NaAlH4-TiF3复合催化Mg (AlH4$-$) 2的正交试验探究
南开大学新能源材料化学研究所,天津化学化工协同创新中心,先进能源材料教育部重点实验室,金属与分子基材料化学天津市重点实验室
南开大学新能源材料化学研究所天津化学化工协同创新中心先进能源材料教育部重点实验室金属与分子基材料化学天津市重点实验室
天津职业大学电子信息工程学院
摘 要:
Mg (AlH4) 2是一种理想的储氢材料, 理论储氢容量高达7.5% (质量分数) 。然而较高的起始放氢温度在很大程度上制约了Mg (AlH4) 2的应用。正交试验设计方法能够在较少的试验次数中掌握可靠的实验数据以及各因素之间的内在联系从而确定最优的实验方案, 特别适用与多因素多水平的实验条件研究。利用高能球磨法成功地制备了Mg (AlH4) 2, 并将NaAlH4和TiF3引入到该体系中。利用傅里叶红外转换测试仪 (FTIR) 对产物的结构进行表征, 程序控温脱附 (TPD) 对产物的放氢温度和放氢量进行测定。此外, 采用三因素三水平的L9 (33) 正交试验法, 以Mg (AlH4) 2的起始放氢温度为指标, 以NaAlH4的添加量、TiF3的添加量和球磨间隔时间为因素, 同时考察以上3项重要因素对降低Mg (AlH4) 2起始放氢温度的影响。通过对正交试验的系统分析发现, NaAlH4的添加量对降低Mg (AlH4) 2的起始放氢温度影响最显著, 其次为TiF3的添加量, 最后为球磨间隔时间。得到最佳试验条件, 在最佳条件下Mg (AlH4) 2的起始放氢温度仅为72℃, 与未添加的相比放氢温度降低了67℃, 放氢性能明显提高。
关键词:
正交试验;因素水平;Mg (AlH4) 2;放氢温度;复合催化;
中图分类号: TQ032
作者简介:王迎 (1987-) , 女, 河北邯郸人, 博士研究生, 研究方向:新能源材料;E-mail:wangying2011@mail.naikai.edu.cn;;王一菁, 教授;电话:022-23503639;E-mail:wangyj@nankai.edu.cn;
收稿日期:2013-07-15
基金:国家高技术研究发展计划 (2012AA051901);国家自然科学基金 (51071087, 51171083, 21103124);天津市应用基础及前沿技术研究项目 (11JCYBJC077000);高等学校学科创新引智基地 (111计划) (B12015) 资助;
Orthogonal Test Analysis of NaAlH4-TiF3 Co-Catalyzed Mg ( AlH4) 2
Wang Ying Xu Changchang Li Jia Wang Yijing Jiao Lifang Yuan Huatang
Institute of New Energy Material Chemistry, Collaborative Innovation Center of Chemical Science and Engineering ( Tianjin) , Key Laboratory of Advanced Energy Materials Chemistry, Tianjin Key Laboratory on Metal and Molecule-based Material Chemistry, Nankai University
School of Electronic and Information Engineering, Tianjin Vocational Institute
Abstract:
Mg ( AlH4) 2was a kind of ideal hydrogen storage material, and it had a high hydrogen storage capacity of 7. 5% ( mass fraction) . However, its application was blocked by its tough dehydrogenation temperature. Mg ( AlH4) 2was successfully prepared by high energy ball-milling, and further doped with NaAlH4and TiF3. The Fourier infrared spectrometer ( FTIR) and temperature-programmed desorption system ( TPD) were used to determine the structure and the onset dehydrogenation temperature of the product, respectively. In addition, the L9 ( 33) orthogonal experimental method was used to determine the optimum conditions to decrease the desorption temperature of Mg ( AlH4) 2, with the onset dehydrogenation temperature as the indictor, as well as the additive amount of NaAlH4, the additive amount of TiF3, and the pause time during the ball-milling as the factors. The orthogonal experimental results were systematically investigated. The additive amount of NaAlH4was found to be the key factor, the next was the additive amount ofTiF3, the final ore was the pause time during ball-milling. And the optimal preparation condition was determined. For the Mg ( AlH4) 2, prepared under the optimum conditions, the onset desorption temperature reduced to 72 ℃, which was about 67 ℃ lower than the pure Mg ( AlH4) 2. The hydrogen storage properties were improved.
Keyword:
orthogonal experimental; factors and levels; Mg (AlH4) 2; dehydrogenation temperature; co-catalyst;
Received: 2013-07-15
氢能被认为是最具有发展前景的可再生清洁能源之一[1 - 4]。近年来, 以Mg ( Al H4) 2为代表的轻金属配位氢化物由于具有较高的储氢密度和环境友好等优异性能而成为研究的热点[5 - 10]。研究发现, Mg ( Al H4) 2的放氢分两步进行[11], 反应历程如方程式 ( 1) , ( 2) 所示。Mg ( Al H4) 2的起始放氢温度由于制备条件不同而不同[11 - 15]。但是在实际应用中, 它的操作温度相对较高, 因此, 在保持Mg ( Al H4) 2储氢量的同时有效降低其放氢温度是亟需解决的问题。
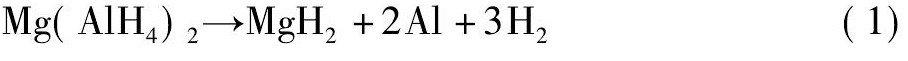
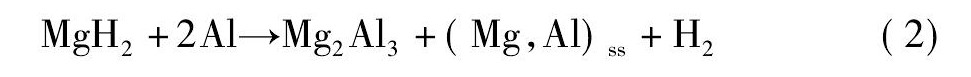
改善储氢化合物性能的常用方法包括: ( 1) 在体系中加入其他种类的金属氢化物[15 - 16], Liu等[16]研究发现Li ( BH4) 2体系中引入适量的Mg ( Al H4) 2可以显著降低其放氢温度; ( 2) 引入适当催化剂[17 - 19], 自Bogdanovi 'c等[19]首次发现Ti-基催化剂的引入可以使Na Al H4体系可逆之后, Ti-基催化剂便得到了广泛的应用; ( 3) 改变制备条件[11 - 20], 球磨间隔时间对Mg ( Al H4) 2也有重要的影响。正交试验设计是在科研和实际应用中最具价值的一种试验设计方法, 具有条件试验量小的优点, 特别适用于多因素、多水平试验条件的研究[21 - 22]。