DOI: 10.11817/j.issn.1672-7207.2020.09.003
姜黄素二聚体载药纳米粒的制备与性能研究
文纳川1,刘珍宝2,杜沛芳1,侯姣姣1,刘艳飞1
(1. 中南大学 化学化工学院,湖南 长沙,410083;
2. 中南大学 湘雅药学院,湖南 长沙,410013)
摘 要:
药物姜黄素载药效率,以姜黄素为单元合成新型姜黄素二聚体(CUR2-TK),并以聚乙二醇-聚乳酸羟基乙酸共聚物(PEG-PLGA)为载体,通过单乳液溶剂挥发法,制备姜黄素二聚体缓释纳米粒,研究不同药物CUR2-TK与聚合物PEG-PLGA的质量比(m(CUR2-TK):m(PEG-PLGA))等对纳米粒性能的影响。研究结果表明:通过姜黄素二聚体构建的载药纳米粒具备极高的载药效率,在m(CUR2-TK):m(PEG-PLGA)为3:1时,载药量和包封率分别达到(61.9±2.9)%和(80.1±3.8)%,且纳米粒形貌规整均一,粒径可控在50~100 nm之间,释药时间达4 d以上。
关键词:
姜黄素二聚体;聚乙二醇聚乳酸羟基乙酸;纳米粒;药物传递体系;
中图分类号:O633 文献标志码:A
文章编号:1672-7207(2020)09-2389-07
Preparation and properties of curcumin dimer loaded nanoparticles
WEN Nachuan1, LIU Zhenbao2, DU Peifang1, HOU Jiaojiao1, LIU Yanfei1
(1. School of Chemistry and Chemical Engineering, Central South University, Changsha 410083, China;
2. Xiangya School of Pharmaceutical Sciences, Central South University, Changsha 410013, China)
Abstract: In order to improve the drug loading efficiency of antitumor drug curcumin, a new curcumin dimer (CUR2-TK) was synthesized using curcumin as a unit, and polyethylene glycol-polylactic acid-glycolic acid copolymer (PEG-PLGA ) used as a carrier, curcumin dimer loaded sustained-release nanoparticles were prepared by a single emulsion solvent volatilization method, and the effects of different experimental conditions such as the mass ratio of different drugs to polymer (m(CUR2-TK): m(PEG-PLGA)) on the performance of nanoparticles were studied. The results show that the drug-loaded nanoparticles constructed by curcumin dimer have extremely high drug-loading efficiency. Under the condition of m(CUR2-TK): m(PEG-PLGA)=3:1, the drug load and entrapment efficiency reaches (61.9 ± 2.9)% and (80.1 ± 3.8)%, respectively, and the nanoparticles are uniform in appearance and the particle size can be controlled between 50 and 100 nm. The drug release of the nanoparticles is up to 4 d.
Key words: curcumin dimer; PEG-PLGA; nanoparticles; drug delivery system
姜黄素(CUR)是一种从姜黄中分离出来的植物化学物质,因其对膀胱癌、肺癌、乳腺癌、宫颈癌、卵巢癌等具有抗肿瘤效果[1-3],以及对正常组织细胞具有低毒性,近年来引起了人们极大的研究兴趣并被广泛用于癌症治疗[4]。研究表明,姜黄素具有很好的抗癌活性。然而,姜黄素的药代动力学性质差,存在水溶性低、代谢速度快,消除速度快和生物利用度低的缺陷,而常规通过脂质体或聚合物等包封CUR制成纳米粒,虽在一定程度上能延长体循环,提高生物利用度,但载药量不理想[5],包封药物的大量载体材料存在材料相关的毒性、代谢及降解问题[6],目前鲜有CUR高载药量的纳米载药体系的报道。自2015年CAI等[6]阐述药物制成二聚体形式的聚合物纳米粒子具有特别高的载药量和包封率后,人们对药物缀合成二聚体前药结构的研究产生了极大兴趣。PEI等[7-8]设计了二聚体药物缀合物,并将其用作纳米核心构成单元,形成具有二聚体药物核心的聚合物纳米粒子。这种方法一般能将药物载药量从低于10%提高到50%以上,如PEI等[7]合成的PEG-b-PDLLA载PTX二聚体载药量达到了85%;FANG等[9]开发了基于二聚喜树碱甘油磷酸胆碱(di-CPT-GPC)前药的喜树碱(CPT)的新脂质体制剂载药量达到了62%。人们对姜黄素构建二聚体的研究很少,一般药物构成二聚体都是通过酯键或酰胺键与含二硫键的响应子连接[7-9]。姜黄素因具备酚羟基,可通过酯键连接响应型结构域,因此,可以通过二聚体形式解决姜黄素载药量问题。该二聚体能在肿瘤的微环境解聚,发挥姜黄素的抗癌活性。同时,研究表明纳米粒子的粒径对其体循环稳定性、肿瘤部位的积累与穿透都有很大影响,已报道粒度大于200 nm的纳米粒容易被补体系统及网状内皮系统快速清除[10-11],而小于5 nm的微小纳米粒容易被肾脏排泄和肾小球滤过清理[12-13],且只有大于50 nm的粒子才能有效地通过EPR效应实现肿瘤积累,并随着粒径的增大肿瘤渗透能力降低[14-15],因此,抗肿瘤载药纳米粒适宜的粒径为50~100 nm。本文作者通过合成姜黄素二聚体,并以PEG-PLGA为载体,通过乳化溶剂挥发法制备(CUR2-TK)PEG-PLGA纳米粒,并探讨纳米粒制备工艺中药物聚合物比例等对纳米粒性能的影响,在提高载药效率的同时控制得到最佳粒径,并对药物的体外释药进行研究。
1 实验
1.1 样品与试剂
姜黄素粉末(西安昌岳生物生产);1-(3-二甲氨基丙基)-3-乙基碳二亚胺盐酸盐、4-二甲氨基吡啶、三巯基丙酸(上海安耐吉化学生产);聚乙二醇-聚乳酸羟基乙酸共聚物(PEG-PLGA)(相对分子质量为20 000,其中,PEG和 PLGA的相对分子质量分别为5 000和15 000,山东岱罡生物生产);其他试剂和溶剂均为分析纯。
1.2 姜黄素二聚体的制备
将无水3-巯基丙酸(5.31 g,50 mmol)和无水丙酮(5.81 g,100 mmol)加入25 mL三颈烧瓶中,然后用1 mL三氟乙酸(TFA)催化。在室温下搅拌3 h后,将烧瓶置于冷却的冰浴中进行产物结晶并过夜。抽滤过滤晶体,用正己烷和冷水冲洗。在真空烘箱中干燥得到白色产物缩硫酮TK[16]。
通过一锅反应制备姜黄素二聚体[17],将CUR(姜黄素,80.0 mg,0.22 mmol)溶解在20 mLCH2Cl2中,然后加入缩硫酮TK(25.2 mg,0.1 mmol),1-(3-二甲氨基丙基)-3-乙基碳二亚胺盐酸盐(EDC·HCl)(76.8 mg,0.40 mmol)和4-二甲氨基吡啶(DMAP)(2.5 mg,0.02 mmol)。于30 ℃下搅拌1 h之后,加入EDC·HCl(38.4 mg,0.20 mmol)和DMAP(2.5 mg,0.02 mmol),并在相同条件下搅拌反应24 h。反应后,旋转蒸发有机溶剂,再溶于3 mL 二甲基亚砜与12 mL纯水中,使用透析膜(500 D,MWCO)透析24 h除去小分子杂质,反应产物以CH2Cl2与MeOH的体积比为94:6为洗脱液,用硅胶柱层析法纯化,冻干得到深红色产物。
1.3 纳米粒的制备
使用溶剂挥发法制备(CUR2-TK)PEG-PLGA NPs[18]。分别将30 mg CUR2-TK溶解在0.3 mL DMSO中,将60,30,15和10 mg PEG-PLGA溶解在1 mL二氯甲烷中(其中,m(CUR2-TK):m(PEG-PLGA)分别为1:2,1:1,2:1和3:1,m为质量),然后,将二者混合后逐滴加入10 mL质量分数为1%的聚乙烯醇(PVA)溶液中,使用超声液体破碎仪(SONICS-VCX500)超声混合3 min,再加入40 mL质量分数为0.1% PVA溶液中,在转速为600 r/min磁力下搅拌4 h,挥发有机溶剂,然后用10 000 r/min低温离心15 min,并用蒸馏水洗涤重复3次,收集(CUR2-TK)PEG-PLGA NPs纳米粒子,冻干24 h,产物NPs储存在-20 ℃冰箱中。
1.4 纳米粒表征
通过纳米粒的形态、粒度、多分散系数、zeta电位、纳米粒载药量和包封率来对不同配比下合成的纳米粒(m(CUR2-TK):m(PEG-PLGA)为1:2,1:1,2:1和3:1)进行表征:采用马尔文粒度分析仪(Malvern Zeta-sizer Nano)对粒径和多分散指数(PDI)进行表征;采用透射电镜TEM(Tecnai G2 20S-Twin,FEI Czech Republic)研究纳米粒子形态;考察聚合物分子结构和载药纳米粒子制备条件对纳米粒子粒径的影响,通过调节载药条件对粒径性能进行调控,考察不同投料比下纳米粒的载药量与包封率;取冷冻干燥载药纳米粒加入二氯甲烷,离心、取上清液,采用紫外分光光度法计算纳米粒中药物的载药量与包封率。
1.5 体外释药性能研究
采用智能药物溶出仪进行纳米粒体外释放的性能研究。准确称取 100 mg载药纳米粒,置于透析袋中(2 000 D,MWCO),再加入5 mL pH=7.3的磷酸缓冲溶液,扎紧两端,加入释放液pH=7.3的磷酸缓冲溶液500 mL。调节温度为37 ℃,转速为 130 r/min,每隔一定时间取5 mL释放液,在波长415 nm处测定紫外吸光度,同时补充等量释放液,根据标准曲线计算累积溶出率。
2 实验结果与讨论
2.1 姜黄素二聚体的合成分析
利用姜黄素的酚羟基与缩硫酮的羧基进行酯化反应生成姜黄素二聚体,将2个单分子姜黄素连接起来,其反应式如图1所示。
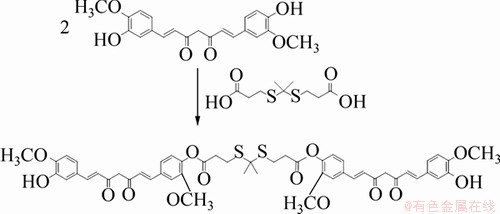
图1 姜黄素二聚体合成路线图
Fig. 1 Synthesis route of curcumin dimer
通过红外光谱(FTIR)和核磁共振氢谱 (1HNMR)表征姜黄素二聚体,分别如图2和图3所示。FTIR光谱(图2)表明在1 627 cm-1附近的峰与羰基的C=O延伸有关;1 592,1 507和1 429 cm-1附近的峰是苯环的C=C拉伸振动引起的。在CUR2-TK的光谱中,在1 730 cm-1处出现了新的吸收峰,这是酯键形成引起的C=O拉伸振动所致。姜黄素二聚体CUR2-TK的1HNMR谱(图3)表明:在核磁共振氢谱中,在化学位移6~8之间的区域中存在CUR芳族基团的多个特征性质子共振峰,化学位移2.5处的峰为氘代DMSO溶剂峰,化学位移3.3处的峰为水峰,而作为插入的中间连接基团的缩硫酮TK接头的特征峰出现在化学位移1.62和2.92处,这表明成功合成了目标产物。通过色谱柱纯化,姜黄素二聚体CUR2-TK的产率为76.37%。
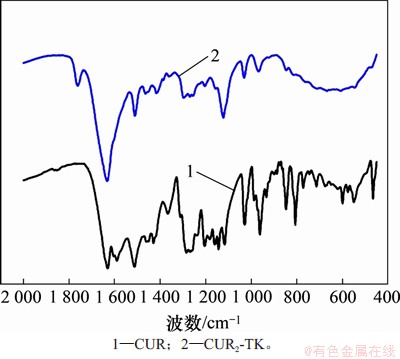
图2 CUR2-TK的红外光谱图
Fig. 2 FTIR spectrum of CUR2-TK
