
J. Cent. South Univ. (2019) 26: 3225-3237
DOI: https://doi.org/10.1007/s11771-019-4248-7
Effect of coal moisture content on coke’s quality and yields of products during coal carbonization
FANG Hong-ming(方红明)1, 2, HAN Jun(韩军)1, ZHANG Hong-jie(张洪杰)1,ZHAO Bo(赵波)1, QIN Lin-bo(秦林波)1
1. Hubei Key Laboratory for Efficient Utilization and Agglomeration of Metallurgic Mineral Resources, Wuhan University of Science and Technology, Wuhan 430081, China;
2. Industrial Safety Engineering Technology Research Center of Hubei Province, Wuhan University of Science and Technology, Wuhan 430081, China
 Central South University Press and Springer-Verlag GmbH Germany, part of Springer Nature 2019
Central South University Press and Springer-Verlag GmbH Germany, part of Springer Nature 2019
Abstract:
The coal with low moisture during carbonization could not only increase the yield of coke, but also promote the coke quality and reduce the energy consumption. In this paper, the influence of the moisture in the blend coal (1.8%-10.13%) on the product yields and coke quality during coal carbonization were investigated. The results show that the coke yield is increased from 75.90% to 77.16%, and the coke qualities such as coke strength after reaction with CO2 (CSR), coke reactivity index (CRI), fragmentation index (M25) and abrasion index (M10)) are also improved when the moisture of the blend coal decreases from 10.13% to 1.80 % in a bench scale reactor. Due to the secondary reaction, tar become lighter when the moisture is decreased. In order to further prove the above results, the blend coal with 1.8% and 9%-10% (common moisture used in coke plant) moisture is carbonized in a coke oven with 6 m height, the results show that CRI are 23.4% and 27.3%, CRS are 67.1% and 62.2% under 1.8% and 9%-10% moisture of blend coal. Moreover, the variation of the moisture in blend coal has a limited influence on dust emission at the ascension pipe and the charging car.
Key words:
coking coals; low moisture; carbonization; coke; coke qualities;
Cite this article as:
FANG Hong-ming, HAN Jun, ZHANG Hong-jie, ZHAO Bo, QIN Lin-bo. Effect of coal moisture content on coke’s quality and yields of products during coal carbonization [J]. Journal of Central South University,2019, 26(12): 3225-3237.
DOI:https://dx.doi.org/https://doi.org/10.1007/s11771-019-4248-71 Introduction
Metallurgical coke, obtained by carbonizing and appropriate blend of coals at temperatures up to 1100 °C, is used as fuel, reducing agent and support of blast furnace during the iron-making process [1, 2]. In 2015, the production and consumption of coke in China were 448 and 393 million tons, respectively [3]. Recently, the coal moisture control (CMC) has been paid attention due to the need of improving coke quality (coke strength after reaction with CO2, CSR), coke reactivity index (CRI), fragmentation index (M25) and abrasion index (M10)) and increasing the use of weak caking coal [4]. Moreover, the coal moisture control (CMC) has the advantage, such as reducing wastewater production, and reducing energy consumption. It was reported that the energy consumption could reduce 45-60 MJ each ton blend coal during coal carbonization if the moisture of the blend coal was reduced by 1%, and the wastewater could be reduced by 30-40 kg/t coal [5].
Nippon Steel developed the first CMC and installed at No. 1-2 coke oven batteries of Oita Works in 1983, and found that the CMC equipment could reduce the moisture from 9% to 5% of 260 t/h coal, which caused 334.40-388.74 MJ/t coal heat consumption reduction, 1.5% increase of the drum strength of coke (DI15150) and 11% increase of the coke yield [6]. NOMURA et al [7] stated that the decrease in coal moisture leads to an increase of the bulk density of coal in the coke oven chamber, which caused the improvement of coke quality. Moreover, Nippon Steel also developed the secondary CMC at Nakayama plant, the moisture was reduced to 6.1% by means of coke flue gas with direct heat transfer [8]. However, if the coal moisture is too low, the problem of dust pollution, carbon deposition on coking oven, and tar quality deterioration will occur [9]. Hence, the dry-cleaned and agglomerated pre-compaction system (DAPS) was developed for drying and selective pelletization of coal fines. In DAPS, the fine coals were separated and pelleted, the dust pollution was suppressed. At the same time, the moisture of blended coal was decreased from 8% to 4.7%, and the percentage of non- or slightly-caking coal ratio had expanded to 47%. Moreover, the strength of DAPS coke was better than that of CMC coke [10]. KATO et al [11] thought the decrease in coke porosity and increase in cohesion strength between grains due to increased charging density was responsible for the coke strength improvement. In China, CMC has been applied in most of coke plants, and the moisture is generally controlled at 6%-8% [12]. According to the experimental results of NOMURA et al [4, 7], the bulk density of coal was increased with the decrease of moisture. It was assumed that the strength of coke would be further improved if the moisture was below 5%. However, the researches concerning the low moisture (below 5%) coal charging were fewer reported due to the problems of dust emissions during coal charging process.
In this paper, the blend coals with 1.8%-10.13% moisture in coal were carbonized, and influences of the coal moisture on the coke quality, yields and morphology of by-products (coke, wastewater, coking gas, and tar) were also considered. Moreover, the comparison of different moisture coal charges was also carried out in a coke oven with 6 m height, and the dust emissions during coal charging process were monitored.
2 Materials and methods
2.1 Materials
The blended coals (A-D) were sampled from a coke plant, which were composed of four different rank coals (gas coal, 1/3 coking coal, coking coal and lean coal). Before the experiments, the blend coal was mixed according to the ratio of 26% gas coal, 23% 1/3 coking coal, 43% coking coal and 8% lean coal, mass fraction. The blend coals had the same properties except for the difference of the moisture, and the moisture was controlled by adding water or dry. The basic properties of coals are listed in Table 1.
Table 1 Basic properties of coals
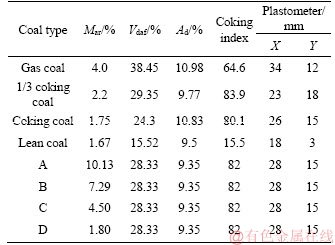
2.2 Carbonization tests
The yields and components of by-products (such as coke, gaseous, water and liquid tar) were studied in a fixed bed reactor, which was thoroughly described in our previous paper [13]. In brief, the reactor consisted of a stainless steel reactor with a diameter of 60 mm and a height of 100 mm, a heater and a cooling system for separating water/tar from gas. The heater was electrically heated, and the temperature was controlled by a thermocouple. The temperature program was determined according to the real temperature program in the coke plant: 1) the temperature increased from 40 to 800 °C at 20 °C/min; 2) the temperature was isothermally held at 800 °C for 30 min; 3) the temperature ramped from 800 to 900 °C at the rate of 1.5 °C/min; 4) the temperature was isothermally held at 900 °C for 30 min; and 5) the temperature ramped at 2 °C/min to 1150 °C and held at 1150 °C for 90 min.
Before the experiment, 30 g prepared coal sample was fed into the fixed bed reactor. The gas released from coals carbonization was collected by a gas bag. Prior to entering the gas bag, the gas was cooled by a condenser, water and liquid tar were collected by a glass tube. The low heating values and components of gas were analyzed by an infrared gas analyzer (Gasboard-3100, China). After the distillation, tar was separated from water and analyzed by GC-MS (Agilent 19091S-433, Agilent Technologies, Inc., Santa Clara, CA.).
The yields of coke, tar and water were calculated according to their weights, while the yield of the non-condensable gases was calculated by different methods. The low heating values of tar and coke were also measured by a calorimeter (YX-ZR/Q 9704, Li Thermal Company, China) according to GB/T213-2008. As for the non- condensable gases, the low heating value was calculated on the basis of CO, H2, CH4, CmHn concentrations and their caloric values.
In order to study the effects of coal moisture on coke quality parameters (such as CSR, CRI, M25, M10), coal carbonization were carried out in a 40 kg laboratory-scale coke oven. The detailed description of the coke oven was also found in previous paper [13]. The temperature program was the same to the above.
Before the experiment, the blend coal from a coke plant was dried in a fluidized bed reactor, then pelleted with an average diameter of 6 mm by pressure. Then, 35 ton pellet coal was carried to the charging car, and charged into one chamber of a top charging coke oven with 6 m height. The dimensions of the chamber were 0.45 m×15.48 m. During the charge process, the dust emissions at the coal charging car and ascension pipe were recorded with an isokinetic sampler (Wuhan Tianhong Instruments Co., Ltd, TH-880W). At the same time, the coke quality was also characterized.
2.3 Product pre-treatment and analysis
According to Chinese standard (GB/T 2006-2008), the cold mechanical strength index of coke such as M25 index (the amount of coke with a particle size greater than 40 mm) and M10 index (the amount of coke with a particle size lower than 10 mm) were calculated. CRI and CSR were characterized according to GB/T2006-94. In order to ensure the reliability, each experiment was repeated five times, and the average data were used. After distillation, water in tar can be calculated, then tar was analyzed by GC-MS. The detailed description of preparation and analysis can also be found in previous paper [14].