本文采用三因素三水平的L9 ( 33) 正交试验法, 考察以上3 种改善方法同时作用于Mg ( Al H4) 2时对降低其放氢温度的影响。以Na Al H4添加量、Ti F3添加量、球磨间隔时间为因素, 以Mg ( Al H4) 2起始放氢温度为指标。对正交试验结果进行系统分析, 优化实验条件, 从而最大可能的降低Mg ( Al H4) 2的放氢温度, 改善其放氢性能。
1 实验
1. 1 正交试验设计
以Mg ( Al H4) 2起始放氢温度为指标, 以Na Al H4添加量、Ti F3添加量以及球磨间隔时间为因素, 并分别选取各因素的适当水平。设计L9 ( 33) 三因素三水平的正交试验来优化实验条件, 寻找改善Mg ( Al H4) 2放氢性能的最佳实验方案。具体的实验安排如表1 所示。
1. 2 Mg ( Al H4) 2的制备和表征
根据Na Al H4∶ Mg Cl2= 2∶ 1 的置换反应制备Mg ( Al H4) 2。样品用2 MPa氢气保护, 在高能行星式球磨机上球磨5 h, 控制球料比为50∶ 1, 转速为450 r·min- 1。改变球磨条件, 每球磨12 min后间隔0 或6 或12 min后继续球磨; 调整Na Al H4的添加量, 过量添加不同比例的Na Al H4 ( 以Mg ( Al H4) 2为基) ; 并在球磨产物中加入不同量的Ti F3 ( 以Mg ( Al H4) 2为基) , 研磨使样品充分混合。
采用傅里叶红外转换测试仪 ( FTIR, FTIR-650) 对样品的物相进行表征, 程序升温脱附 ( TPD, PX200) 在Ar气流下测定样品的起始放氢温度和放氢曲线。所有的制备和转移过程都在氩气填充的手套箱中进行, 并且控制水、氧的含量低于10 ×10- 6。
表1 正交试验设计表Table 1 Designing scheme of orthogonal experimental 下载原图
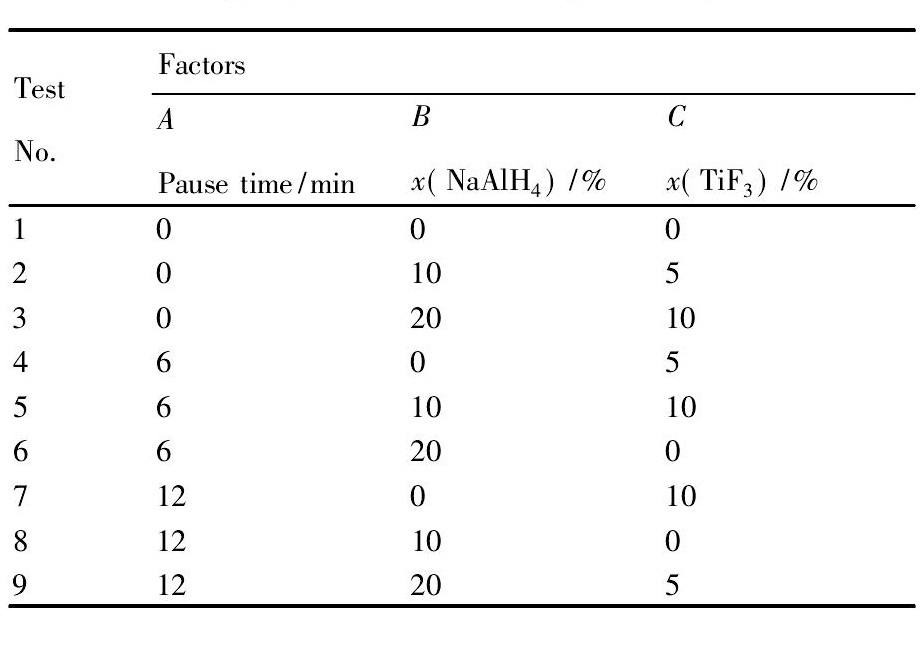
表1 正交试验设计表Table 1 Designing scheme of orthogonal experimental
2 结果与讨论
2. 1 样品的结构表征和放氢温度的测定
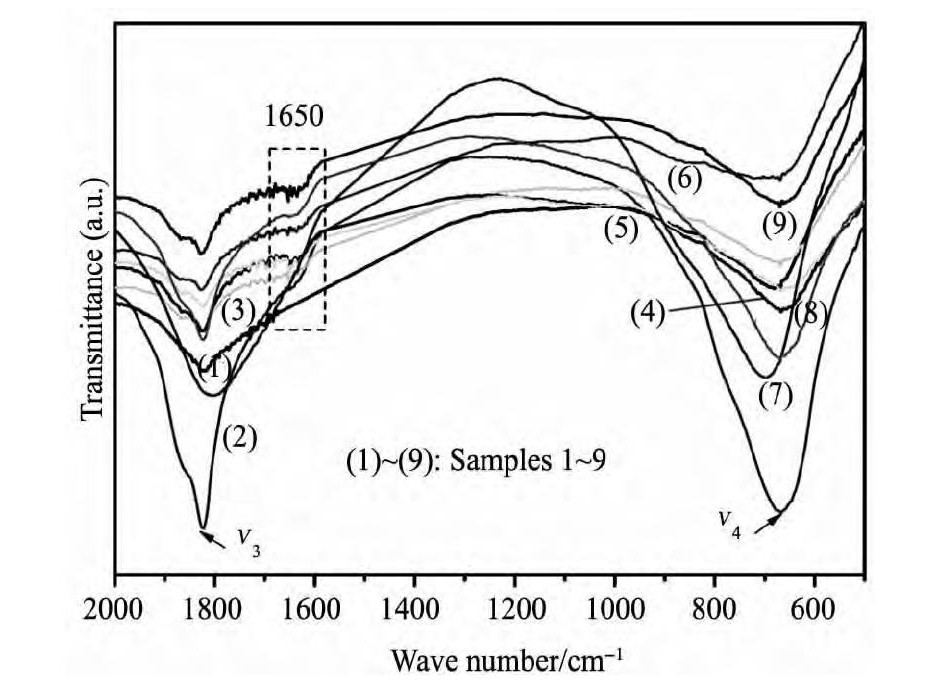
通过FTIR对不同条件制备产物结构进行表征, 结果如图1 所示。从图中可以观察到所有样品在1810 ~ 1840, 640 ~ 660 cm- 1处出现特征吸收峰, 上述两个吸收峰分别对应Mg ( Al H4) 2中Al - H的ν3伸缩振动和 ν4弯曲振动[23]。以上结果表明各试验条件下均得到了Mg ( Al H4) 2。随着Na Al H4的过量添加 ( 2, 3, 5, 6, 8, 9 号样品) 在1650 cm- 1处出现了吸收峰, 但吸收强度较低, 该处对应过量添加的Na Al H4中Al - H的特征峰。
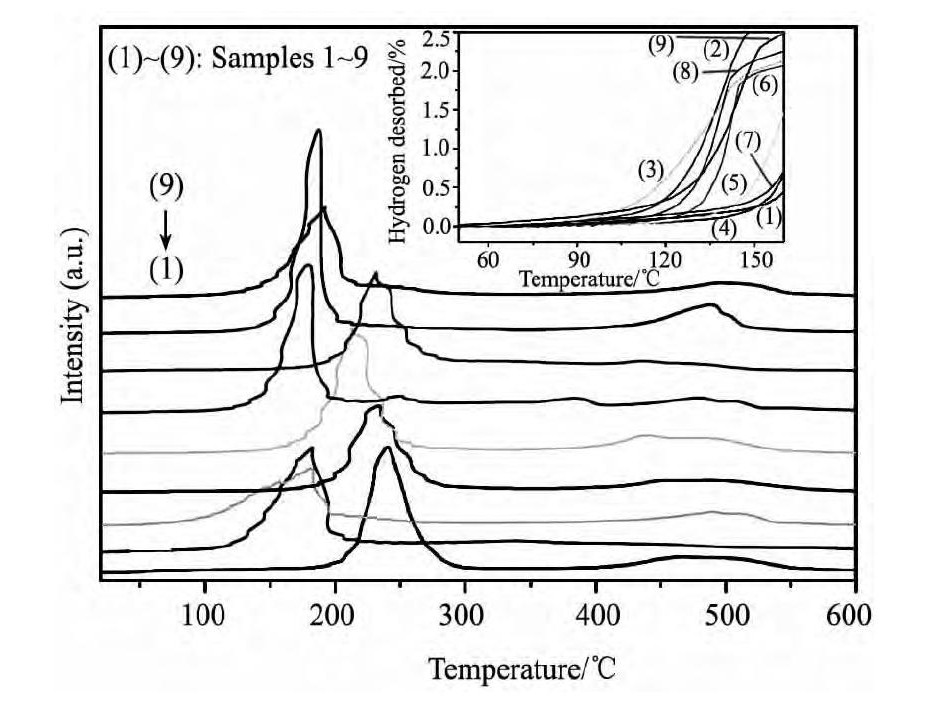
样品的起始放氢温度通过TPD测试来确定。结果如图2, 显然Mg ( Al H4) 2分解放氢分两步进行, 不同实验条件下制备样品的起始放氢温度在139 ~ 72 ℃ 间变化。为了方便观察各样品起始放氢温度的变化, 将TPD曲线处理得到局部放氢曲线, 如图2 中内嵌图所示。具体的放氢温度详见表2。
2. 2 正交试验结果分析
根据TPD测定的放氢温度补充完成三因素三水平的L9 ( 33) 正交试验结果分析表。表2 中K1, K2, K3代表各对应因素分别在水平1, 2, 3 下的实验结果之和。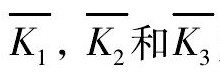
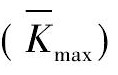
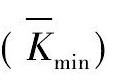
图1 不同制备条件下样品的FTIR谱图Fig. 1 FTIR images of Samples 1 ~ 9 under different conditions
图2 样品的TPD谱图 ( 内嵌图片为样品的局部放氢曲线, 升温速率为2 ℃·min- 1) Fig. 2 TPD curves of Samples 1 ~ 9 ( Insert: partial dehydro-genation curves of samples, heating at 2 ℃·min- 1)
表2 正交试验结果分析表Table 2 Analysis of the orthogonal experimental results 下载原图
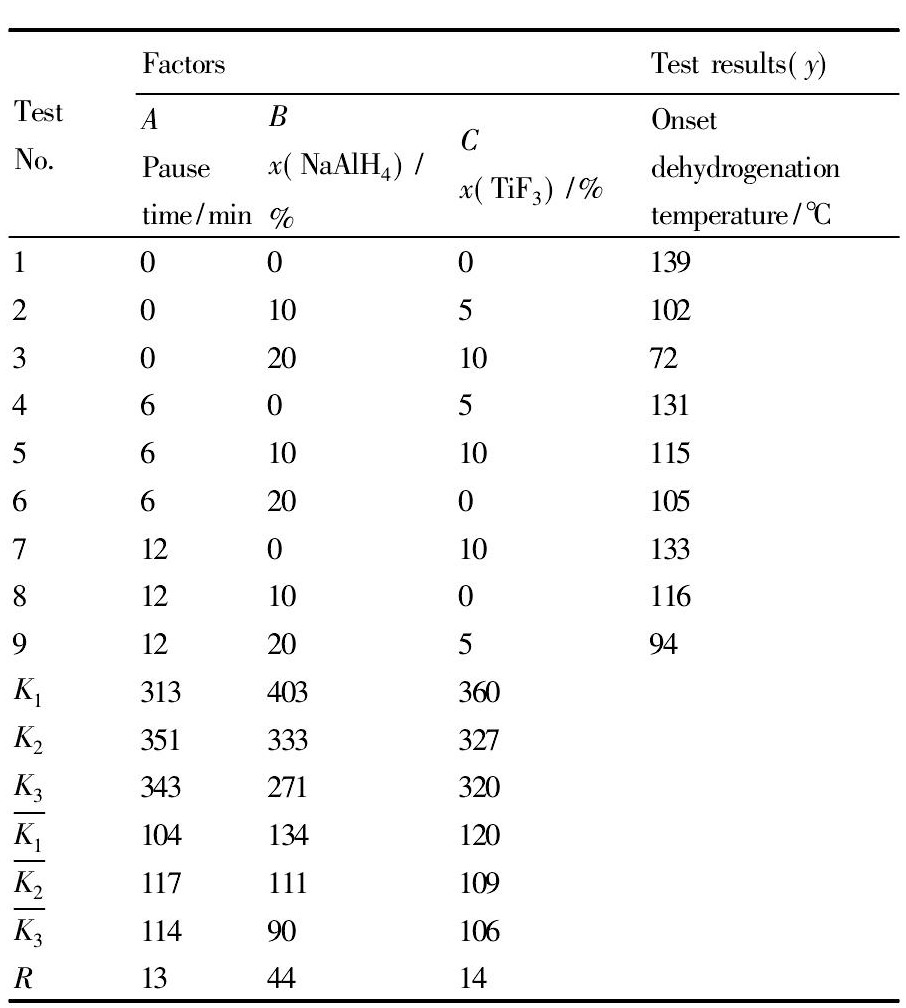
表2 正交试验结果分析表Table 2 Analysis of the orthogonal experimental results



将有关数值代入式 (3) ~ (5) , 得:

根据表2 中A, B, C这3 种因素R值的不同可以判断以上3 种因素对降低Mg ( Al H4) 2放氢温度的影响强弱。RB> RC> RA, 所以因素B对于降低Mg ( Al H4) 2的放氢温度影响最大, 因素C影响次之, 因素A的影响最小。
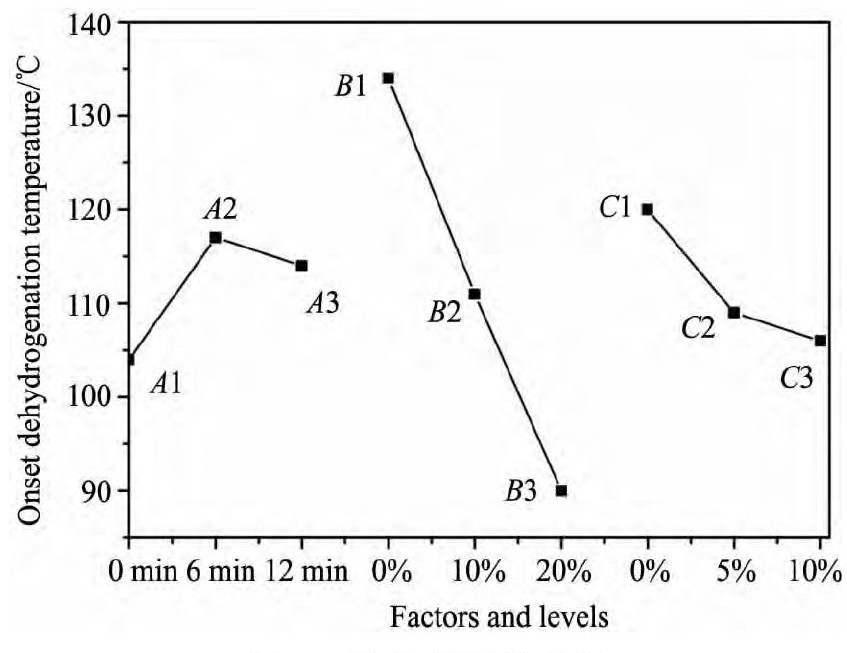
为了直观的得到各因素在不同水平下和指标的关系, 用因素的水平作为横坐标, 相应指标的平均值作纵坐标, 画出因素-指标关系图。图3 中因素A随着球磨间隔时间的增长放氢温度先增加后减小, 在球磨间隔为0 min时放氢温度最低。因素B随着Na Al H4加入量的增加放氢温度逐渐降低, 在添加量20% 时放氢温度最低。因素C随着Ti F3加入量的增加放氢温度逐渐降低, 但是降低速率明显减慢, Ti F3加入量为10% 时放氢温度最低。一定量Na Al H4的加入可以显著降低Mg ( Al H4) 2的起始放氢温度, 这可能是由于在分解过程中Mg ( Al H4) 2和Na Al H4的分解产物发生自催化作用[24], 而Ti F3的存在对Mg ( Al H4) 2的分解也起到一定的促进作用。
综合对比表2 和图3 的试验数据, 得到在A, B, C 3 种因素同时作用于Mg ( Al H4) 2时放氢温度最低的组合为A1B3C3, 即球磨间隔时间为0 min, 加入20% Na Al H4和10% Ti F3。最优条件和试验3吻合, 此时放氢温度为72 ℃ 明显低于其他样品。值得注意的是, 在球磨间隔时间为0 min且长时间的连续球磨条件下, 高能球磨产生的热量大量聚集导致温度的升高, 最终导致Mg ( Al H4) 2的分解。因此一定的球磨间隔时间是非常必要的, 但是在较短球磨时间下 ( 5 h) 热量聚集并不明显。
图3 因素-指标关系图Fig. 3 Relationship between factors and indicators
3 结论
本文采用L9 ( 33) 正交试验的方法, 以球磨间隔时间、Na Al H4添加量、Ti F3添加量为因素探讨了Na Al H4-Ti F3复合催化剂的引入对Mg ( Al H4) 2放氢温度的影响。通过对正交试验结果的分析发现, 在探讨的3 个因素中Na Al H4添加量对降低Mg ( Al H4) 2放氢温度影响最为显著, 其次为Ti F3添加量, 最后为球磨间隔时间。确定了在球磨时间较短 ( 5 h) 时最优的实验条件; 球磨间隔时间0 min, Na Al H4添加量20% , Ti F3添加量10% 。最佳条件下Mg ( Al H4) 2的起始放氢温度为72 ℃, 放氢性能得到较大改善。
参考文献