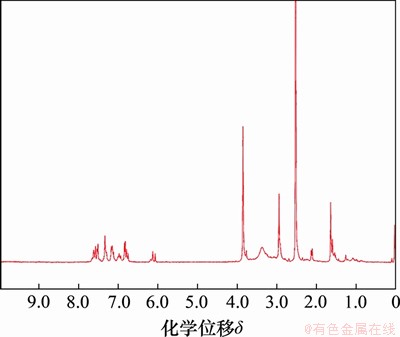
图3 CUR2-TK的核磁共振氢谱图
Fig. 3 1H NMR spectrum of CUR2-TK
2.2 纳米粒的制备与粒径、形貌分析
姜黄素为疏水性药物,因此,制备(CUR2-TK)PEG-PLGA纳米粒采用水包油(O/W)单乳液溶剂挥发法。在纳米粒制备过程中,药物姜黄素二聚体与载体PEG-PLGA的质量比、油相DMSO与二氯甲烷的用量、超声搅拌的强度与时间、水分散相中稳定剂PVA含量以及搅拌挥发有机溶剂的时间等因素都会影响最终制备的纳米粒性质,为了能通过EPR效应有效地实现肿瘤部位的药物积累,并加强纳米粒的肿瘤渗透能力,需要严格控制纳米粒的粒径,粒径为50~100 nm的纳米粒能达到最佳的肿瘤摄取效率。通过对实验条件的探索,确定得到纳米级纳米粒的最佳条件如下:油相聚合物PEG-PLGA在二氯甲烷溶剂中的质量分数为2.2%,水分散相中PVA的质量分数为1%,油相与水相以质量比为1.3:10进行超声搅拌3 min,再加入质量分数为0.1%的PVA水溶液中以600 r/min的搅拌速度挥发有机溶剂4 h,可以得到粒径小于100 nm的纳米粒。
通过透射电镜观察制备的(CUR2-TK)PEG-PLGA纳米粒,纳米粒水溶液的TEM照片如图4所示。从图4可以发现:纳米粒为球形结构,较为圆整,粒度较均一,表明获得了稳定的、具有良好球形的纳米粒。图4(a)所示为在m(CUR2-TK):m(PEG-PLGA)=1:2条件下制备的纳米粒的透射电镜图。从图4(a)可以发现纳米粒的平均粒径约为40 nm,而当m(CUR2-TK):m(PEG-PLGA)由1:2逐渐增加到3:1时,纳米粒的平均粒径逐渐增加到76 nm左右,这表明随着疏水性药物姜黄素二聚体的增加,制备的纳米粒粒径随之增大。
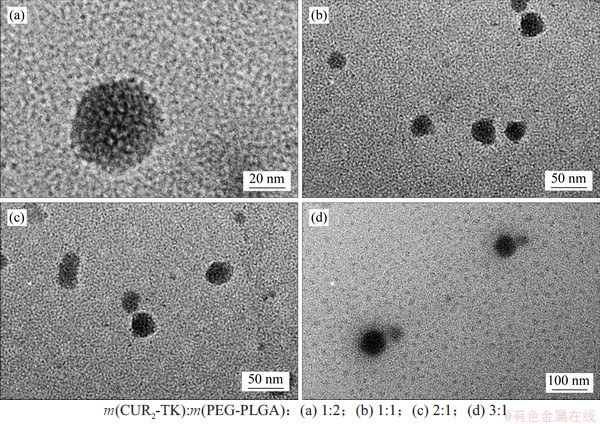
图4 载药纳米粒的TEM图像
Fig. 4 TEM images of (CUR2-TK) PEG-PLGA NPs
采用马尔文粒度分析仪(Malvern Zeta-sizer Nano)对制备的纳米粒的水合粒径与多分散系数进行表征,DLS粒径分析结果如图5所示,其结果与透射电镜观察到的纳米粒粒径较为吻合。在m(CUR2-TK):m(PEG-PLGA)分别为1:2,1:1,2:1和3:1的情况下,平均水合粒径分别为(41.0±2.4),(47.8±3.1),(58.3±4.5)和(76.6±5.3) nm,表明随着药物姜黄素二聚体含量的增加,纳米粒的粒径随之增大。图6所示为多分散系数PDI随药物姜黄素二聚体含量增加而增大,不同投料比下的多分散系数分别为0.168±0.009,0.215±0.013,0.323±0.021和0.409±0.025,表明随着载药量增加,粒径均一性变差。
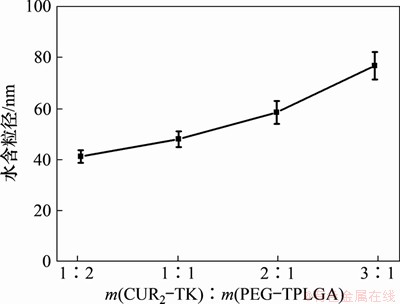
图5 不同药物、赋形剂投料比下纳米粒的粒径
Fig. 5 Size with different mass ratios of CUR2-TK and PEG-PLGA
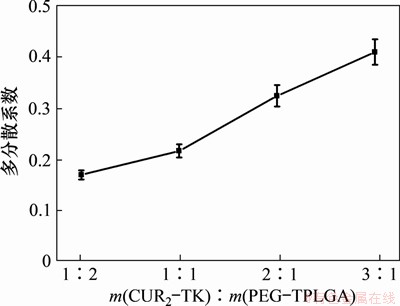
图6 不同药物、赋形剂投料比下纳米粒的多分散系数(PDI)变化
Fig. 6 PDI with different mass ratios of CUR2-TK and PEG-PLGA
2.3 纳米粒的载药量与包封率
疏水性药物单体负载于聚合物的载药量通常为10 %左右,姜黄素也是如此。而将药物单体合成二聚体后,载药量可大幅增加到50%以上。因此,通过药物姜黄素二聚体CUR2-TK与聚合物PEG-PLGA以不同的质量比制备纳米粒,其载药量与包封率随m(CUR2-TK):m(PEG-PLGA)的变化如表1所示。
表1 不同CUR2-TK与PEG-PLGA投料比下纳米粒载药量和包封率
Table 1 Drug loding and encapsulation efficiency with different mass ratios of CUR2-TK and PEG-PLGA
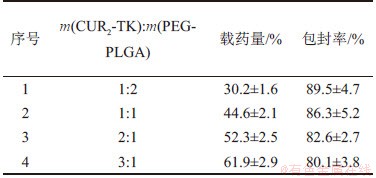
影响纳米粒的载药量与包封率的主要因素为药物CUR2-TK与聚合物PEG-PLGA的投料比。为了提高药物的载药量,减少赋形剂的使用及其毒性,需要在加大药物占比的同时保证一定的包封率。不同CUR2-TK与PEG-PLGA投料比下的纳米粒载药量和包封率见表1。从表1可知:随着CUR2-TK质量的增加,纳米粒的载药量逐步提高,但包封率随之降低。这可能归因于CUR2-TK比例的增加使O/W体系中CUR2-TK的浓度梯度增大;随着浓度梯度的作用,更多的药物CUR2-TK扩散至水相而未被油相包裹,因而造成药物损失,这意味着随m(CUR2-TK):m(PEG-PLGA)增加,药物在O/W体系中的浓度增大,因而包封率减小。结果显示,当药物与聚合物投料质量比为3:1时,载药效率高达(61.9±2.9)%,远超常规姜黄素单体构建的10%左右的载药量[5, 19-20];包封率为(80.1±3.8)%,变化不明显;且该投料比下得到的纳米粒粒径较均一,平均粒径为76 nm,表明该实验条件下制备的姜黄素二聚体纳米粒具有比较好的应用潜力。
2.4 纳米粒的体外药物释放性能
(CUR2-TK)PEG-PLGA纳米粒的体外释药研究在pH为7.3时的磷酸缓冲溶液中进行,采用m(CUR2-TK):m(PEG-PLGA)=3:1组进行释药研究。图7(a)所示为纳米粒累积药物释放度Qt与时间t的特性关系。从图7(a)可知:在开始阶段,由于纳米粒膜内外高的药物浓度差,导致药物迅速扩散,释药速率达到顶峰;随着浓度差减小,转变为缓释阶段,经100 h释药后,累积药物释放度Qt达到(84.87±3.15)%。
图7(b)所示为t1/2与累积药物释放度Qt的拟合直线。从图7(b)可知其与Higuchi释药模型相关性很好,释药模型拟合结果表明纳米粒在此缓冲液中的释放符合Higuchi方程:Qt=14.45+6.95 t1/2(相关系数R=0.998)。
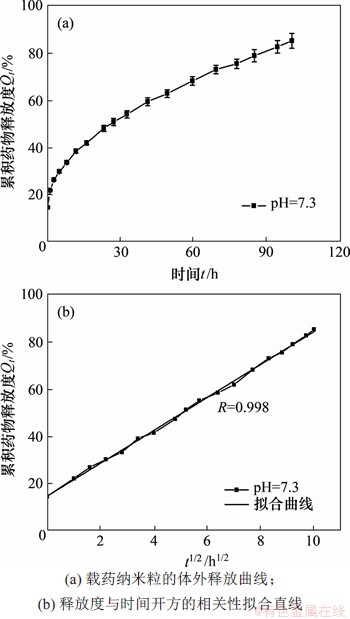
图7 载药纳米粒的体外释放曲线和拟合直线
Fig. 7 Release profiles of CUR2-TK from drug loaded nanoparticles and linear fitting chart
3 结论
1) 合成了姜黄素二聚体CUR2-TK,并以此为药物单元,以聚合物PEG-PLGA作为药物载体,通过单乳液溶剂挥发法制备了(CUR2-TK)PEG-PLGA纳米纳米粒。
2) 探讨了纳米粒制备过程中不同实验条件对纳米粒性能的影响,确定了优化后的实验条件,提高了姜黄素载药量。通过投料比调节,纳米粒载药量和包封率分别达到(61.9%±2.9)%和(80.1%±3.8)%,纳米粒形貌规整较均一,平均粒径为76 nm左右,且药物缓释时间达4 d以上。
3) (CUR2-TK)PEG-PLGA纳米粒有效提高了姜黄素的载药量,减少了载体的使用及可能的毒性,且粒径可控制在50~100 nm之间,在增强肿瘤部位积累的同时有较强的肿瘤穿透能力,可望作为一种抗肿瘤的药物传递系统。
参考文献:
[1] NGAI S C. Curcumin sensitizes cancers towards TRAIL-induced apoptosis via extrinsic and intrinsic apoptotic pathways[J]. Current Drug Targets, 2020, 21: DOI:10.2174/1389450121666200302124426.
[2] HU Yuzhu, HE Yihong, JI Jianrui, et al. Tumor targeted curcumin delivery by folate-modified MPEG-PCL self-assembly micelles for colorectal cancer therapy[J]. International Journal of Nanomedicine, 2020, 15: 1239-1252.
[3] MUKHOPADHYAY R, SEN R, PAUL B, et al. Gemcitabine Co-encapsulated with curcumin in folate decorated PLGA nanoparticles:a novel approach to treat breast adenocarcinoma[J]. Pharmaceutical Research, 2020, 37(3): 56.
[4] YANG Jingzhe, WANG Chengli, ZHANG Zhijie, et al. Curcumin inhibits the survival and metastasis of prostate cancer cells via the Notch-1 signaling pathway[J]. Apmis, 2017, 125(2): 134-140.
[5] MAITI C, PARIDA S, KAYAL S, et al. Redox-responsive core-cross-linked block copolymer micelles for overcoming multidrug resistance in cancer cells[J]. ACS Applied Materials & Interfaces, 2018, 10(6): 5318-5330.
[6] CAI Kaimin, HE Xi, SONG Ziyuan, et al. Dimeric drug polymeric nanoparticles with exceptionally high drug loading and quantitative loading efficiency[J]. Journal of the American Chemical Society, 2015, 137(10): 3458-3461.
[7] PEI Qing, HU Xiuli, LIU Shi, et al. Paclitaxel dimers assembling nanomedicines for treatment of cervix carcinoma[J]. Journal of Controlled Release, 2017, 254: 23-33.
[8] PEI Qing, HU Xiuli, WANG Lei, et al. Cyclodextrin/paclitaxel dimer assembling vesicles: reversible morphology transition and cargo delivery[J]. ACS Applied Materials & Interfaces, 2017, 9(32): 26740-26748.
[9] FANG Shuo, HOU Yongpeng, LING Longbing, et al. Dimeric camptothecin derived phospholipid assembled liposomes with high drug loading for cancer therapy[J]. Colloids and Surfaces B: Biointerfaces, 2018, 166: 235-244.
[10] LI S D, HUANG L. Nanoparticles evading the reticuloendothelial system: role of the supported bilayer[J]. Biochimica et Biophysica Acta(BBA)-Biomembranes, 2009, 1788(10): 2259-2266.
[11] MATSUMOTO Y, NICHOLS J W, TOH K, et al. Vascular bursts enhance permeability of tumour blood vessels and improve nanoparticle delivery[J]. Nature Nanotechnology, 2016, 11(6): 533-538.
[12] ZHOU Chen, LONG M, QIN Yanping, et al. Luminescent gold nanoparticles with efficient renal clearance[J]. Angewandte Chemie International Edition, 2011, 50(14): 3168-3172.
[13] CHOI H S, LIU Wenhao, MISRA P, et al. Renal clearance of quantum dots[J]. Nature Biotechnology, 2007, 25(10): 1165-1170.
[14] TANG Li, YANG Xujuan, YIN Qian, et al. Investigating the optimal size of anticancer nanomedicine[J]. Proceedings of the National Academy of Sciences of the United States of America, 2014, 111(43): 15344-15349.
[15] MAEDA H, WU J, SAWA T, et al. Tumor vascular permeability and the EPR effect in macromolecular therapeutics: a review[J]. Journal of Controlled Release, 2000, 65(1/2): 271-284.
[16] YUAN Youyong, LIU Jie, LIU Bin. Conjugated-polyelectrolyte-based polyprodrug: targeted and image-guided photodynamic and chemotherapy with on-demand drug release upon irradiation with a single light source[J]. Angewandte Chemie International Edition, 2014, 53(28): 7163-7168.
[17] PEI Qing, HU Xiuli, ZHENG Xiaohua, et al. Light-activatable red blood cell membrane-camouflaged dimeric prodrug nanoparticles for synergistic photodynamic/chemotherapy[J]. ACS Nano, 2018, 12(2): 1630-1641.
[18] PHAECHAMUD T, TUNTARAWONGSA S. Transformation of eutectic emulsion to nanosuspension fabricating with solvent evaporation and ultrasonication technique[J]. International Journal of Nanomedicine, 2016, 11: 2855-2865.
[19] LIU Lisha, BI Yunke, ZHOU Muru, et al. Biomimetic human serum albumin nanoparticle for efficiently targeting therapy to metastatic breast cancers[J]. ACS Applied Materials & Interfaces, 2017, 9(8): 7424-7435.
[20] YAO Qing, DAI Zhi, CHOI J H, et al. Building stable MMP2-responsive multifunctional polymeric micelles by an all-in-one polyme-lipid conjugate for tumor-targeted intracellular drug delivery[J]. ACS Applied Materials & Interfaces, 2017, 9(38): 32520-32533.
(编辑 杨幼平)
收稿日期: 2020 -04 -01; 修回日期: 2020 -06 -05
基金项目(Foundation item):湖南省自然科学基金资助项目(2020JJ4680);湖南省研究生自主探索创新项目(CX20190184);湖湘青年英才项目(2018RS3005);中南大学升华育英计划项目(CX20190242) (Project(2020JJ4680) supported by the Natural Science Foundation of Hunan Province; Project(CX20190184) supported by the Graduate Independent Exploration and Innovation of Hunan Province; Project(2018RS3005)supported by Huxiang Youth Talent; Project(CX20190242)supported by the Sublimation Education of Central South University)
通信作者:刘艳飞,博士,副教授,从事药物化学与药物制剂研究;E mail: liuyf@csu.edu.cn
摘要:为了提高抗肿瘤药物姜黄素载药效率,以姜黄素为单元合成新型姜黄素二聚体(CUR2-TK),并以聚乙二醇-聚乳酸羟基乙酸共聚物(PEG-PLGA)为载体,通过单乳液溶剂挥发法,制备姜黄素二聚体缓释纳米粒,研究不同药物CUR2-TK与聚合物PEG-PLGA的质量比(m(CUR2-TK):m(PEG-PLGA))等对纳米粒性能的影响。研究结果表明:通过姜黄素二聚体构建的载药纳米粒具备极高的载药效率,在m(CUR2-TK):m(PEG-PLGA)为3:1时,载药量和包封率分别达到(61.9±2.9)%和(80.1±3.8)%,且纳米粒形貌规整均一,粒径可控在50~100 nm之间,释药时间达4 d以上。