The CO, CO2, H2, CH4, CmHn (m≤3, n≤8), O2 and low heating value of the non-condensable gases produced from coal carbonization were also recorded by the gas analyzer (Gas board-3100, China), which was also described in our previous paper [15]. In Gas board-3100 infrared gas analyzer, CO, CO2, CH4, CmHn were analyzed on the basis of non-dispersion infrared (NDIR) method for micro-TCD gas sensor for H2 and O2 by fuel cell method. The above experiments were repeated five times, and the average data were used in this paper.
3 Results and discussion
3.1 Effect of moisture of blend coal on yields
The effect of coal moisture on the yield of products could be found in Figure 1. In this experiment, the yield is calculated based on the dry basis and the mass balance of coke, tar, water and gas was 89%-113%. Figure 1 demonstrates that the yield of coke is increased with the decrease of the coal moisture. When the coal moisture was 10.13%, the yield of coke is 75.9%. The yield of coke is increased to 77.16% under 1.8% of coal moisture. Figure 1 also presents that the yield of tar is slightly decreased with the decrease of coal moisture. The tar yields are 6.9% and 6.4% under 10.13% and 1.8% of coal moisture. KREBS et al [16] reported that the heating rate was improved when the coal moisture was increased due to the thermal conductivity enhancement, and the higher heating rate would result in decreasing coke yield [17]. Meanwhile, the moisture vaporized from the coal center would have to travel through a layer of semi-coke [18]. The moisture could be immediately reacted with semi-coke or tar, which also caused the decrease of coke and tar yield [19]. As displayed in Figure 1, gas yield is slightly decreased when the moisture decreases, which means that the moisture in coal is a favor for gas generation [20, 21]. The gas yields at 10.13%, 7.29%, 4.5% and 1.8% are 16.14%, 16.62%, 16.28% and 15.50%, respectively. LIU et al [22] also thought that there was free water, which could have reacted with solid or liquid compounds from coal pyrolysis and formed gaseous compounds.
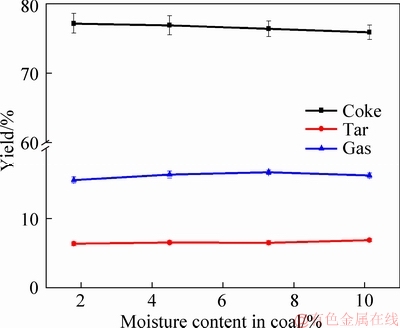
Figure 1 Influence of coal moisture on yield
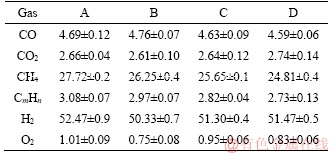
In order to further understand the influence of the moisture, gas components are also measured, as summarized in Table 2. DING et al [23] stated most of the water in coals was removed when the temperature was above 300 °C, and therefore the moisture in coal might not affect the release mechanisms of hydrocarbon (CxHy) and H2 in the subsequent devolatilization process. In this run, it is found out that H2 and CxHy (n<4) concentrations in coke gas are slightly decreased with reducing the moisture in coal. H2 concentration in coke gas is 52.47% and 51.47% when the coal moisture is 10.13% and 1.8%, respectively. CxHy concentration is decreased from 3.08% to 2.73% when the coal moisture is decreased from 10.13% to 1.8%. The main reaction is described as follows [15]:
CO+H2O CO2+H2 (1)
CO2+H2 (1)
CH4+H2O CO+3H2 (2)
CO+3H2 (2)
CH4+2H2O CO2+4H2 (3)
CO2+4H2 (3)
CxHy+xH2O xCO+(x+0.5y)H2 (4)
xCO+(x+0.5y)H2 (4)
CxHy+2xH2O xCO2+(2x+0.5y)H2 (5)
xCO2+(2x+0.5y)H2 (5)
C+H2O CO+H2 (6)
CO+H2 (6)
C+2H2O CO2+2H2 (7)
CO2+2H2 (7)
Tar+H2O CO+H2 (8)
CO+H2 (8)
Table 2 Gas component variation with coal moisture
Tar CxHy+H2 (9)
CxHy+H2 (9)
Moreover, the heating value of coke gas was also decreased due to the decomposition of CxHy and the reduction of H2. The heating value of coke gas was decreased from 4242 to 4040 kJ/m3 when the coal moisture was decreased from 10.13% to 1.8%. DAS et al [24] also proved that the heating value of coke gas had a strong relation with the moisture.
