 Effects of feed particle size distribution on recoveries and grades of Mo (a) and Cu (b) in Cu-Mo rougher concentrates
As shown in Fig. 3, with an increase of the mass ratio passing 74 μm particles, the recoveries of molybdenum and copper have a corresponding increase trend. When the distribution of particle sizes is over 68% passing 74 μm, the increase tendencies of Mo and Cu recoveries gradually slow down while the grades of Mo and Cu sharply decrease. When a grind fineness of 68% passing 74 μm is used in the rougher flotation, the liberation degree of molybdenite is 84% while that of copper sulfides is 73%-80%. It can well explain the reason why the recovery of Mo (91.28%) is higher than that of Cu (77.34%) in the Cu-Mo rougher concentrate. Also, it is indicated that the subsequent cleaner concentrate should be reground to obtain high quality products in the Mo/Cu flotation separation. Therefore, at the Cu-Mo IFF stage, the feed particle size distribution of 68% passing 74 μm is suitable for the rougher flotation.
3.2.2 Effects of rougher pH
To inhibit the flotation of pyrite and gangue minerals in the rougher flotation process, pulp pH values should be adjusted from 6.5 to 11.0 by adding calcium oxide from 0 to 3000 g/t. Under the rougher flotation conditions of feed particle size distribution of 68% passing 74 μm, transformer oil of 30 g/t and pine oil of 18 g/t, the effects of calcium oxide dosage on the recoveries of molybdenum and copper in the Cu-Mo rougher concentrates were examined and the results are shown in Fig. 4.

Fig. 4 Effects of rougher pH values on recoveries of Mo and Cu in Cu-Mo rougher concentrates
As shown in Fig. 4, the recoveries of molybdenum and copper are significantly affected by pulp pH values. With adding calcium oxide from 0 to 1500 g/t, the pulp pH value increases from 6.5 to 8.5 and the recovery of Mo increases from 60.38% to 92.78%. At the same time, the recovery of Cu also shows an uptrend and increases from 65.95% to 77.69%. The inhibition of pyrite flotation by adding calcium oxide is conducive to obtain high-grade Cu-Mo rougher concentrates. However, a further increase of calcium oxide dosage will lead to the decrease in the recoveries of Mo and Cu. It is clear that the flotation behaviors of molybdenum and copper sulfide minerals are impacted markedly by calcium oxide at the high-alkaline pH. When the rougher pH value increases from 8.5 to 11.0, the Mo recovery decreases from 92.78% to 84.57%, which may be resulted from the negative influence of overdosing lime on the floatability of molybdenite. Hence, the appropriate dosage of calcium oxide is 1500 g/t, corresponding to the rougher pH value of 8.5.
3.2.3 Effects of collector dosage
With other flotation conditions fixed (feed particle size distribution of 68% passing 74 μm, pine oil of 18 g/t and rougher pH value of 8.5), the influence of collector dosage on the recoveries and grades of molybdenum and copper in the Cu-Mo rougher concentrates is depicted in Fig. 5.
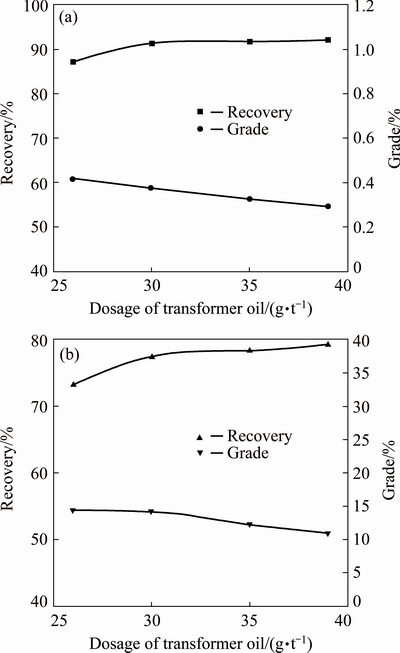
Fig. 5 Effects of dosage of transformer oil on recoveries and grades of Mo (a) and Cu (b) in Cu-Mo rougher concentrates
Figure 5 indicates that with an increase of transformer oil amount, the Mo recoveries increase while the Mo grades decrease. Furthermore, the changing trends of the recovery and grade of Cu are similar to those of Mo. When the transformer oil amount exceeds 35 g/t, the recoveries of Mo and Cu rise slightly. However, the grades of Mo and Cu drop markedly due to the growth of rougher concentrate yields. As a result, 35 g/t transformer oil is considered as the proper dosage in the Cu-Mo rougher flotation operation.
