
J. Cent. South Univ. (2021) 28: 2037-2051
DOI: https://doi.org/10.1007/s11771-021-4751-5

Bioleaching and dissolution kinetics of pyrite, chalcocite and covellite
SHANG He(尚鹤)1, 2, 3, 4, GAO Wen-cheng(高文成)1, 2, 3, 4,WU Biao(武彪)1, 2, 3, 4, WEN Jian-kang(温建康)1, 2, 3, 4
1. National Engineering Laboratory of Biohydrometallurgy, GRINM Group Co., Ltd., Beijing 100088, China;
2. GRINM Resources and Environment Tech. Co., Ltd., Beijing 101407, China;
3. GRIMAT Engineering Institute Co., Ltd., Beijing 101407, China;
4. General Research Institute for Nonferrous Metals, Beijing 100088, China
 Central South University Press and Springer-Verlag GmbH Germany, part of Springer Nature 2021
Central South University Press and Springer-Verlag GmbH Germany, part of Springer Nature 2021
Abstract:
In this work, the bioleaching process of pyrite, chalcocite and covellite which were the main phase compositions for Zijin copper mineral was comprehensively studied. The influence parameters, such as leaching temperature, Fe3+ concentration, pH of solution and bacteria concentration were investigated. The leaching kinetics of the pyrite, chalcocite and covellite under the studied conditions was successfully modeled by an empirical diffusion-like equation, respectively. The apparent activity energy of pyrite leaching, chalcocite leaching (stage II) and covellite leaching was calculated to be 69.29, 65.02 and 84.97 kJ/mol, respectively.
Key words:
pyrite; chalcocite; covellite; bioleaching; kinetics;
Cite this article as:
SHANG He, GAO Wen-cheng, WU Biao, WEN Jian-kang. Bioleaching and dissolution kinetics of pyrite, chalcocite and covellite [J]. Journal of Central South University, 2021, 28(7): 2037-2051.
DOI:https://dx.doi.org/https://doi.org/10.1007/s11771-021-4751-51 Introduction
In the last decades, bioleaching technology has been widely applied in the processing mineral resources of low grade in copper melting industry. The bio-hydrometallurgical process is often considered relatively convenient, cost-effective and eco-friendly [1-5]. An annual output of 3×104 t of cathode copper heap bioleaching plant has been built in Zijin copper mine, Shanghang County, Fujian province, China.
In the bioleaching technology, microorganisms, such as Acidithiobacillus ferrooxidans [6-10], Acidithiobacillus thiooxidans [11-14], Acidithiobacillus caldus [15-20], Leptospirillum ferrooxidans [21-24], and Ferroplasma spp. [25-27], were often employed to enhance the dissolution of copper sulfide ores. In the meantime, some other sulfide ore containing pyrite was also dissolved in the bioleaching process, and many iron ions were leached into the circulating solution, causing the problems to separation and recovery of iron, which was difficult to tackle with.
Dynamic condition could be the most defining factor in the process of a bioleaching system. There exists a big difference of leaching behaviors among the sulfide ores because of lattice energy [28], minerogenetic condition and course of dissolution. In order to restrain the dissolving of pyrite and to accelerate the leaching process of copper from chalcocite and covellite, it is necessary to study the bioleaching behavior of pyrite, chalcocite and covellite, and their dissolution kinetics.
To achieve this goal, the leaching process of pyrite, chalcocite and covellite separated from Zijin copper mine was studied systemically. The influence factors, such as leaching temperature, Fe3+ concentration, pH of solution, bacteria concentration were comprehensively investigated. Then, the bioleaching kinetics of pyrite, chalcocite and covellite was successfully modeled by an empirical diffusion-like equation and the apparent activity energy was calculated, respectively.
It was noticed that, there are mainly two stages in the dissolution of chalcocite. In Stage I, the chalcocite was leached quickly and transferred into covellite; in Stage II, the covellite was dissolved in the leaching process. Thus, Stage II was the key leaching step of the chalcocite, and was the main object of research in this work.
2 Materials and methods
2.1 Experimental materials
Bulk copper ore from the Zijin copper mine was adopted in bioleaching tests. The material was treated by crushing, grinding, gravity separation and step-flotation, and the pyrite, chalcocite and covellite concentrations were obtained respectively.
Analytically pure (NH4)2SO4 (>98.5%), K2HPO4 (99.0%), MgSO4·7H2O (≥99.0%), Ca(NO3)2·2H2O (≥99.0%), FeSO4·7H2O (>98.5%), Fe2(SO4)3 (>99.0%) were supplied by Tianjin Guangfu Fine Chemical Research Institute,and sulfuric acid (95.0%–98.0%) was obtained from Beijing Chemical Plant. Distilled water was used. All solutions in this study were prepared by dissolving analytical-grade chemicals directly without further purification.
2.2 Characterization
The chemical composition of pyrite, chalcocite and covellite concentrations samples were dissolved according to the standard process and determined by ICP-OES (Agilent Technologies 700 series ICP-OES). The structure and morphology of the particles were characterized by powder X-ray diffraction (XRD), the XRD (X’Pert PRO MPD, PANalytical, Almelo, The Netherlands) patterns were recorded on a diffractometer (using Cu Kα radiation) operating at 40 kV, 30 mA, scanning rate of 0.02 °/s in 2θ angle range of 5°-90°. Mineral liberation analyzer (MLA) was employed to identify the process mineralogy characteristic of the ore sample.
2.3 Bacteria and growth medium
A mixed culture of mesophilic bacteria (Acidithiobacillus sp., Leptopirillum sp., Ferroplasma sp.) obtained from the bioleaching heap of Zijin copper was used. If not stated otherwise, the bacteria were cultivated in 250 mL flasks with 100 mL modified medium 9K containing 0.5 g MgSO4·7H2O, 3.0 g (NH4)2SO4, 0.1g KCl, 0.5 g K2HPO4, 0.01 g Ca(NO3)2·2H2O, and 44.22 g FeSO4·7H2O. The pH of the medium was adjusted to 1.8-2.0 by adding H2SO4 (98 wt%) and measured with an electrode. After breeding and domestication, a kind of high activity of bacteria was obtained for the next experimental study, and the optimum growth temperature for the bacteria was 30-45 °C and pH was 1.2-2.5.
2.4 Experimental procedure
The bioleaching tests were carried out in a continuous-stirring reactor equipped with a water-cooled condenser, controller, recorder, dissolved oxygen electrode, pH/ORP instrument, etc., and the sketch of the reactor is shown in Figure 1. All the reactors were equipped with propeller agitator. The speed of stirring was kept at 200 r/min to satisfy the hydrodynamic requirements of maintaining a stable and intensive mixing.
Firstly, 2.0 L water and 100 g sample ore were put into the reactor (slurry concentration of 5%). The system was maintained at a desired temperature 30-35 °C with ±0.5 °C by circulating heating water through the reactor’s jackets. After that, the pH of the system was adjusted to 1.5-2.0 by 20% H2SO4 solution. When the pH was maintained stable, the bacteria (inoculation of 10%) were put into the system for the bioleaching kinetic study. In the experiments, the air and nitrogen were pumped alternately to keep a high level of equilibrium of dissolved oxygen, and the pH and ORP were recorded online, respectively.
Liquid samples (about 5 mL) were collected at predetermined time intervals (about 2 d), and filtered using 0.22 μm syringe filters. The concentrations of Cu2+ and total Fe of the clear filtrate were analyzed by ICP-OES (Agilent Technologies 700 series ICP-OES), and the leaching extents of metals can be calculated.
When the experiments were finished, the leaching residue was then collected, filtered off, and washed with distilled water three times to remove the ionic remnants, and dried slowly in an oven at 60 °C for 12 h. After that, the obtained sample was characterized by XRD and MLA to identify the phase composition.

Figure 1 Sketch of bio-leaching reactor
3 Modeling of leaching kinetics
In a general way, hydrometallurgical leaching steps were controlled by diffusion through the fluid and/or inert solid layer of the reactant, or by chemical surface reaction. The slowest step was thought to be the rate controlling step [29].
Aiming at establishing the bioleaching kinetics and rate controlling step for the dissolution of copper ores, the experimental data were analyzed by using the un-reacted shrinking core model. According to the shrinking core model, the reaction was considered to take place first at the outer surface of the particle [28, 29]. The region of the reaction goes into the solid and the reacting particle shrinks during the reaction. The rate can be described by film diffusion, chemical reaction, or product layer diffusion characteristics equations, which can be described as follows [30]:
Film diffusion:
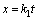 (1)
(1)
Product layer diffusion:
 (2)
(2)
Chemical reaction controls:
 (3)
(3)
where k is the kinetic constant; t is the reaction time; x is the leaching extent of concentration.
The activation energy of the dissolution reaction can be calculated by the Arrhenius equation [31]:
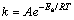 (4)
(4)
The values of A and Ea can be calculated from the intercept and slope of the plot of lnk against T-1.
For the purpose of understanding the effects of the parameters on the rate constant of bioleaching reaction, a semiempirical kinetic relation was also developed to correlate experimental data for the leaching process [32-34].
 (5)
(5)
where C is the concentration of Fe3+ or bacteria; pH is the pH value of leaching solution; and a, b and c are the parameters of the above model. By combining Eq. (3) with Eq. (5), the expression can be written as follows:
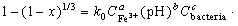
 (6)
(6)
4 Results and discussion
4.1 Characterization of sample
The elements analysis of obtained pyrite, chalcocite and covellite concentrations are listed in Table 1. Also, the theoretical compositions of pure pyrite, chalcocite and covellite are listed as comparison. We can see that all of the obtained pyrite, chalcocite and covellite concentrations have a high purity of >92%.
Table 1 Main compositions and purity of ore sample
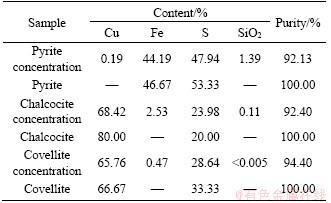
Figure 2 shows the XRD pattern of pyrite, chalcocite and covellite. As demonstrated, all of the compositions have a high purity.
To understand the mineral constituents of obtained pyrite, chalcocite and covellite concentration ores, the concentrations were analyzed by mineral liberation analyzer (MLA), and the quantitative analyses of the sample are shown in Tables 2-4, respectively.
As shown in Table 2, the MLA analysis of pyrite shows that it consists mainly of pyrite (94.93%), with a little amount of pyrrhotite (2.16%), chalcocite (0.06%), quartz (1.29%), mica (0.20%), rutile (0.09%) and zircon (0.02%), etc. From Table 3, the results show that the content of chalcocite in chalcocite concentration was in a high level of 93.91%, and the content of covellite was 2.48%. Also small amounts of other ores such as bornite (1.86%), native copper (0.16%) and pyrite (0.48%) were found in the chalcocite concentration.
According to the results of MLA analysis for covellite concentration, the major mineral was covellite, accounting for 91.94%. Also, small amounts of chalcocite (3.49%), digenite (3.36%), pyrite (0.66%) and chalcocite (0.18%) were determined.
On the basis of the characterization, the purity of obtained pyrite, chalcocite and covellite concentrations were in a high level, which satisfied the requirements of bioleaching kinetic research for the next step.
4.2 Leaching kinetics of pyrite
Generally, the bioleaching mechanism of pyrite was considered as follows:
 (7)
(7)
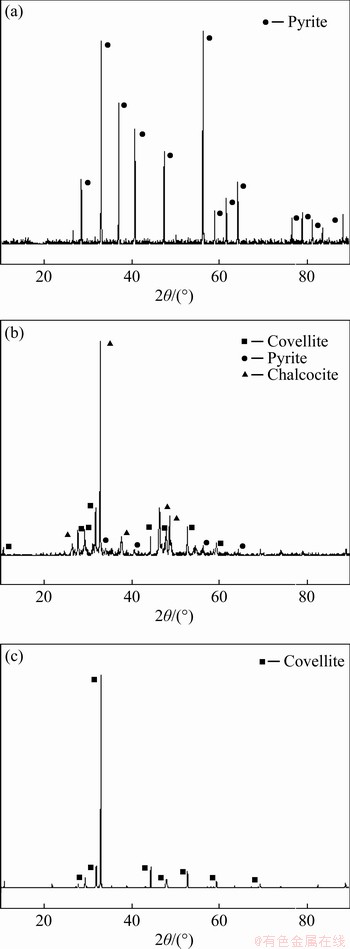
Figure 2 XRD patterns of pyrite concentrate (a), chalcocite concentrate (b) and covellite concentrate (c)
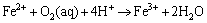 (8)
(8)
 (9)
(9)
At a lower pH, the oxidation effect of Fe3+ was higher than O2, but the dissolved O2 was the key point to enhance the reactions by supplying Fe3+.
4.2.1 Effect of leaching temperature
The temperature varying from 30 to 75 °C was adopted to investigate the effect of leaching temperature. In these experiments, the slurry concentration, initial pH of solution, particle size,speed of stirring were kept at 5%, 1.5, <0.074 mm (about 80%) and 120 r/min, respectively.
Table 2 Composition of pyrite concentration (wt%)

Table 3 Composition of chalcocite concentration (wt%)
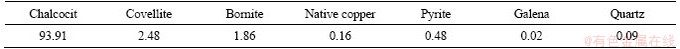
Table 4 Composition of covellite concentration (wt%)

The variation of pyrite dissolution with the leaching time at different leaching temperature is present in Figure 3. It is clear that a higher leaching temperature has a greater positive effect on leaching pyrite in the test duration.

Figure 3 Effect of temperature on leaching of pyrite
4.2.2 Effect of Fe3+ concentration
To investigate the effect of Fe3+ concentration on the leaching of pyrite, a certain amount of Fe2(SO4)3 was added in the leaching system, and the reaction condition was controlled as follows: slurry concentration of 5%, stirring speed of 120 r/min, solution initial pH of 1.50, particle size <0.074 mm (about 80%), and leaching temperature of 30 °C. The results are shown in Figure 4.
It is found that a higher Fe3+ concentration generates a higher leaching extent of pyrite as shown in Figure 4.
4.2.3 Effect of pH
The effect of pH on the leaching of pyrite was studied in a range of 0.5-2.0, and the reaction condition was kept as follows: slurry concentration 5%, stirring speed 120 r/min, particle size <0.074 mm (about 80%), and leaching temperature 30 °C. As shown in Figure 5, a lower pH makes a benefit for leaching of pyrite.
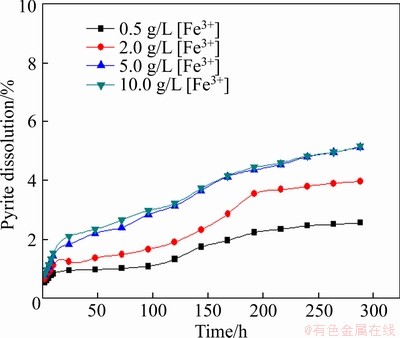
Figure 4 Effect of Fe3+ concentration on leaching of pyrite
4.2.4 Effect of bacteria concentration
The bacteria concentration of 5%-20% was investigated under condition of slurry concentration 5%, at stirring speed of 120 r/min, particle size <0.074 mm (about 80%), and leaching temperature of 30 °C, and the results are demonstrated in Figure 6. It can be concluded that the inoculation of bacteria generated a positive effect on the leaching of pyrite, and the bacteria concentration had a little effect of pyrite leaching.
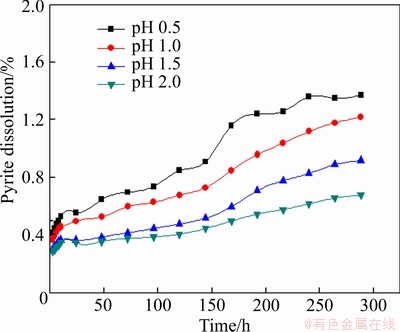
Figure 5 Effect of pH on leaching of pyrite

Figure 6 Effect of bacteria concentration on leaching of pyrite
4.2.5 Leaching process kinetics of pyrite
The leaching process kinetic experiments for pyrite leaching were performed in leaching temperature range of 30-75 °C, Fe3+ concentration of 0.5-10.0 g/L, pH 0.5-2.0, and bacteria concentration of 5%-20%. The experimental kinetic data under various conditions were correlated by the film diffusion model (Eq. (1)), product layer diffusion model (Eq. (2)), and chemical reaction control model (Eq. (3)). The analysis results demonstrated that the experimental data fitted well to Eq. (3) (see Figures 7-10, and Table 5), showing that the leaching of pyrite was controlled by the chemical reaction control step. From the slopes of straight lines in Figures 7-10, the values of apparent rate constants and regression coefficients were obtained as shown in Table 5.
The activation energy of the dissolution reaction was calculated from the Arrhenius equation (Eq. (4)). The value of E can be calculated from the slope of the plot of lnk against T-1, and E was calculated to be 69.29 kJ/mol (see Figure 11). The result was in the range of 33-92 kJ/mol [35, 36], indicating a feasible result in this work.
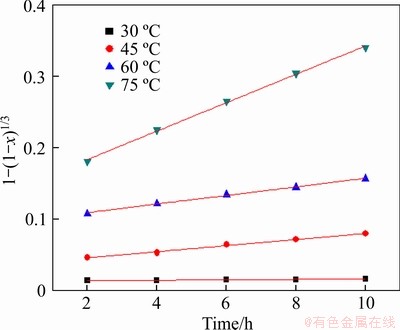
Figure 7 Variation of 1-(1-x)1/3 with time for different temperature

Figure 8 Variation of 1-(1-x)1/3 with time for different Fe3+ concentration

Figure 9 Variation of 1-(1-x)1/3 with time at different pH value

Figure 10 Variation of 1-(1-x)1/3 with time with different bacteria concentration
In order to understand the influence of the parameters on the rate constant of dissolution, Eq. (5) was adopted to correlate experimental data for the leaching process. The parameters of a, b and c in Eq. (5) can be estimated by multiple linear regression analysis of the main reaction data of pyrite (see Figures 12-14), and the semi-empirical equation can be given as:

 (10)
(10)
According to Eq. (7), the exponents of Fe3+ concentration, pH, and bacteria concentration are 0.279, -0.409 and 0.477, respectively. The leaching kinetics correlation indicated that higher concentration of Fe3+, lower pH, and higher bacteria concentration lead to a higher leaching extent of pyrite.
Table 5 Apparent rate constant (k) of pyrite and correlation coefficients
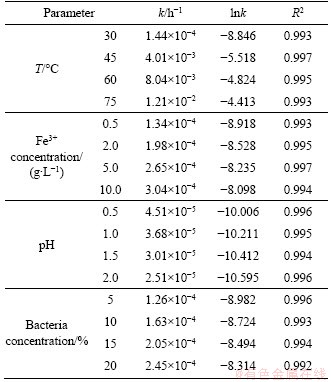