CO and CO2 concentrations are seen to be independent with the variation of moisture in coal and the heating value H and moisture M has the relationship:
H=184.4M+24.26 (10)
3.2 Effect of moisture of blend coal on coke quality
NOMURA et al [7] reported that the decrease of coal moisture would cause the increase of coal bulk density in coke oven chamber, the bulk densities, however, were 0.85 and 0.67 g/cm3 when the moisture content were 3% and 9%. The high bulk density could promote the interaction between the fused coal particle and other particles, and cause to form strong bonds. Thus, the coke quality is improved. Moreover, the decrease in coal moisture would increase the plastic layer thickness and consequently promote strong coke formation [24]. In this experiment, the moisture in coal is above 7.29%, CRI seems to be constant. Then, CRI is slightly decreased from 19.3% to 19.1% when the moisture varies from 7.29% to 4.5%. However, CRI is sharply decreased from 19.1% to 18.0% as the moisture decreases from 4.5% to 1.8%. CSR is 61.3%, 66.7%, 73.3% and 74.4% at 10.13%, 7.29%, 4.5% and 1.8% moisture in coal. M25 is increased with decreasing coal moisture, while M10 is decreased with enhancing the coal moisture, as presented in Table 3. Moreover, the effect of moisture content in the coal on the morphology and porosity of coke is given in Table 4 and Figure 2.
Table 3 Coke quality variation with coal moisture
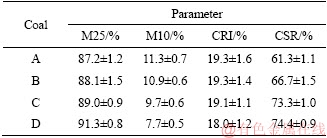
Table 4 Porosity of coke derived from coal with different moisture contents

The porosity of coke significantly decreases when the moisture content in the coal decreases. At the same time, Figure 2 shows that the coke derived from the coal with 10% moisture content has more and larger pores.
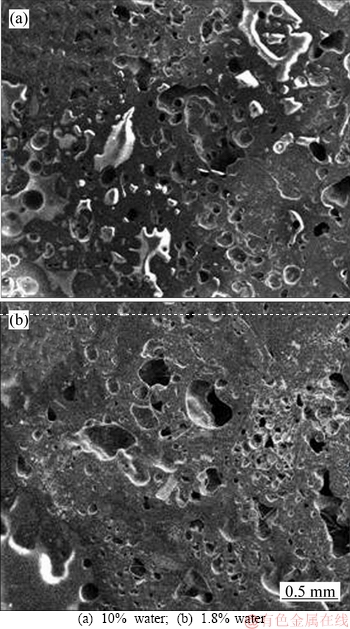
Figure 2 SEM images of coke derived from coal with different moisture contents:
3.3 Effect of moisture of blend coal on tar components
Tar is a complex mixture which contains thousands of organic compounds varying from a wide variety of chemical groups [15, 25, 26]. In this paper, the detail information of components and morphology distribution of tar obtained from different moisture coals could be found in Tables 5-8 and Figures 3-6. In order to clarify the variation of tar components, tar is divided into four groups as follows: aliphatics, aromatics, oxygenated groups and asphaltene [13].
Figure 7 depicts that the moisture in coal has a slight influence on tar components distribution. As for the oxygenated groups, the content is firstly increased, followed by decreasing with the increase of the moisture in coal. The maximum content of the oxygenated groups occurs at 4.5% moisture, about 39.71%. Aliphatic content in tar is slightly increased with the enhancement of the moisture, which is 23.42% and 26.28% under 1.8% and 10.13% moisture. On the contrary, aromatics and asphaltene content are slightly decreased with the increase of the moisture. Generally, the components of tar from coal with low moisture are lighter than those with high moisture, as shown in Tables 5-8. Moreover, the influence of coal moisture content on naphthalene proportion in tar is similar with Krebs’s results [16]; naphthalene proportion in tar was firstly decreased with the increase of the moisture, followed by decreasing when the moisture was further increased.
3.4 Effect of moisture of blend coal on wastewater generation
The effect of the coal moisture on the yield of wastewater is summarized in Table 9. It is seen that the water produced from coal carbonization is higher than that in coal. It is well known that there is the inherent water and water of constitution in coal except for the external water [15, 27]. The inherent water, external water and the water of constitution would transform into wastewater during coal carbonization. Moreover, Table 9 also demonstrates that the decrease of the moisture in coal would significantly reduce the effluent water during coal carbonization, which also reduces the cost of treating wastewater. At the same time, the energy consumption is also reduced.