3.2.4 Effects of frother dosage
Under the fixed flotation conditions: feed particle size distribution of 68% passing 74 μm, transformer oil of 35 g/t and rougher pH value of 8.5, the effects of frother dosage on the recoveries and grades of Mo and Cu in the Cu-Mo rougher concentrates were investigated. The results are shown in Fig. 6.
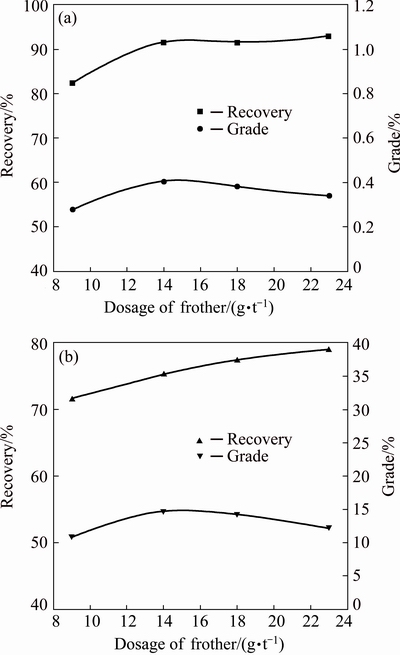
Fig. 6 Effects of frother dosage on recoveries and grades of Mo (a) and Cu (b) in Cu-Mo rougher concentrates
As shown in Fig. 6, when the dosage of pine oil is over 14 g/t, the Mo recoveries increase slowly and the Cu recoveries rise significantly; meanwhile, the grades of Mo and Cu in the rougher concentrates turn to decrease gradually. Apparently, the higher Mo recovery and lower Cu recovery in the Cu-Mo rougher concentrate are beneficial to simplifying the subsequent separation process of Mo/Cu minerals. Therefore, an appropriate dosage of pine oil is 14 g/t, corresponding to the Mo recovery of 91.31% and Cu recovery of 75.17%.
3.3 Enhancing flotation of Cu
At the IFF stage, the recoveries of Cu in the Cu-Mo rougher concentrates cannot reach 80%, indicating that a Cu recovery rate of above 20% is lost in the IFF tailings. Therefore, the enhancing flotation of residual copper minerals is necessary. Because of the weak floatability of copper-bearing minerals in the tailings, sodium butyl xanthate (SBX) and ammonium dibutyl dithiophosphate (ABDTP) mixed with the mass ratio of 3:1 were used as the collectors of copper minerals. Under the conditions of collector dosage of 80 g/t and regrinding fineness of <45 μm reaching 96%, the closed-circuit test of enhancing flotation of Cu was conducted as the flowsheet given in Fig. 1. The test results are listed in Table 5. The results indicate that a copper concentrate containing 15.88% Cu with 9.77% Cu recovery is achieved, and the Cu content of final tailing is reduced to 0.066%. Therefore, through enhancing flotation recovery of copper minerals from the IFF tailings, the residual copper minerals can be effectively retrieved, and the total recovery of copper from the feed reaches over 86%.
Table 5 Closed-circuit test results for enhancing flotation of Cu

3.4 Mo/Cu flotation separation
The Cu-Mo cleaner concentrates were obtained by three blank cleaner flotations of Cu-Mo rougher concentrates. The selected Cu-Mo cleaner concentrates with 0.80% Mo and 28.77% Cu, collected from the closed-circuit test of IFF and enhancing flotation of Cu, were used as the feed of Mo/Cu separation process. To acquire a satisfied Mo concentrate and an excellent Cu product, valuable minerals in the Cu-Mo cleaner concentrates were needed to be liberated fully, thus the approach of regrinding cleaner concentrates followed by Mo/Cu flotation separation was adopted.
3.4.1 Effects of Na2S dosage
Under the flotation conditions of regrinding fineness of cleaner concentrate of <45 μm reaching 98%, overall sodium hexametaphosphate (SHMP) of 250 g/t and transformer oil of 75 g/t, the effects of Na2S dosage on the flotation separation of Mo/Cu minerals were carried out as closed-circuit flowsheet given in Fig. 1. The results are plotted in Fig. 7. It is clear that with the increase of Na2S amount, the Cu grades and recoveries decrease significantly, while the Mo grades increase markedly and the Mo recoveries rise slightly. When the dosage of Na2S used in rougher flotation separation is over 500 g/t, the recovery and grade of Mo in the Mo concentrate turn to decrease gradually, but the recovery and grade of Cu do not change reasonably. Consequently, the suitable dosage of Na2S used in the rougher flotation of Mo/Cu separation is chosen to be 500 g/t.
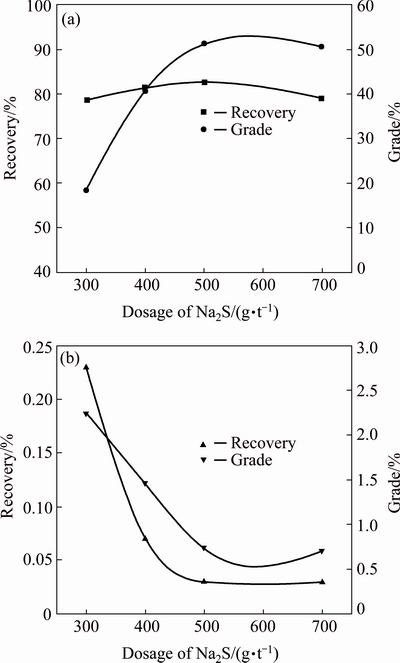
Fig. 7 Effects of dosage of Na2S used in rougher flotation separation on recoveries and grades of Mo (a) and Cu (b) in Mo concentrates
3.4.2 Effects of sodium hexametaphosphate dosage
Under the fixed flotation conditions: regrinding fineness of cleaner concentrate of <45 μm reaching 98%, overall Na2S dosage of 1 kg/t and transformer oil of 75 g/t, the influence of SHMP dosage on the recoveries and grades of Mo and Cu in the Mo concentrates was investigated. The results are shown in Fig. 8.
Figure 8 indicates that the use of SHMP as the slurry dispersant can significantly improve the cleaner operation and increase the Mo grades of molybdenum concentrates. With adding SHMP from 0 to 250 g/t, the Mo grades and recoveries increase by 34.16% and 6.17%, respectively. At the same time, the Cu grades decrease markedly from 2.66% to 0.82% and the Cu recoveries decrease slightly from 0.29% to 0.03%. However, when the dosage of SHMP exceeds 250 g/t, the Mo grades start to drop while the Cu grades increase slowly. Therefore, in consideration of minimizing the Cu content and maximizing the Mo grade in the Mo concentrates, the optimal SHMP dosage should be 250 g/t.

Fig. 8 Effects of dosage of SHMP on recoveries and grades of Mo (a) and Cu (b) in Mo concentrates
3.5 Closed-circuit tests
Based on the recommended flotation conditions, the locked cycle tests of Cu-Mo IFF followed by separation and enhancing flotation of copper were conducted using the flowchart and reagent scheme given in Fig. 1. Moreover, the contrast tests of bulk flotation followed by
separation were also carried out. The experimental conditions were as follows: 80 g/t SBX, 20 g/t kerosene and 10 g/t pine oil were used in the rougher circuit, about 11 of pulp pH was maintained by lime in the Cu/Fe separation, and 10 kg/t Na2S and 150 g/t kerosene were used in the Mo/Cu separation. The results of close circuit tests are displayed in Tables 6 and 7.
As given in Table 6, a high Mo recovery of 92% and Mo grade of 0.33% in the rougher concentrate are achieved by the IFF approach, and the Mo recovery and grade in the cleaner concentrate are 90.77% and 0.80%, respectively. In comparison with the IFF approach, bulk flotation only obtains 75.89% Mo recovery with 0.55% Mo grade in the cleaner concentrate (after Cu/Fe separation). The differences of the recovery and grade of Mo in the cleaner concentrate for two flotation approaches are 14.88% and 0.25%, respectively. This may be due to the fact that the floatability of molybdenite is significantly impaired in the Cu/Fe separation of bulk flotation approach and lime may play a crucial role in depression of pyrite and molybdenite [2]. Moreover, Table 6 also indicates that the Cu recovery and grade in the Cu concentrates produced by the two flotation approaches are similar, but the differences of Mo recovery and grade in the Mo concentrates increase to 18.91% and 5.22%, respectively. These results mean that compared with bulk flotation approach, the IFF approach has a significant advantage in improving the Mo grade and recovery of molybdenum concentrate.
As seen from Table 7, the cleaner concentrate produced by bulk flotation is treated by Mo/Cu separation under the high dosage of reagents such as 10 kg/t Na2S and 150 g/t kerosene, while an inferior Mo concentrate containing 45.78% Mo with 84.1% Mo operation recovery is returned. By contrast, the IFF approach can obtain a relatively high grade of molybdenum in the Cu-Mo cleaner concentrate uncontaminated with thiol collectors, thus it is easier to separate molybdenite from copper sulfide minerals under low consumption of Na2S. As given in Table 7, the IFF approach achieves the Mo grade of 51% and operation recovery of 91% in the Mo concentrate, and the Mo/Cu flotation separation only consumes 1 kg/t Na2S and 75 g/t transformer oil.
From these results, it can be concluded that compared to bulk flotation approach, the IFF approach using transformer oil as the molybdenum collector can obtain superior Mo grade and recovery not only in the Cu-Mo recovery stage, but also in the Mo/Cu separation stage, can increase the Mo recovery by 18.91%, and can save about 90% Na2S consumption as well as 50% oily collector dosage.
Table 6 Results of close circuit tests for two different flotation approaches

Table 7 Results of close circuit tests for Mo/Cu flotation separation
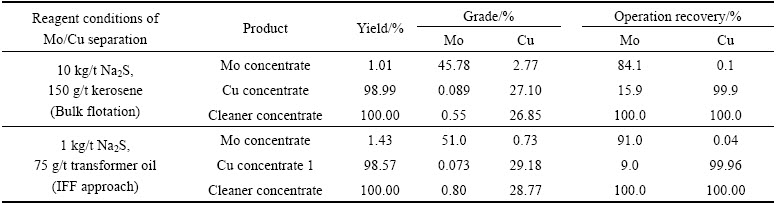
Table 8 Flotation results of pilot-scale testing

3.6 Pilot-scale testing of IFF approach
Pilot-scale testing of processing 1000 t/d porphyry Cu-Mo ores was conducted as the flowchart and reagent scheme given in Fig. 1. In the pilot-scale test, the grades of Cu, Mo, Au and Ag in the crude ores were 0.45%, 0.0136%, 0.24 g/t and 3.37 g/t, respectively. The statistical results of the continuous operation 9 shifts are listed in Table 8.
The results of pilot-scale testing are consistent to those of laboratory closed-circuit test. The Mo grade and recovery in the Mo concentrate are 51.22% and 79.18%, respectively, and the Cu grade is only 0.78%. Moreover, the attained Cu concentrates (Cu concentrate 1 + 2) have a high Cu recovery of 91.88%. As shown in Table 8, both molybdenite and copper minerals in crude ore are concentrated effectively; meanwhile, 74.61% Au and 61.69% Ag as co-present metal values are also recovered.
4 Conclusions
1) The recovery of molybdenum and copper from Duobaoshan porphyry Cu-Mo ores was investigated by a novel process of copper-molybdenum iso-flotability flotation followed by separation and enhancing flotation of copper. The effects of flotation approach, type of collector, feed particle size distribution, rougher pH value and reagent dosage on the molybdenite recovery were evaluated systematically.
2) Based on the interfacial interaction free energy between minerals, collectors and water, the dispersion capability and flotation selectivity of three collectors in water media follow the order: transformer oil > diesel oil > kerosene, which plays a more important role in the flotation recovery of molybdenite compared with its adsorption abilities on the molybdenite surface. Moreover, compared with kerosene and diesel oil, transformer oil provides superior flotation indexes under low reagent dosage.
3) The closed-circuit bench tests demonstrate that compared to bulk flotation approach, the IFF approach using transformer oil as the molybdenum collector can obtain superior Mo recovery (90.77%) and grade (0.80%) in the cleaner concentrate, and increase the Mo recovery and grade over 18% and 5% in the final Mo concentrate, respectively. Because of using transformer oil instead of thiol collector and kerosene in the Mo circuit, the IFF approach can easily perform the Mo/Cu flotation separation, which returns a superior Mo concentrate with Mo grade of 51% and operation recovery of 91% and saves about 90% Na2S consumption as well as 50% oily collector dosage. Pilot-scale testing further indicates that the IFF approach is a rational and effective route to beneficiate the porphyry Cu-Mo ores.
References
[1] BULATOVIC S M. Handbook of flotation reagents: Chemistry, theory and practice: Flotation of sulfide ores [M]. Amsterdam: Elsevier, 2007: 235-293.
[2] LIU Guang-yi, ZHONG Hong, XIA Liu-yin, WANG Shuai, XU Zheng-he. Improving copper flotation recovery from a refractory copper porphyry ore by using ethoxycarbonyl thiourea as a collector [J]. Minerals Engineering, 2011, 24(8): 817-824.
[3] LI Ming-yang, WEI De-zhou, SHEN Yan-bai, LIU Wen-gang, GAO Shu-ling, LIANG Guang-quan. Selective depression effect in flotation separation of copper-molybdenum sulfides using 2,3-disulfanylbutanedioic acid [J]. Transactions of Nonferrous Metals Society of China, 2015, 25(9): 3126-3132.
[4] LIU Guang-yi, LU Yi-ping, ZHONG Hong, CAO Zhan-fang, XU Zheng-he. A novel approach for preferential flotation recovery of molybdenite from a porphyry copper-molybdenum ore [J]. Minerals Engineering, 2012, 36-38: 37-44.
[5] ZHAO Cui-hua, CHEN Jian-hua, WU Bo-zeng, LONG Xian-hao. Density functional theory study on natural hydrophobicity of sulfide surfaces [J]. Transactions of Nonferrous Metals Society of China, 2014, 24(2): 491-498.
[6] CASTRO S, LOPEZ-VALDIVIESO A, LASKOWSKI J S. Review of the flotation of molybdenite. Part I: Surface properties and floatability [J]. International Journal of Mineral Processing, 2016, 148: 48-58.
[7] YIN Wan-zhong, ZHANG Li-rong, XIE Feng. Flotation of Xinhua molybdenite using sodium sulfide as modifier [J]. Transactions of Nonferrous Metals Society of China, 2010, 20(4): 702-706.
[8] DU Shu-hua, LUO Zhen-fu. Flotation technology of refractory low-grade molybdenum ore [J]. International Journal of Mining Science andTechnology, 2013, 23(2): 255-260.
[9] HE Ting-shu, WAN He, SONG Nian-ping, GUO Lin. The influence of composition of nonpolar oil on flotation of molybdenite [J]. Minerals Engineering, 2011, 24(13): 1513-1516.
[10] YOUNG T L , GREENE M G, BAUER K, REBER N R, YOUNG S K. Floatation of sulphide mineral species with oils [P]. US 6827220B1. 2004-12-07.
[11] RUBIO J, CAPPONI F, RODRIGUES R T, MATIOLO E. Enhanced flotation of sulfide fines using the emulsified oil extender technique [J]. International Journal of Mineral Processing, 2007, 84(1-4): 41-50.
[12] SUYANTARA G P W, HIRAJIMA T, ELMAHDY A M, MIKI H, SASAKI K. Effect of kerosene emulsion in MgCl2 solution on the kinetics of bubble interactions with molybdenite and chalcopyrite [J]. Colloids and Surfaces A: Physicochemical and Engineering Aspects, 2016, 501: 98-113.
[13] CHEN Li-juan, XU Qiu-sheng, LIU Yan-ying, MA Ling-shan, ZHONG Zai-ding. The experimental study on magnetized molybdenum collector [J]. China Molybdenum Industry, 2013, 37(4): 21-25. (in Chinese)
[14] ZHANG Wen-zheng. Searching for collectors for the flotation of molybdenite [J]. China Molybdenum Industry, 2006, 30(2): 3-6. (in Chinese)
[15] TRIFFETT B, VELOO C, ADAIR B J I, BRADSHAW D. An investigation of the factors affecting the recovery of molybdenite in the Kennecott Utah copper bulk flotation circuit [J]. Minerals Engineering, 2008, 21(12-14): 832-840.
[16] ZANIN M, AMETOV I, GRANO S, ZHOU L, SKINNER W. A study of mechanisms affecting molybdenite recovery in a bulk copper/molybdenum flotation circuit [J]. International Journal of Mineral Processing, 2009, 93(3-4): 256-266.
[17] JORJANI E, BARKHORDARI H R, KHORAMI M T, FAZELI A. Effects of aluminosilicate minerals on copper-molybdenum flotation from Sarcheshmeh porphyry ores [J]. Minerals Engineering, 2011, 24(8): 754-759.
[18] RAMOS O, CASTRO S, LASKOWSKI J S. Copper-molybdenum ores flotation in sea water: Floatability and frothability [J]. Minerals Engineering, 2013, 53: 108-112.
[19] FU Jian-gang, CHEN Kai-da, WANG Hui, GUO Chao, LIANG Wei. Recovering molybdenite from ultrafine waste tailings by oil agglomerate flotation [J]. Minerals Engineering, 2012, 39: 133-139.
[20] van OSS C J, CHAUDHURY M K, GOOD R J. Monopolar surfaces [J]. Advances in Colloid and Interface Science, 1987, 28: 35-64.
[21] van OSS C J, CHAUDHURY M K, GOOD R J. Interfacial Lifshitz-vander Waals and polar interactions in macroscopic systems [J]. Chemical Reviews, 1988, 88(6): 927-941.
[22] JANCZUK B, BRUQUE J M, GONZALEZ-MARTIN M L, ROMAN-GALAN E. The contribution of double layers to the free energy of interactions in the cassiterite-SDS solution system [J]. Colloids and Surfaces A: Physicochemical and Engineering Aspects, 1995, 100(6): 93-103.
[23] BIALOPIOTROWICZ T, JANCZUK B. The changes of the surface free energy of the adsorptive gelatin films [J]. European Polymer Journal, 2001, 37(5): 1047-1051.
[24] WANG Chong-qing, WANG Hui, GU Guo-hua, FU Jian-gang, LIN Qing-quan, LIU You-nian. Interfacial interactions between plastic particles in plastics flotation [J]. Waste Management, 2015, 46: 56-61.
[25] WANG Hui, GU Guo-hua, FU Jian-gang, CHEN Li, HAO Ye. Study of the interfacial interactions in the molybdenite floatation system [J]. Journal of China University of Mining and Technology, 2008, 18(1): 82-87.
[26] SMIT F J, BHASIN A K. Relationship of petroleum hydrocarbon characteristics and molybdenite flotation [J]. International Journal of Mineral Processing, 1985, 15(1-2): 19-40.
斑岩型铜钼矿等可浮浮选回收钼和铜
林清泉1,顾帼华1,王 晖2,刘有才2,王重庆2,符剑刚2,赵俊瑶2,黄锣锣2
1. 中南大学 资源加工与生物工程学院,长沙 410083;
2. 中南大学 化学化工学院,长沙 410083
摘 要:为了有效提高多宝山斑岩铜钼矿的辉钼矿回收率,开发了铜钼等可浮浮选的工艺流程。系统地考察了浮选方案、捕收剂种类、磨矿细度、粗选pH值及药剂用量对辉钼矿浮选回收的影响。结果表明,与煤油、柴油相比,变压器油在水中具有较强的分散能力,且对辉钼矿的浮选选择性较优,因而在低用量条件下便可获得较高的钼回收率。另外,与混合浮选方案相比,等可浮浮选方案利用变压器油作捕收剂不仅可获得较优的铜钼混合精矿(钼回收率和品位分别为90.77%和0.80%),而且能提高钼精矿的钼回收率在18%以上、钼品位在5%以上。工业浮选试验结果进一步表明,采用等可浮浮选方案选别该斑岩铜钼矿是行之有效的。
关键词:铜钼矿;等可浮浮选;油类捕收剂;界面作用;浮选分离
(Edited by Bing YANG)
Foundation item: Project (51374249) supported by the National Natural Science Foundation of China; Project (2016zzts103) supported by the Fundamental Research Funds for the Central Universities of China; Project (2015BAB12B02) supported by the National Science-Technology Support Plan, China; Project (2013B090800016) supported by Guangdong Provincial Science and Technology Plan, China
Corresponding author: Guo-hua GU; Tel: +86-13975151469; E-mail: guguohua@126.com
DOI: 10.1016/S1003-6326(17)60252-8
Abstract: A copper-molybdenum iso-flotability flotation process has been developed to efficiently improve the recovery of molybdenite from Duobaoshan porphyry Cu-Mo ores. The effects of flotation approach, type of collector, feed particle size distribution, rougher pH value and reagent dosage on the recovery of molybdenite were evaluated systematically. The results suggest that compared with kerosene and diesel oil, transformer oil has stronger dispersion capability in water media and better flotation selectivity for molybdenite, providing a higher molybdenum recovery under low reagent dosage. Moreover, compared with bulk flotation approach, the iso-flotability flotation approach using transformer oil as a collector can obtain superior Mo recovery (90.77%) and grade (0.80%) in the cleaner concentrate, and increase the Mo recovery and grade by over 18% and 5% in the final Mo concentrate, respectively. The results of commercial flotation further indicate that the iso-flotability flotation approach is a rational and effective route to beneficiate the porphyry Cu-Mo ores.