Figure 11 Napierian logarithm of reaction rate constant versus reciprocal of absolute temperature

Figure 12 Napierian logarithm of reaction rate constant versus napierian logarithm of Fe3+ concentration

Figure 13 Napierian logarithm of reaction rate constant versus napierian logarithm of pH

Figure 14 Napierian logarithm of reaction rate constant versus napierian logarithm of bacteria concentration
4.3 Leaching kinetics of chalcocite
There are mainly two stages in the dissolution of chalcocite as reported [37]. Stage I is significantly fast and involves the conversion of chalcocite into covellite (reaction (11)); Stage II is bioleached slowly (reaction (12)). Thus, Stage II is the key leaching step of the chalcocite.
 (11)
(11)
 (12)
(12)
4.3.1 Effect of leaching temperature
The leaching temperature range from 30 to 75 °C was chosen to investigate its effect on the leaching of chalcocite. In these experiments, the slurry concentration, initial pH of solution, particle size, speed of stirring were kept at 5%, 1.5,<0.074 mm (about 80%) and 120 r/min, respectively. The relationship of pyrite dissolution with leaching time at different leaching temperature is shown in Figure 15. It is found that the leaching extent of chalcocite increased quickly in the initial 2 min, showing the transformation of chalcocite to covellite. After that, a higher leaching temperature generated a greater positive effect on leaching chalcocite with increasing time.
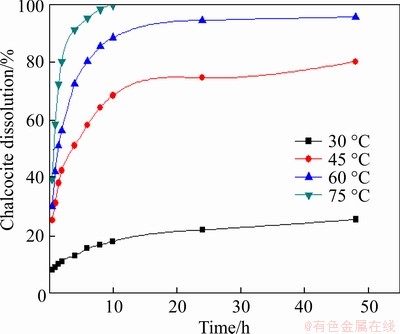
Figure 15 Effect of temperature on leaching of chalcocite
4.3.2 Effect of Fe3+ concentration
To investigate the effect of Fe3+ concentration on the leaching of chalcocite, a certain amount of Fe2(SO4)3 was added in the leaching system, and the leaching condition was controlled as follows: slurry concentration of 5%, stirring speed of 120 r/min, initial pH of solution 1.50, particle size <0.074 mm (about 80%), and leaching temperature of 30 °C. The results are shown in Figure 16. Similarly, we can see that a higher Fe3+ concentration generated a higher leaching extent of chalcocite.
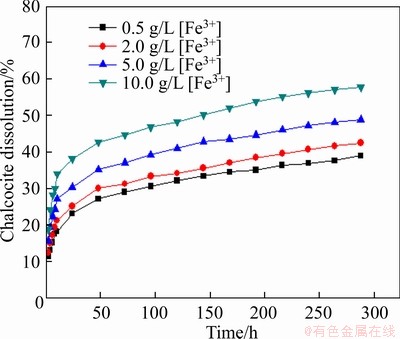
Figure 16 Effect of Fe3+ concentration on leaching of chalcocite
4.3.3 Effect of pH
The effect of pH on the leaching of pyrite was performed in pH range of 0.5-2.0, and the reaction condition was controlled as follows: slurry concentration of 5%, stirring speed of 120 r/min, particle size <0.074 mm (about 80%), and leaching temperature of 30 °C. As listed in Figure 17, a lower pH made a positive effect for leaching chalcocite.
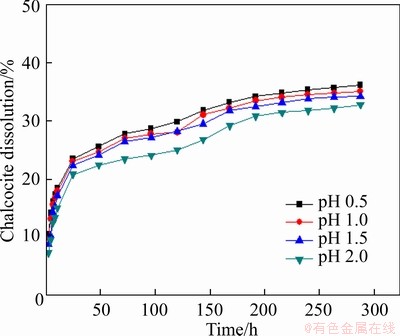
Figure 17 Effect of pH on leaching of chalcocite
4.3.4 Effect of bacteria concentration
To check the effect of bacteria, the bacteria concentration of 5%-20% was tested in the condition of slurry concentration of 5%, stirring speed of 120 r/min, particle size <0.074 mm (about 80%), and leaching temperature of 30 °C.
The results are present in Figure 18. It can be seen that the inoculation of bacteria made a positive effect on the leaching of chalcocite, and the bacteria concentration had a little effect on chalcocite leaching.
4.3.5 Leaching process kinetics of pyrite
Similarly, the leaching process kinetic experiments for chalcocite dissolution were performed under the condition of leaching temperature of 30-75 °C, Fe3+ concentration of 0.5-10.0 g/L, pH 0.5-2.0, and bacteria concentration of 5%-20%. The experimental kinetic data under various conditions were correlated by chemical reaction controls model (Eq. (3)), and the experimental data fitted well to Eq. (3) (see Figures 19-22, and Table 6), showing that the leaching process Stage II of chalcocite was controlled by the chemical reaction controls step. From the slopes of straight lines in Figures 19-22, the values of apparent rate constants and regression coefficients were obtained as shown in Table 6.
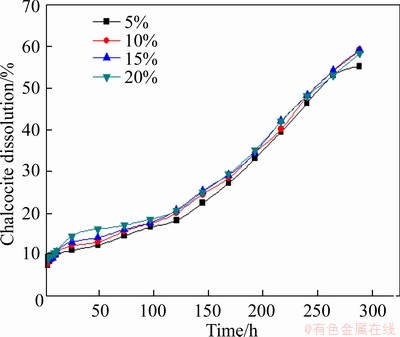
Figure 18 Effect of bacteria concentration on leaching of chalcocite
The activation energy of the dissolution Stage II of chalcocite was calculated from the Arrhenius equation (Eq. (4)), and the E was calculated to be 65.02 kJ/mol (see Figure 23), which was close to the data of Ref. [38], showing a reasonable result in this calculation.
Similar with the estimation and calculation of pyrite leaching (see Figures 24-26), the semiempirical kinetic equation can be obtained as follows:
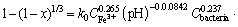
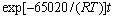 (13)
(13)

Figure 19 Variation of 1-(1-x)1/3 with time at different temperatures

Figure 20 Variation of 1-(1-x)1/3 with time with different Fe3+ concentrations
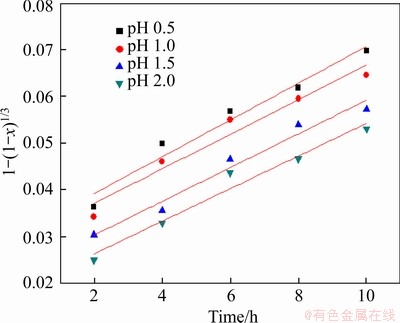
Figure 21 Variation of 1-(1-x)1/3 with time with different pH values
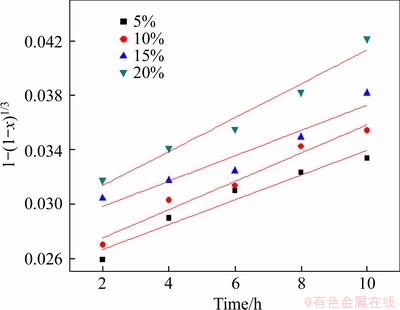
Figure 22 Variation of 1-(1-x)1/3 with time with different bacteria concentrations
Table 6 Apparent rate constant (k) of chalcocite and correlation coefficients

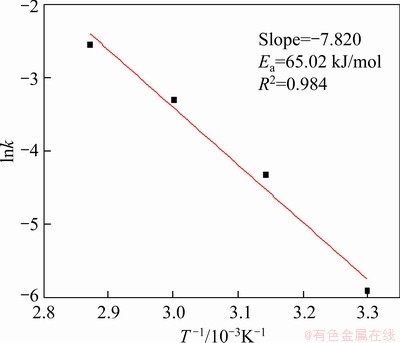
Figure 23 Napierian logarithm of reaction rate constant versus reciprocal of absolute temperature

Figure 24 Napierian logarithm of reaction rate constant versus napierian logarithm of Fe3+ concentration

Figure 25 Napierian logarithm of reaction rate constant versus napierian logarithm of pH value

Figure 26 Napierian logarithm of reaction rate constant versus napierian logarithm of bacteria concentration
According to Eq. (13), the exponents of Fe3+ concentration, pH, and bacteria concentration are 0.265, -0.0842 and 0.237, respectively. The leaching kinetics correlation indicated that higher concentration of Fe3+, lower pH, and higher bacteria concentration lead to a higher leaching extent of chalcocite.
4.4 Leaching kinetics of covellite
The leaching mechanism of covellite was listed as reaction (12), and the effects of leaching temperature, Fe3+ concentration and pH were investigated to establish the leaching kinetic equation.
4.4.1 Effect of leaching temperature
In order to understand the effect of leaching temperature, temperature range of 30-75 °C was adopted to investigate its effect on the leaching of covellite. In this work, the slurry concentration, initial pH of solution, particle size, stirring speed were kept 5%, 1.5, <0.074 mm (about 80%) and 120 r/min, respectively. The relationship between covellite dissolution and leaching time at different leaching temperature is demonstrated in Figure 27. It is obvious that a higher leaching temperature generated a greater positive effect on leaching of covellite with increasing reaction time.

Figure 27 Effect of temperature on leaching of covellite
4.4.2 Effect of Fe3+ concentration
To investigate the influence of Fe3+ concentration on the leaching of covellite, a certain amount of Fe2(SO4)3 was added in the leaching system, and the experimental condition was slurry concentration of 5%, stirring speed of 120 r/min, initial pH of solution 1.50, particle size <0.074 mm (about 80%), and leaching temperature of 30 °C, and the results are listed in Figure 28.
A similar tendency of data is found in Figure 28 compared with Figures 4 and 16. With increasing Fe3+ concentration, the leaching extent of covellite increased accordingly.
4.4.3 Effect of pH
The effect of pH on the leaching of covellite was studied with pH ranging from 0.5 to 2.0, under the condition of slurry concentration of 5%, stirring speed of 120 r/min, particle size <0.074 mm (about 80%), and leaching temperature of 30 °C.
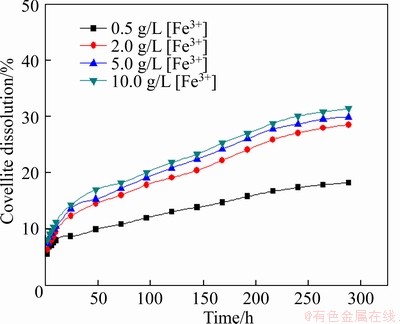
Figure 28 Effect of Fe3+ concentration on leaching of covellite
As shown in Figure 29, a lower pH, namely a higher sulfuric acid concentration made a benefit for leaching covellite.

Figure 29 Effect of pH on leaching of covellite
4.4.4 Effect of bacteria concentration
The bacteria concentration of 5%-20% was examined under condition of slurry concentration of 5%, stirring speed of 120 r/min, particle size <0.074 mm (about 80%), and leaching temperature of 30 °C. The results are demonstrated in Figure 30. It was also can be concluded that the inoculation of bacteria made a favorite effect on the leaching of covellite, and the bacteria concentration had a little effect on covellite leaching.
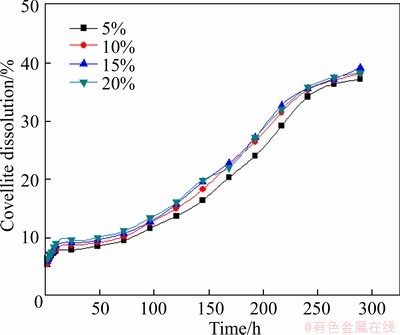
Figure 30 Effect of bacteria concentration on leaching of covellite
4.4.5 Leaching process kinetics of covellite
According to the kinetic analysis of pyrite and chalcocite, the same experimental condition and treatment of experimental data were adopted to analyze the leaching process kinetic analysis of covellite. The experimental kinetic data under various conditions were correlated by chemical reaction controls model (Eq. (3)), and the experimental data fitted well to Eq. (3) (see Figures 31-34, and Table 7), showing that the leaching process Stage II of chalcocite was also controlled by the chemical reaction control step.
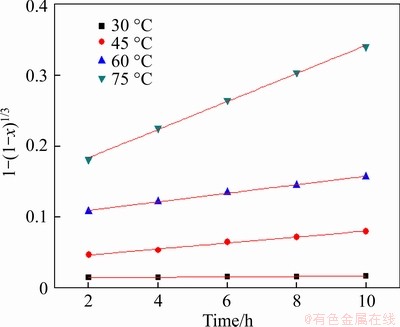
Figure 31 Variation of 1-(1-x)1/3 with time at different temperature

Figure 32 Variation of 1-(1-x)1/3 with time with different Fe3+ concentration
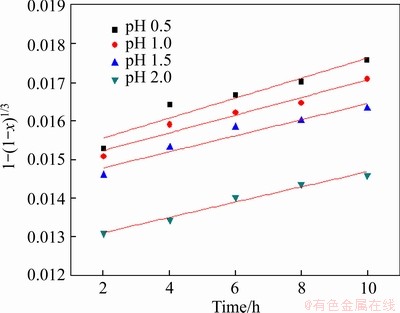
Figure 33 Variation of 1-(1-x)1/3 with time for different pH values

Figure 34 Variation of 1-(1-x)1/3 with time with different bacteria concentration
From the slopes of straight lines in Figures 31-34, the values of apparent rate constants and regression coefficients were obtained as shown in Table 7.
Table 7 Apparent rate constant (k) of covellite and correlation coefficients

The activation energy of the dissolution of covellite was calculated from the Arrhenius equation (Eq. (4)), and E was calculated to be 84.97 kJ/mol (see Figure 35), which was similar with Refs. [39, 40].
Similar with the estimation and calculation of pyrite leaching (see Figures 36-38), the semiempirical kinetic equation can be obtained as follows:

Figure 35 Napierian logarithm of reaction rate constant versus reciprocal of absolute temperature

Figure 36 Napierian logarithm of reaction rate constant versus napierian logarithm of Fe3+ concentration
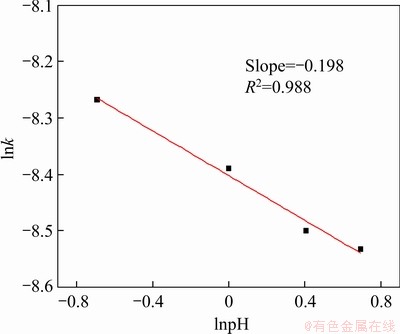
Figure 37 Napierian logarithm of reaction rate constant versus napierian logarithm of pH value
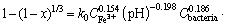
 (14)
(14)
From Eq. (14), we can see the exponents of Fe3+ concentration, pH, and bacteria concentration are 0.154, -0.198 and 0.186, respectively. The leaching kinetics correlation indicated that higher concentration of Fe3+, lower pH, and higher bacteria concentration lead to a higher leaching extent of covellite.

Figure 38 Napierian logarithm of reaction rate constant versus napierian logarithm of bacteria concentration
5 Conclusions
1) In this work, the leaching process of pyrite, chalcocite and covellite are experimentally investigated, and the leaching kinetics of the ores at the studied conditions is modeled by an empirical diffusion-like equation.
2) It is found that the bacteria concentration, Fe3+ concentration and pH of solution have a little effect on leaching of pyrite, and temperature is the major influence factor. The apparent activity energy of pyrite leaching is calculated to be 69.29 kJ/mol.
3) There are two stages in the leaching of chalcocite. The reaction in Stage I performed quickly while the chalcocite changed into covellite; while in Stage II, the leaching temperature is the main effect factor and the covellite dissolves completely in 10 h at leaching temperature of 75 °C. The apparent activity energy of chalcocite leaching in Stage II is calculated to be 65.02 kJ/mol.
4) Compared with chalcocite, the covellite is much more difficult to dissolve. The apparent activity energy of covellite leaching is 84.97 kJ/mol, which is higher than the chalcocite leaching in Stage II, indicating that the native covellite is very difficult to leach.
Contributors
The overarching research goals were developed by SHANG He, GAO Wen-cheng, WU Biao and WEN Jian-kang. SHANG He and WU Biao provided the concept and edited the draft of manuscript. SHANG He conducted the literature review and GAO Wen-cheng wrote the first draft of the manuscript. SHANG He and GAO Wen-cheng analyzed the measured data. SHANG He, GAO Wen-cheng, WU Biao and WEN Jian-kang edited the draft of manuscript. All the authors replied to reviewers’ comments and revised the final version.
Conflict of interest
SHANG He, GAO Wen-cheng, WU Biao and WEN Jian-kang declare that they have no conflict of interest.
References
[1] ROSSI G. Biohydrometallurgy [M]. Hamburg: McGraw-Hill, 1990.
[2] RAWLINGS D E, JOHNSON B D. Biomining [M]. Springer-Verlag Berlin Heidelberg, 2007.
[3] EDGARDO R, DONATI, WOLFGANG S. Microbial processing of metal sulfides [M]. Springer, 2007.
[4] ZHAO H B, WANG J, HU M H, QIN W Q, ZHANG Y S, QIU G Z. Synergistic bioleaching of chalcopyrite and bornite in the presence of Acidithiobacillus ferrooxidans [J]. Bioresource Technology, 2013, 149: 71-76. DOI: 10.1016/j.biortech.2013. 09.035.
[5] WU B, SHANG H, WEN J K, LIU M L, ZHANG Q D, CUI X L. Well-controlled stirring tank leaching to improve bio-oxidation efficiency of a high sulfur refractory gold concentrate [J]. Journal of Central South University, 2020, 27(5): 1416-1423. DOI: 10.1007/s11771-020-4377-z.
[6] GAN M, LI J Y, SUN S J, CAO Y Y, ZHENG Z H, ZHU J Y, LIU X X, WANG J, QIU G Z. The enhanced effect of Acidithiobacillus ferrooxidans on pyrite based Cr(VI) reduction [J]. Chemical Engineering Journal, 2018, 341: 27-36. DOI: 10.1016/j.cej.2018.02.014.
[7] YANG B J, LIN M, FANG J H, ZHANG R Y, LUO W, WANG X X, LIAO R, WU B Q, WANG J, GAN M, LIU B, ZHANG Y, LIU X D, QIN W Q, QIU G Z. Combined effects of jarosite and visible light on chalcopyrite dissolution mediated by Acidithiobacillus ferrooxidans [J]. Science of the Total Environment, 2020, 698: 134175. DOI: 10.1016/ j.scitotenv.2019.134175.
[8] YANG B J, ZHAO H B, FANG J H, ZHAO C X, LUO W, LIAO R, GAN M, WANG J, LIU X D, QIU G Z. Catalytic effect of silver on copper release from chalcopyrite mediated by Acidithiobacillus ferrooxidans [J]. Journal of Hazardous Materials, 2020, 392: 122290. DOI: 10.1016/j.jhazmat.2020. 122290.
[9] WU B, WEN J K, CHEN B W, YAO G C, WANG D Z. Control of redox potential by oxygen limitation in selective bioleaching of chalcocite and pyrite [J]. Rare Metals, 2014, 33(5): 622-627. DOI: 10.1007/s12598-014-0364-6.
[10] ZHAO H B, WANG J, QIN W Q, HU M H, QIU G Z. Electrochemical dissolution of chalcopyrite concentrates in stirred reactor in the presence of Acidithiobacillus ferrooxidans [J]. International Journal of Electrochemical Science, 2015, 10: 848-858. DOI: 10.1.1.666.6424.
[11] POGLIANI C, DONATI E. Immobilisation of Thiobacillus ferrooxidans: Importance of jarosite precipitation [J]. Process Biochemistry, 2000, 35(9): 997-1004. DOI: 10.1016/S0032-9592(00)00135-7.
[12] BACELAR-NICOLAU P, JOHNSON D B. Leaching of pyrite by acidophilic heterotrophic iron-oxidizing bacteria in pure and mixed cultures [J]. Applied & Environmental Microbiology, 1999, 65(2): 585-590. DOI: 10.1128/aem.65.2. 585-590.1999.
[13] WANG J, ZHU S, ZHANG Y S, ZHAO H B, HU M H, YANG C R, QIN W Q, QIU G Z. Bioleaching of low-grade copper sulfide ores by Acidithiobacillus ferrooxidans and Acidithiobacillus thiooxidans [J]. Journal of Central South University, 2014, 21: 728-734. DOI: 10.1007/s11771-014-1995-3.
[14] RZHEPISHEVSKA O I, LINDSTROM E B, TUOVINEN O H, DOPSON M. Bioleaching of sulfidic tailing samples with a novel, vacuum-positive pressure driven bioreactor [J]. Biotechnology & Bioengineering, 2005, 92(5): 559-67. DOI: 10.1002/bit.20609.
[15] HALLBERG K B, LINDSTROM E B. Characterization of Thiobacillus caldus sp. nov., a moderately thermophilic acidophile [J]. Microbiology, 1994, 140(4): 3451-3456. DOI: 10.1099/13500872-140-12-3451.
[16] HALLBERG K B, THOMSON H E C, BOESELT I, DOPSON M. Aerobic and anaerobic sulfur metabolism by acidophilic bacteria [M]. Elsevier Science BV, 2001.
[17] RAWLINGS D E. Characteristics and adaptability of iron- and sulfur-oxidizing microorganisms used for the recovery of metals from minerals and their concentrates [J]. Microbial Cell Factories, 2005, 4(1): 54-56. DOI: 10.1186/1475-2859-4-13.
[18] KARAVAIKO G I, BOGDANOVA T I, TOUROVA T P, KONDRAT`EVA T F, TSAPLINA I A, EGOROVA M A, KRASIL`NIKOVA E N, ZAKHARCHUK L M. Reclassification of Sulfobacillus thermosulfidooxidans subsp. Thermotolerans’ strain K1 as Alicyclobacillus tolerans sp. nov. and Sulfobacillus disulfidooxidans Dufresne et al. 1996 as Alicyclobacillus disulfidooxidans comb. nov., and emended description of the genus Alicyclobacillus [J]. International Journal of Systematic & Evolutionary Microbiology, 2005, 55(2): 941-947. DOI: 10.1099/ ijs.0.63300-0.
[19] HONG M X, WANG X X, WU L B, FANG C J, HUANG X T, LIAO R, ZHAO H B, QIU G Z, WANG J. Intermediates transformation of bornite bioleaching by Leptospirillum ferriphilum and Acidithiobacillus caldus [J], Minerals, 2019, 9: 159. DOI: 10.3390/min9030159.
[20] QIN W Q, WANG J, ZHANG Y S, ZHEN S J, SHANG H, LIU Q, SHI H B, ZHANG J W, QIU G Z. Electrochemical behavior of massive bornite bioleached electrodes in the presence of acidithiobacillus ferrooxidans and acidithiobacillus caldus [J]. Advanced Materials Research, 2009, 71-73: 417-420. DOI: 10.4028/www.scientific.net/ AMR.71-73.417.
[21] NORRIS P R, MURRELL J C, HINSON D. The potential for diazotrophy in iron and sulfur oxidizing acidophilic bacteria [J]. Archives of Microbiology, 2010, 164(4): 294-300. DOI: 10.1007/BF02529964.
[22] PARRO V, MORENO-PAZ M. Gene function analysis in environmental isolates: The nifregulon of the strict iron oxidizing bacterium Leptospirillum ferrooxidans [J]. Proceedings of the National Academy of Sciences, 2003, 100(13): 7883-7888. DOI: 10.1073/pnas.1230487100.
[23] ROHWERDER T, GEHRKE T, KINZLER K, SAND W. Bioleaching review part A: Progress in bioleaching: fundamentals and mechanisms of bacterial metal sulfide oxidation [J]. Applied Microbiology & Biotechnology, 2003, 63(3): 239-48. DOI: 10.1007/s00253-013-4954-2.
[24] RAWLINGS D E, CORAM N J, GARDNER M N, DEANE S M. Thiobacillus caldus and Leptospirillum ferrooxidans are widely distributed in continuous-flow biooxidation tanks used to treat a variety of metal- containing ores and concentrates [J]. Process Metallurgy, 1999, 9(9): 777-786. DOI: 10.1016/ S1572-4409(99)80080-7.
[25] GOLYSHINA O V, PIVOVAROVA T A, GOLYSHINA P N. Ferroplasma acidiphilum gen. nov., sp. nov., an acidophilic, autotrophic, ferrous-iron-oxidizing, cell-wall-lacking, mesophilic member of the Ferroplasmaceae fam. nov., comprising a distinct lineage of the Archaea [J]. International Journal Evolutionary Microbiology, 2000, 50: 997-1006. DOI: 10.1099/ 00207713-50-3-997.
[26] DOPSON M, BAKER-AUSTIN C, HIND A, BOWMAN J P, BOND P L. Characterization of Ferroplasma isolates and Ferroplasma acidarmanus sp nov., extreme acidophiles from acid mine drainage and industrial bioleaching environments [J]. Applied & Environmental Microbiology, 2004, 70(4): 2079-88. DOI: 10.1128/AEM.70.4.2079-2088.2004.
[27] JOHNSON D B, HALLBERG K B. The microbiology of acidic mine waters [J]. Research in Microbiology, 2003, 154(7): 466-473. DOI: 10.1016/S0923-2508(03)00114-1.
[28] WU B, WEN J K, CHEN B W. Control of redox potential by oxygen limitation in selective bioleaching of chalcocite and pyrite [J]. Rare Metals, 2014, 33(5): 622-627. DOI: 10.1007/s12598-014-0364-6.
[29] LEVENSPIEL O. Chemical reaction engineering [M]. 2nd ed. New York: John Wiley and Sons, 1972.
[30] MA R J. Principle on hydrometallurgy [M]. Beijing: Metallurgical Industry Press, 2007. (in Chinese)
[31] TIAN Y W, ZHAI X J, LIU K R. Short course of metallurgical physical chemistry [M]. Beijing: Chemical Industry Press, 2007.
[32] DEMIRKIRAN N. A study on dissolution of ulexite in ammonium acetate solutions [J]. Chemical Engineering Journal, 2008, 141: 180-186. DOI: 10.1016/j.cej.2007.12.012.
[33] GAO W C, WEN J K, LI Z B. Dissolution kinetics of magnesium from calcined serpentine in NH4Cl solution [J]. Industrial & Engineering Chemistry Research, 2014, 53: 7947-7955. DOI: dx.doi.org/10.1021/ie4043533.
[34] GAO W C, WEN J K, WU B, SHANG H, LIU X. A novel approach to extract Nb, Y and Ce from a niobium-bearing ore of low grade by roasting KHSO4–H2SO4 system [J]. Rare Metals, 2021, 40, 1979–1986. DOI: 10.1007/s12598-020-01435-z.
[35] ANTONIJEVIC M M, DIMITRIJEVIC M, JANKOVIC Z. Leaching of pyrite with hydrogen peroxide in sulphuric acid [J]. Hydrometallurgy, 1997, 46(46): 71-83. DOI: 10.1016/S0304-386X(96)00096-5.
[36] SYLVIE C B, BERNY F R V, DAVID G D. Leaching kinetics and stoichiometry of pyrite oxidation from a pyrite–marcasite concentrate in acid ferric sulfate media [J]. Hydrometallurgy, 2006, 84(3, 4): 225-238. DOI: 10.1016/j.hydromet.2006. 05.008.
[37] TANDA B C, EKSTEEN J J, ORABY E A. Kinetics of chalcocite leaching in oxygenated alkaline glycine solutions [J]. Hydrometallurgy, 2018, 178: 264-273. DOI 10.3390/ min8100461.
[38] CHENG C Y, LAWSON F. The kinetics of leaching covellite in acidic oxygenated sulphate–chloride solutions [J]. Hydrometallurgy, 1991, 27(3): 269-284. DOI: 10.1016/0304-386X(91)90054-P.
[39] CARLOS A. Kinetics of leaching of covellite in ferric-sulfate-sulfuric acid media [D]. The university of British Columbia, 2015. DOI: 10.14288/1.0166616.
[40] YU S C, YANG B J, FANG C J, ZHANG Y S, LIU S T, ZHANG Y S, SHEN L, XIE J P, WANG J. Dissolution mechanism of the oxidation process of covellite by ferric and ferrous ions [J]. Hydrometallurgy, 2021, 201: 105585. DOI: 10.1016/j.hydromet.2021.105585.
(Edited by FANG Jing-hua)
中文导读
黄铁矿、辉铜矿和铜蓝的生物浸出及其浸出动力学
摘要:研究紫金山铜矿主要矿物黄铁矿、辉铜矿和铜蓝,考察温度、细菌接种浓度、pH值和 Fe3+浓度等对矿物溶解动力学的影响,查明影响矿物溶解动力学的主要因素,获得控制氧化速率的关键步骤,建立半经验动力学模型,经计算,黄铁矿、辉铜矿和铜蓝在浸出过程中的表观活化能分别为69.29, 65.02 and 84.97 kJ/mol。
关键词:黄铁矿;辉铜矿;铜蓝;生物浸出;浸出动力学
Foundation item: Project(51574036) supported by the National Natural Science Foundation of China
Received date: 2019-06-27; Accepted date: 2020-04-20
Corresponding author: GAO Wen-cheng, PhD, Senior Engineer; Tel: +86-10-60662775; E-mail: gaowc1984@163.com; ORCID: https://orcid.org/0000-0002-5811-8250
Abstract: In this work, the bioleaching process of pyrite, chalcocite and covellite which were the main phase compositions for Zijin copper mineral was comprehensively studied. The influence parameters, such as leaching temperature, Fe3+ concentration, pH of solution and bacteria concentration were investigated. The leaching kinetics of the pyrite, chalcocite and covellite under the studied conditions was successfully modeled by an empirical diffusion-like equation, respectively. The apparent activity energy of pyrite leaching, chalcocite leaching (stage II) and covellite leaching was calculated to be 69.29, 65.02 and 84.97 kJ/mol, respectively.
[1] ROSSI G. Biohydrometallurgy [M]. Hamburg: McGraw-Hill, 1990.
[2] RAWLINGS D E, JOHNSON B D. Biomining [M]. Springer-Verlag Berlin Heidelberg, 2007.
[3] EDGARDO R, DONATI, WOLFGANG S. Microbial processing of metal sulfides [M]. Springer, 2007.
[29] LEVENSPIEL O. Chemical reaction engineering [M]. 2nd ed. New York: John Wiley and Sons, 1972.