Table 5 GC-MS analysis of tar from coal with 10.13% moisture
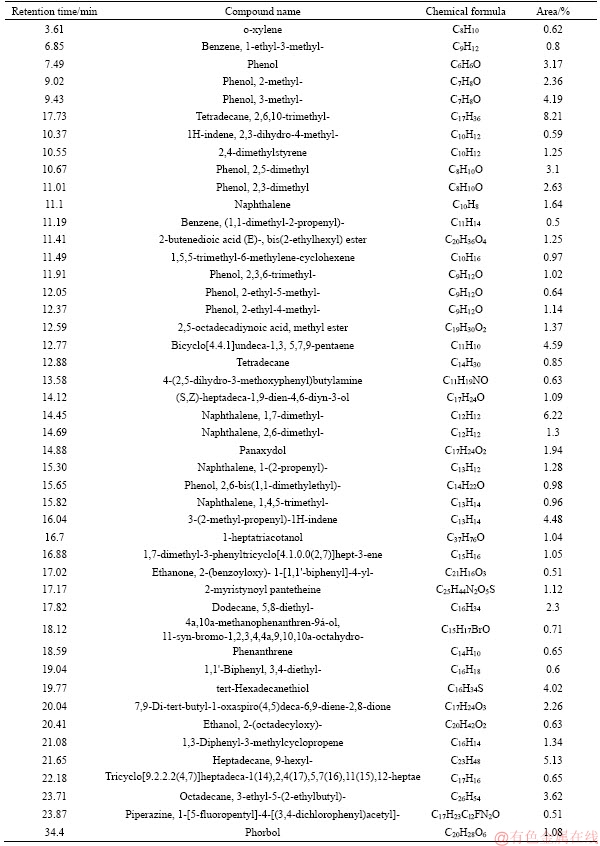
Table 6 GC-MS analysis of tar from coal with 7.29% moisture

Continued
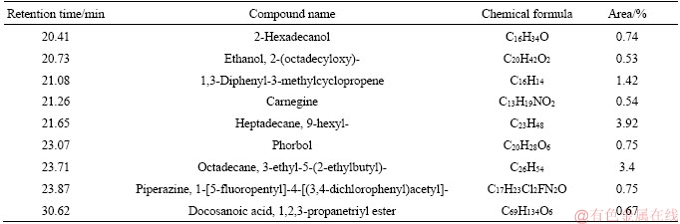
Table 7 GC-MS analysis of tar from coal with 5.40% moisture
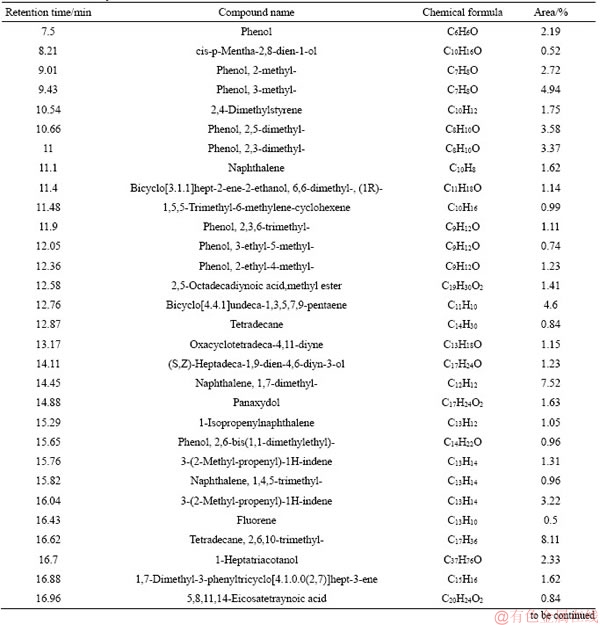
Continued
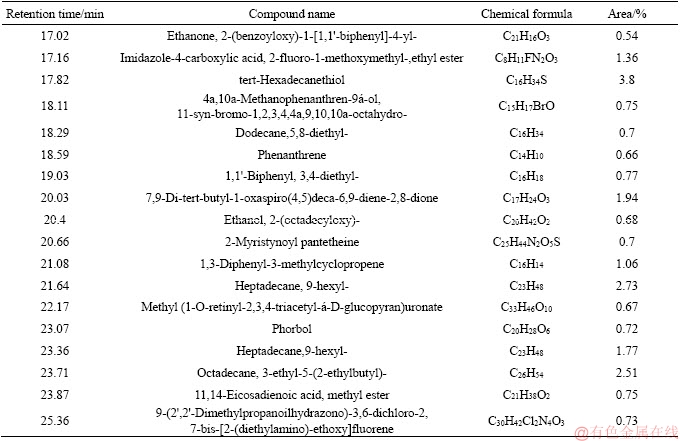
Table 8 GC-MS analysis of tar from coal with 1.80% moisture
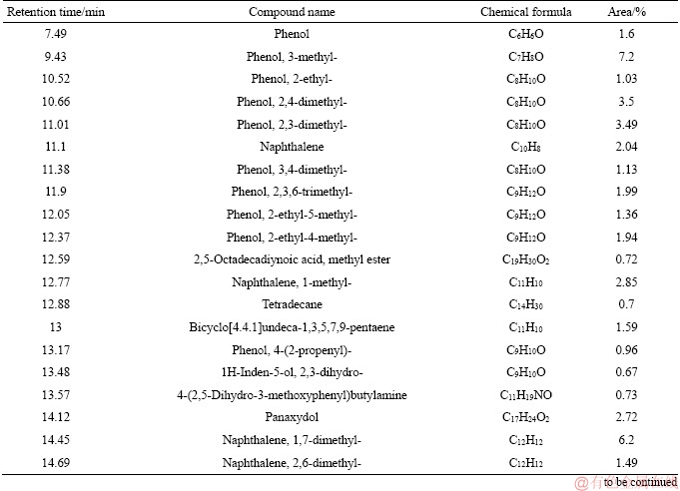
Continued
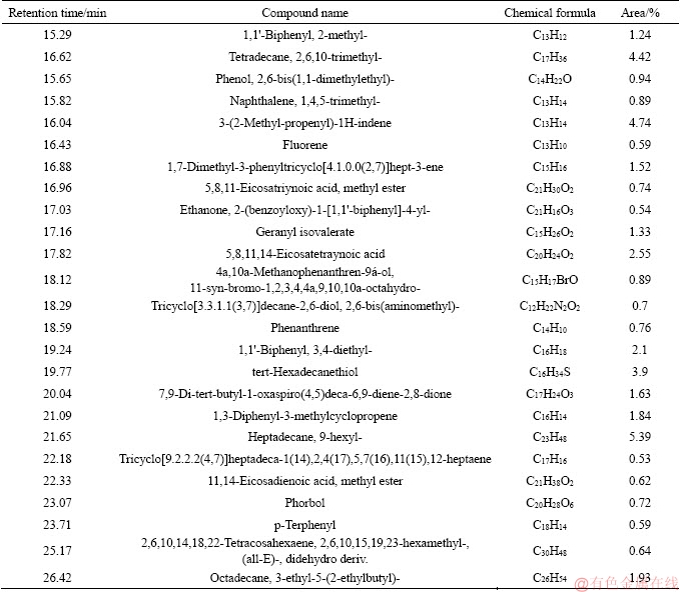
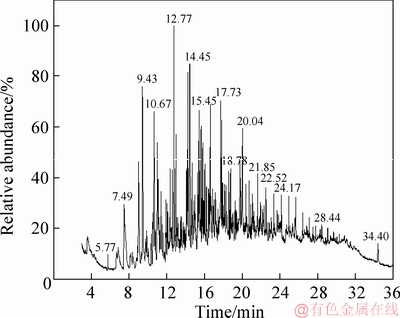
Figure 3 GC-MS analysis of tar from coal with 10.13% moisture
3.5 Plant trial at 6 m height top charge coke oven
In this test, the blend coal from a coke plant was dried in a fluidized bed reactor, then pelleted with an average diameter of 6 mm by pressure. The dried blend coal had a moisture of 1.8%. The bulk density of pelleted dried coal increases to 950 kg/m3 (the density is 650 kg/m3 under 9%-10% moisture content). The prepared coal was transported and charged in a chamber of a top charging coke oven with 6 m height. After carbonization, coke was pushed and cooled by coke dry quenching. Coke was also characterized according to GB/T2006-94. The results demonstrate that CRS and CRI of coke are 67.1% and 23.4%, respectively. However, CRS and CRI of coke from the same chamber with 9%-10% moisture in coal are 62.2% and 27.3%. Hence, it could be said that the low moisture in coal could promote the coke quality [28]. Moreover, the dust emission during coal charging was also compared. The dust concentrations in the 1.8% and 9%-10% moisture coal charge at the ascension pipe are 126.0 and 95.8 g/m3, and the dust concentrations at the charging car are 2.2 and 1.8 g/m3, respectively.
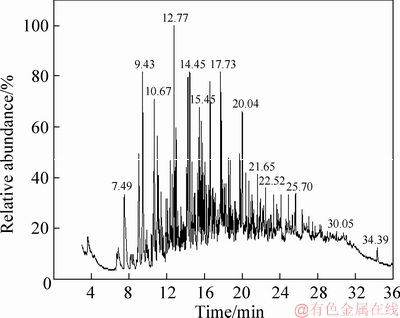
Figure 4 GC-MS analysis of tar from coal with 7.29% moisture
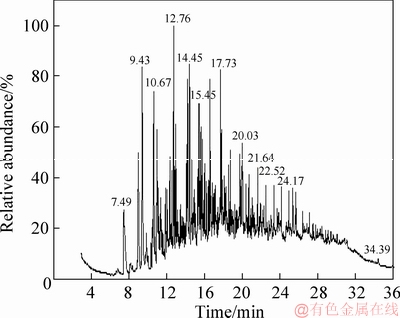
Figure 5 GC-MS analysis of tar from coal with 4.50% moisture
Figure 6 GC-MS analysis of tar from coal with 1.80% moisture
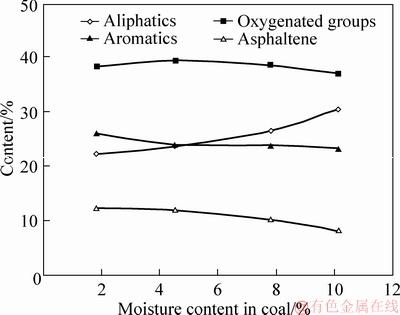
Figure 7 Influence of moisture content of coal on tar components
Table 9 Influence of moisture of coal on production of wastewater

4 Conclusions
In comparison of the carbonization of coal with different moisture, experiments in a bench scale and a coke oven are carried out. It is demonstrated that the low moisture would promote the yield of coke, and suppress the yield of tar and coke gas. Due to the secondary reaction, tar becomes lighter and the heating value of coke gas is decreased. Moreover, it is also found that the low moisture could promote the coke quality. In bench scale experiment, CRI is increased from 18.0% to 28.4% when the moisture in coal is increased from 1.8% to 10.13%. CRS is 74.4%, 73.3%, 66.7% and 39.9% when the moisture in coal is 1.8%, 4.5%, 7.29% and 10.13%. At the same time, the low moisture in coal could promote the level of M25 and M10. In the industrial test, the experimental results also proved that the low moisture in coal could promote the coke quality, CRI and CRS are 67.1% and 23.4%. Meanwhile, the influence of the moisture in coal on dust emission during coal charging is limited.
References
[1] NORTH L, BLACKMORE K, NESBLTT K, MAHONEY M R. Models of coke quality prediction and the relationships to input variables: A review [J]. Fuel, 2018, 219: 446-466. DOI: 10.1016/j.fuel.2018.01.062.
[2] NOMURA S J. The effect of binder (coal tar and pitch) on coking pressure [J]. Fuel, 2018, 220: 810-816. DOI: 10.1016/j.fuel.2018.01.130.
[3] FENG Sheng-dan, LI Ping, LIU Ze-yi, ZHANG Yue, LI Zhuang-mei. Experimental study on pyrolysis characteristic of coking coal from Ningdong coalfield [J]. Journal of the Energy Institute, 2018, 91(2): 233-239. DOI: 10.1016/ j.joei.2016.12.001.
[4] NOMURA S. Recent developments in cokemaking technologies in Japan [J]. Fuel Processing Technology, 2017, 159: 1-8. DOI: 10.1016/j.fuproc.2017.01.016.
[5] GAO Bing, LI Yu-geng, CHEN Peng, KONG De-wen. Application and effect analysis of CMC in coking plant [J]. Fuel & Chemical Processes, 2016, 47(4): 31-33. DOI: 10.3969/j.issn.1001-3709.2016.04.013.
[6] WAKURI S, MORIYOSHI O, HOSOKAWA K, NAKAGAWA K, TAKANOHASHI Y, OHNISHI T, KUSHIOKA K, KONNO Y. New moisture control system of coal for coking [J]. Transactions of the Iron and Steel Institute of Japan, 1985, 25(11): 1111-1115. DOI: 10.2355/ isijinternational1966.25.1111.
[7] NOMURA S, ARIMA T, KATO K. Coal blending theory for dry coal charging process [J]. Fuel, 2004, 83(13): 1771-1776. DOI: 10.1016/j.fuel.2004.03.006.
[8] EREMIN A Y, ZAGAYNOV N, LOBANOV V, CHZHENCHAN V. Coal drying by coke-oven gas at Sanmin Iron and Steel Works [J]. Coke and Chemistry, 2014, 57(7): 284-287. DOI: 10.3103/S1068364X14070023.
[9] BORKER S S, BANDYOPADHYAY P K. Better moisture control in coke manufacturing—A case study [J]. Indian Journal of Science and Technology, 2011, 4: 1147-1154. DOI: 10.17485/ijst/2011/v4i9/30246.
[10] NAITO M, TAKEDA K, MATSUI Y. Ironmaking technology for the last 100 years: Deployment to advanced technologies from introduction of technological know-how, and evolution to next-generation process [J]. ISIJ International, 2015, 55(1): 7-35. DOI: 10.2355/isijinternational.55.7
[11] KATO K, MATSUEDA K. Leading edge of coal utilization technologies for gasification and cokemaking [J]. Powder and Particle, 2018, 35: 112-121. DOI: 10.14356/kona. 2018018.
[12] CUI Ping, QU Ke-ling, LING Qiang, CAO Yin-ping. Effects of coal moisture control and coal briquette technology on structure and reactivity of cokes [J]. Coke and Chemistry, 2015, 58(5): 162-169. DOI: 10.3103/S1068364X15050075.
[13] QIN Lin-bo, HAN Jun, YE Wei, ZHANG Shun, YAN Qian-gu, YU Fei. Characteristics of coal and pine sawdust co-carbonization [J]. Energy & Fuels, 2014, 28: 848-857. DOI: 10.1021/ef401942a.
[14] QIN Lin-bo, HAN Jun, HE Xiang, ZHAN Yi-qiu, YU Fei. Recovery of energy and iron from oily sludge pyrolysis in a fluidized bed reactor [J]. Journal of Environmental Management, 2015, 154: 177-182. DOI: 10.1016/j.jenvman. 2015.02.030.
[15] HUANG Zhi-hang, QIN Lin-bo, XU Zhe, CHEN Wang-sheng, XING Fu-tang, HAN Jun. The effects of Fe2O3 catalyst on the conversion of organic matter and bio-fuel production during pyrolysis of sewage sludge [J]. Journal of the Energy Institute, 2019: 835-842. DOI: 10.1016/j.joei. 2018.06.015.
[16] KRBES V, FURDIN G, MARECHE J F, DUMAY D. Effects of coal moisture content on carbon deposition in coke ovens [J]. Fuel, 1996, 75: 979-986. DOI: 10.1016/0016-2361(96) 00039-7.
[17] OKUMURA Y. Effect of heating rate and coal type on the yield of functional tar components [J]. Proceedings of the Combustion Institute, 2017, 36(2): 2075-2082. DOI: 10.1016/j.proci.2016.09.020.
[18] HAN Jun, ZHANG Li, KIM H J, KASADANI Y, LI Liu-yun, SHIMIZU T. Fast pyrolysis and combustion characteristic of three different brown coals [J]. Fuel Processing Technology, 2018, 176: 15-20. DOI: 10.1016/j.fuproc.2018.03.010.
[19] HASAN M D M, HU Xun, GUNAWAN R, LI Chun-zhu. Pyrolysis of large mallee wood particles: Temperature gradients within a pyrolysing particle and effects of moisture content [J]. Fuel Processing Technology, 2017, 158: 163-171. DOI: 10.1016/j.fuproc.2016.12.018.
[20] HAN Jun, ZHANG Li, ZHAO Bo, QIN Lin-bo, WANG Yu, XING Fu-tang. The N-doped activated carbon derived from sugarcane bagasse for CO2 adsorption [J]. Industrial Crops & Products, 2019, 128: 290-297. DOI: 10.1016/j.indcrop.2018. 11.028.
[21] QIN Lin-bo, HAN Jun, ZHAO Bo, WANG Yu, CHEN Wang-sheng, XING Fu-tang. Thermal degradation of medical plastic waste by in-situ FTIR, TG-MS and TG-GC/MS coupled analyses [J]. Journal of Analytical and Applied Pyrolysis, 2018, 136: 132-145. DOI: 10.1016/ j.jaap.2018.10.012.
[22] LIU Huan, ZHANG Qiang, HU Hong-yun, LI Ai-jun, YAO Hong. Influence of residual moisture on deep dewatered sludge pyrolysis [J]. International Journal of Hydrogen Energy, 2014, 39(3): 1253-1261. DOI: 10.1016/j.ijhydene. 2013.10.050.
[23] DING Lu, ZHOU Zhi-jie, DAI Zheng-hua, YU Guang-suo. Effects of coal drying on the pyrolysis and in-situ gasification characteristics of lignite coals [J]. