Trans. Nonferrous Met. Soc. China 24(2014) 36-41
Effect of Ce on morphology of α(Al)-Al2Cu eutectic in Al-Si-Cu alloy
Maja 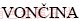 1,
1, 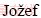 MEDVED1, Tonica
MEDVED1, Tonica 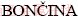 2, Franc
2, Franc 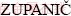 2
2
1. Department for Materials and Metallurgy, Faculty of Natural Science and Engineering, University of Ljubljana,  12, SI-1000 Ljubljana, Slovenia;
12, SI-1000 Ljubljana, Slovenia;
2. University of Maribor, Faculty of Mechanical Engineering, Smetanova 17, SI-2000 Maribor, Slovenia
Received 7 April 2013; accepted 5 August 2013
Abstract:
The effect of Ce addition on the morphology of the α(Al)-Al2Cu eutectic in Al-Si-Cu alloy was investigated using thermal analysis, light microscopy, scanning electron microscopy, focused ion beam and energy dispersive analysis. The results show that the eutectic α(Al)-Al2Cu forms within small space between dendrites, silicon and AlSiFeMn plates. Eutectic Al2Cu is not lamellar but degenerated. However, Al2Cu in Ce-modified alloys is more compact. Ce partially dissolves in Al2Cu, which is a viable reason for the formation of coarser Al2Cu. The addition of Ce also increases the microhardness of the α(Al)-Al2Cu eutectic by almost 10% compared with the basic Al-Si-Cu alloy.
Key words:
Al-Si-Cu alloy; cerium; eutectic; microstructure; focused ion beam;
1 Introduction
Al-Si alloys typically contain copper and sometimes magnesium as the main alloying elements in order to promote precipitation hardening. The types and quantities of intermetallic phases and heterogeneous structures depend largely on the composition and processing of the alloys [1]. Copper is bound mainly in Al2Cu. Al2Cu often appears as a part of a two-phase heterogeneous structure (α(Al)-Al2Cu), which is assigned in the literature as an eutectic constituent. This eutectic usually forms during the terminal stages of the solidification process [2-5]. Rare earth metals, such as cerium (Ce), were found to improve the mechanical properties of Al-Si castings through modifying their microstructures, enhancing their tensile strength [6], ductility [7], heat resistance and extrusion behaviour [8]. It was reported that these Ce-phases may serve as nucleation sites for aluminium or silicon crystals in both hypo- and hypereutectic Al-Si alloys [9]. A high cost of Ce somewhat inhibits its wider application.
The growth of binary α(Al)-Al2Cu eutectic under directional solidification conditions was studied in detail by several authors. The results were compiled in a monograph by ELLIOT [10]. The solidification of a normal α(Al)-Al2Cu eutectic was considered to begin with the nucleation of the Al2Cu phase, followed by the α(Al) nucleated heterogeneously and epitaxially on the Al2Cu phase. Under steady-state conditions, the α(Al)-Al2Cu eutectic possesses faulted lamellar structure because both phases have rather low entropy of solution at the eutectic temperature (15.1 J/(mol·K) for Al2Cu and 12.9 J/(mol·K) for α(Al)). During the early stages of solidification, several degenerate structures can form. The steady-state structure develops only from two of those structures; the others disappear early in the solidification process.
The morphology of α(Al)-Al2Cu eutectic was studied in less detail during equiaxed solidification than during directional solidification. In this case, the steady-state conditions are difficult to be obtained. A profound effect on the eutectic morphology and the primary α(Al) and Al2Cu grains can have available spaces between the primary phase, the cooling rate and the presence of other elements and phases. Thus, the eutectic normally does not have a lamellar morphology but a degenerated one [10]. In degenerated eutectics, one eutectic phase in Al-Cu alloys, the Al2Cu-phase, possesses irregular shape, which strongly deviates from regular lamellar or rod-like shape. The main goal of this work was to determine the effect of Ce on the morphologies of α(Al)-Al2Cu eutectic and the eutectic Al2Cu phase in multiphase structures forming during the final stage of solidification of the alloy Al-Si-Cu, with the composition similar to alloy A383.
2 Experimental
A commercial alloy A383 was melted in an electric induction furnace with the addition of 0.02% Ce and 0.05% Ce (mass fraction) with purity of 99.9%. After 10 min, the melt was poured into a measuring sand cell (made by coning process) with dimensions of 50 mm in height, 35 mm in diameter and volume of 2.52×104 mm3 [11]. In the middle, a K-type thermocouple was placed to measure the temperature upon solidification. The thermocouple was connected to the measuring card national instruments DAQPad-MI0-16XE–50 which was connected to the personal computer, where data were collected with LabVIEW 5.0 program and consequently the cooling curve was recorded. The chemical compositions of the investigated samples are presented in Table 1. In addition, a reference AlCu5 alloy was prepared in the same way to study the solidification of binary α(Al)-Al2Cu eutectic.
The characteristic solidification temperatures were determined from the cooling curves after simple thermal analysis (STA), and the effect of Ce on the α(Al)+Al2Cu eutectic temperature was defined.
The samples were prepared using a standard metallographic procedure for optical microscopy. The surface was investigated using an FEI Quanta 200-3D dual beam microscope (combination of a focused ion beam (FIB) and a scanning electron microscope (SEM)), and an FEI SIRION 400NC scanning electron microscope, equipped with an Oxford Instruments’ INCA 350 energy dispersive analyser. FIB-cross sections were carried out in several steps. For rough milling a current between 3 and 20 nA was applied, for middle milling 0.5-1 nA and for final polishing 0.1-0.5 nA. A low ion current was used (usually 10 pA) for ion microscopy. More details can be obtained in Ref. [12].
The morphologies of Al2Cu and other phases were revealed by deep etching using the methanol-iodine solution. The microhardness of Al2Cu phase with or without Ce was determined using Fischerscope H100C of Helmut Fischer Company.
3 Results and discussion
3.1 Analysis of cooling curves
Figure 1 shows a typical cooling curve (full line) for alloy A383 with 0.02% Ce and its corresponding derivative curve (dotted line). Different solidification parameters can be obtained by analyzing such a curve (Fig. 1). The solidification started with the crystallisation of α(Al) continues with the formation and growth of α(Al)-β-Si eutectic. An intermetallic phase AlSiMnFe formed upon further crystallisation. In the terminal stage of solidification Al2Cu forms. The temperature, at which Al2Cu appears, is denoted with TE3. It will be assigned as a temperature of the eutectic solidification (α(Al)-Al2Cu). However, it is not likely that a simple binary eutectic reaction will take place at the end of the solidification in such complex alloys. Nevertheless, the structures appearing at the end of solidification possess many features similar to those in binary α(Al)-Al2Cu eutectic. The addition of Ce to an A383 alloy shifts the eutectic temperature to slightly higher temperatures (Table 2), indicating that Ce causes some changes to the α(Al)-Al2Cu eutectic. The addition of Ce also makes the solidus temperature shift to a higher value.
Table 1 Chemical compositions of specimens
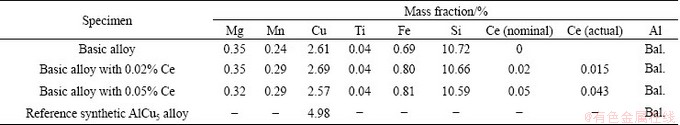
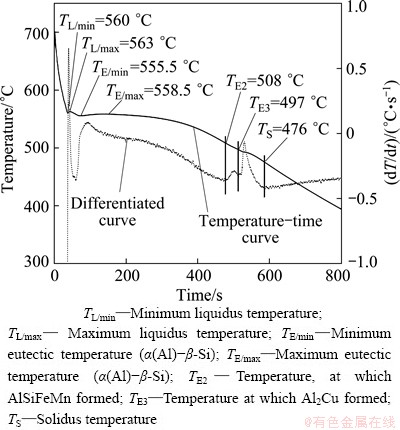
Fig. 1 Cooling curve and differential cooling curve of Al-Si-Cu alloy with 0.02% Ce (mass fraction) cooled with cooling rate of 3.5 °C/s
Table 2 Effect of Ce on eutectic temperature of specimens

3.2 Deep-etched samples
Deep etching revealed the morphology of Al2Cu phase (Fig. 2). Figure 2(a) shows the appearance of the eutectic Al2Cu in binary AlCu5 alloy. The content of Cu in this alloy is sufficient for Al2Cu to form an interconnected network in the spaces between dendrite arms of primary α(Al) dendrites. It can be observed that sidewalls of Al2Cu are smooth, and Al2Cu appears in the form of the degenerated eutectic on fracture surfaces, and the shape of Al2Cu is not lamellar but irregular. The mass fraction of α(Al)-Al2Cu eutectic was 6%-7%. The amount of copper in the basic Al-Si-Cu alloy and its modifications with Ce does not suffice to form a continuous network as in the binary AlCu5 alloy. It was measured that the mass fraction of α(Al)-Al2Cu eutectic is around 4%. Thus, only discrete islands form. However, these islands are not only bounded by α(Al) dendrite arms as in the AlCu5 alloy. Thermal analyses show that before the formation of α(Al)-Al2Cu eutectic, the binary α(Al)-β-Si eutectic forms, and the plates of the Al15(FeMn)3Si2phase form. This phase can be seen as a central rib in Fig. 2(b), and it can also be observed in the Ce-modified basic Al-Si-Cu alloy (see Fig. 2(c)). The main difference in comparison to binary AlCu5 alloy is that the sidewalls are not smooth, but highly fragmented, probably due to constitutional undercooling caused by the elements that enrich the remaining liquid. The structure of Al2Cu alloy is the finest in the basic Al-Si-Cu alloy, and coarser after Ce-addition. In both Ce-free and Ce-containing alloys, the space, in which α(Al)-Al2Cu eutectic forms, is rather limited, which also affects the growth of Al2Cu. This can be nicely seen in Fig. 3 by comparing the microstructures of the alloys with 0.02% Ce and 0.05% Ce, and Fig. 4 by comparing the Ce-free alloy and alloy containing 0.02 % Ce. The α(Al)-Al2Cu eutectic is limited, in all Al-Si-Cu alloys, irrespective of the Ce-content, by α(Al), β-Si and Al15(FeMn)3Si2, which allowed the formation of α(Al)- Al2Cu eutectic in spaces having approximately 20 μm in diameter.
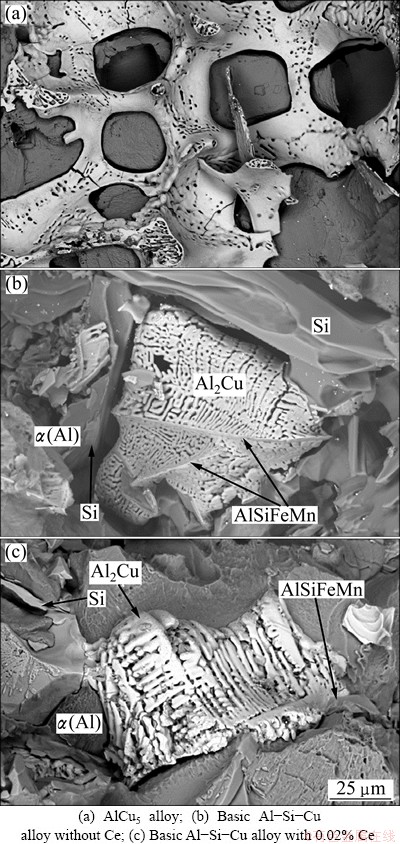
Fig. 2 Secondary electron micrographs of Al2Cu phase in deep-etched samples
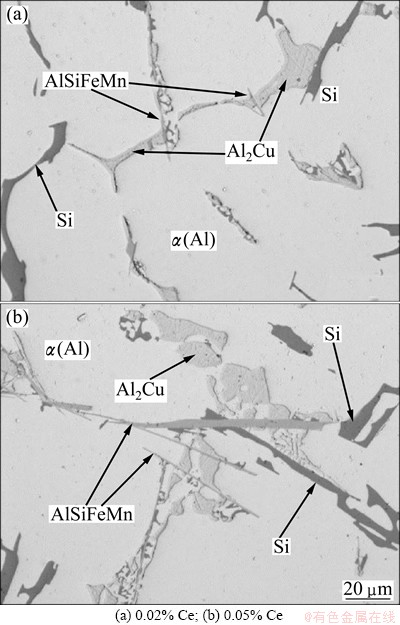
Fig. 3 Optical micrographs of basic Al-Si-Cu alloys with different mass fractions of Ce
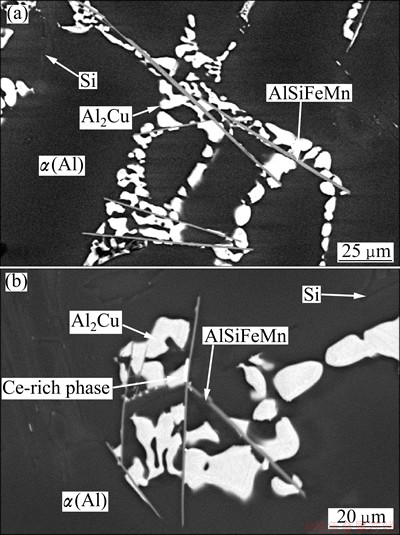
Fig. 4 Backscattered electron image (BSE) of basic Al-Si-Cu alloy without Ce (a) and with 0.02% Ce (b)
Figure 4 shows the microstructures of the basic Al-Si-Cu alloys without Ce, and the basic alloy with 0.02% Ce on the metallographic cross-sections. The morphology of Al2Cu is typical for the degenerated eutectic. Al2Cu forms in the spaces between binary α(Al)-Si eutectic. In these spaces Al15(FeMn)3Si2 is also present, with the shape of platelets, appearing as needles in metallographic cross-sections. A Ce-rich phase is also present. It forms on the sides of Al15(FeMn)3Si2 plates. EDS analysis of some larger particles in the alloy with 0.05% Ti reveals that its composition can be written as Al2CeSi2, which also contains 3% Cu (mole fraction). The Al2Cu is finer in the basic Al-Si-Cu alloy than in the Ce-modified alloys. Figure 5 shows these regions as 3D-microstructure obtained using FIB-milling. These micrographs confirm the previous results.
All results indicate that Al2Cu formed during solidification becomes coarser when Ce is added to A383 alloy. Figure 6 shows the results of a linescan in the basic Al-Si-Cu alloy with 0.02% Ce. It can be clearly seen that Al2Cu contains higher Ce content than the α(Al). The mole fraction of Ce is approximately 1.5%-2.5%. It can be speculated that the variation of morphology is caused by the incorporation of Ce in Al2Cu. The probable reason can be the rise of the surface energy between Al2Cu and α(Al). The dissolution of Ce in Al2Cu can increase the configurational entropy of Al2Cu. This can cause an increase in thermodynamic stability of Al2Cu.
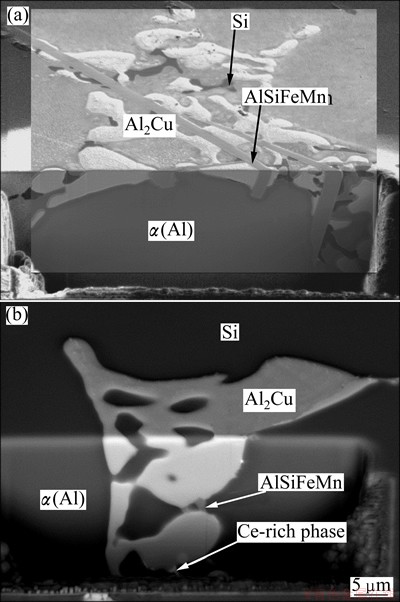
Fig. 5 FIB cross-section of basic Al-Si-Cu alloy without Ce (a) and with 0.02% Ce (b)
Still, further investigations are needed to confirm this assumption.
The microhardness tests reveal that Ce can also make the hardness of the eutectic regions increase. It is proved that the hardness of Al2Cu eutectic phase (or eutectic region) changes from HV481 in Ce-free alloy to HV542 when Al2Cu eutectic phase contains Ce. Higher hardness may be partially attributed to Ce dissolved in Al2Cu, but the main reason may lie in a much smaller fraction of α(Al) within the eutectic islands. The Ce-rich phase is always found outside the α(Al)-Al2Cu eutectic, predominantly on the sides of Al15(FeMn)3Si2 phase, thus it is unlikely to affect the hardness.
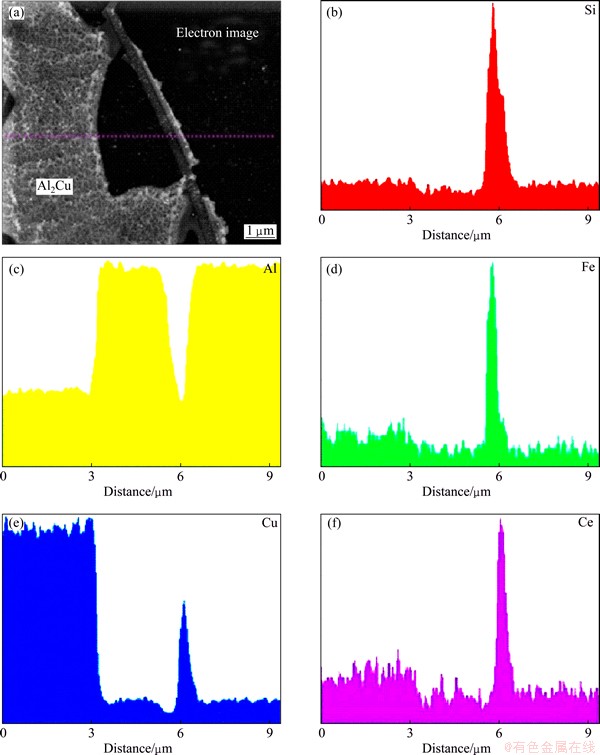
Fig. 6 Line scan through α(Al)-Al2Cu eutectic in basic Al-Si-Cu alloy with 0.02% Ce
4 Conclusions
1) The addition of Ce to basic Al-Si-Cu alloy causes an increase of temperature at which Al2Cu forms, while the solidification sequence is not apparently affected.
2) In all cases, α(Al)-Al2Cu eutectic is present in the degenerated form. It forms in a limited space, confined with α(Al) solid solution, plates of eutectic β-Si and plates of intermetallic phase Al15(FeMn)3Si2.
3) The Al2Cu eutectic is more branched in the basic Al-Si-Cu alloy without Ce addition. In Ce-modified alloy, it is more compact, with much smaller surface area. The addition of 0.02% Ce and 0.05% Ce causes the same change of morphology in comparison to the Ce-free alloy. It is also found that Ce partially dissolves in Al2Cu, which may be the reason why Al2Cu is coarser in Ce-containing alloys.
References
[1] LASA L, RODRIGUEZ-IBABE J M. Characterization of the dissolution of the Al2Cu phase in two Al–Si–Cu–Mg casting alloys using calorimetry [J]. Materials Characterization, 2002, 48: 371–378.
[2]  L, CHAI G, TAMMINEN J. Solidification characteristics of aluminum alloys [M]. De Plaine: AFS/ Skanaluminum, 1990.
L, CHAI G, TAMMINEN J. Solidification characteristics of aluminum alloys [M]. De Plaine: AFS/ Skanaluminum, 1990.
[3] ESMAEILI S, LLOYD D J, POOLE W J. Modelling of precipitation hardening for the naturally aged Al-Mg-Si-Cu alloy AA6111 [J]. Acta Materialia, 2003, 51: 3467–3481.
[4] VORGE C, JUCGUES M, SCHMIDT M P. Corrosion of aluminium [M]. London: Elsevier, 2001.
[5] MA Z, SAMUEL E, MOHAMED A M A, SAMUEL A M, SAMUEL F H, DOTY H W. Parameters controlling the microstructure of Al–11Si–2.5Cu–Mg alloys [J]. Materials and Design, 2010, 31: 902–912.
[6]  M, KORES S, MEDVED J. Influence of Ce addition on the solidification and mechanical properties of AlSi10Mg alloy [C]// Proceedings of Tofa 2010 Discussion Meeting on Thermodynamics. Porto, 2010: 75.
M, KORES S, MEDVED J. Influence of Ce addition on the solidification and mechanical properties of AlSi10Mg alloy [C]// Proceedings of Tofa 2010 Discussion Meeting on Thermodynamics. Porto, 2010: 75.
[7] AMMAR H R, MOREAU C, SAMUEL A M, SAMUEL F H, DOTY H W. Influences of alloying elements, solution treatment time and quenching media on quality indices of 413-type Al–Si casting alloys [J]. Materials Science and Engineering A, 2008, 489, 20: 426–438.
[8] WU Y, XIONG J, LAI R, ZHANG X, GUO Z. The microstructure evolution of an Al–Mg–Si–Mn–Cu–Ce alloy during homogenization [J]. Journal of Alloys and Compounds, 2009, 475: 332–338.
[9]  D, SCHMID-FETZER R. Thermodynamic aspects of the constitution, grain refining, and solidification enthalpies of Al-Ce-Si alloys [J]. Metallurgical and Materials Transactions A, 2004, 35: 3349–3362.
D, SCHMID-FETZER R. Thermodynamic aspects of the constitution, grain refining, and solidification enthalpies of Al-Ce-Si alloys [J]. Metallurgical and Materials Transactions A, 2004, 35: 3349–3362.
[10] ELLIOT R. Eutectic solidification processing, crystalline and glassy alloys [M]. London: Butterworths, 1983.
[11]  M, MEDVED J, MRVAR P. Energy of precipitation of Al[sub]2Cu and [alpha]-AlFeSi phase from the AlCu[sub]3 alloy and the shape of precipitates [J]. Metalurgija (Sisak), 2009, 48(1): 9-13.
M, MEDVED J, MRVAR P. Energy of precipitation of Al[sub]2Cu and [alpha]-AlFeSi phase from the AlCu[sub]3 alloy and the shape of precipitates [J]. Metalurgija (Sisak), 2009, 48(1): 9-13.
[12]  F,
F,  T,
T,  V. UV N2 laser ablation of a Cu–Sn–Zn–Pb alloy: Microstructure and topography studied by focused ion beam [J]. Journal of Alloys and Compounds, 2008, 465: 197–204.
V. UV N2 laser ablation of a Cu–Sn–Zn–Pb alloy: Microstructure and topography studied by focused ion beam [J]. Journal of Alloys and Compounds, 2008, 465: 197–204.
Ce对Al-Si-Cu合金中α(Al)-Al2Cu共晶形貌的影响
Maja  1,
1,  MEDVED1, Tonica
MEDVED1, Tonica  2, Franc
2, Franc  2
2
1. Department for Materials and Metallurgy, Faculty of Natural Science and Engineering, University of Ljubljana,  12, SI-1000 Ljubljana, Slovenia;
12, SI-1000 Ljubljana, Slovenia;
2. University of Maribor, Faculty of Mechanical Engineering, Smetanova 17, SI-2000 Maribor, Slovenia
摘 要:采用热分析、光学显微镜技术、扫描电镜技术、聚焦离子束和能量色散谱分析方法研究Ce对Al-Si-Cu合金中α(Al)-Al2Cu共晶形貌的影响。结果表明,在枝晶、硅和AlSiFeMn之间较小空间内形成了α(Al)-Al2Cu共晶。Al2Cu为非层状的不规则共晶组织。Al2Cu在经Ce改性的合金中更加致密。部分Ce溶解于Al2Cu中,这是粗晶Al2Cu形成的原因。与基体Al-Si-Cu合金相比,Ce的加入能使α(Al)-Al2Cu共晶合金的显微硬度提高约10%。
关键词:Al-Si-Cu合金;Ce;共晶;显微组织;聚焦离子束
(Edited by Wei-ping CHEN)
Corresponding author: Maja  ; Tel: +38-612000418; Fax: +38-614704560; E-mail: maja.voncina@omm.ntf.uni-lj.si
; Tel: +38-612000418; Fax: +38-614704560; E-mail: maja.voncina@omm.ntf.uni-lj.si
DOI: 10.1016/S1003-6326(14)63025-9
Abstract: The effect of Ce addition on the morphology of the α(Al)-Al2Cu eutectic in Al-Si-Cu alloy was investigated using thermal analysis, light microscopy, scanning electron microscopy, focused ion beam and energy dispersive analysis. The results show that the eutectic α(Al)-Al2Cu forms within small space between dendrites, silicon and AlSiFeMn plates. Eutectic Al2Cu is not lamellar but degenerated. However, Al2Cu in Ce-modified alloys is more compact. Ce partially dissolves in Al2Cu, which is a viable reason for the formation of coarser Al2Cu. The addition of Ce also increases the microhardness of the α(Al)-Al2Cu eutectic by almost 10% compared with the basic Al-Si-Cu alloy.


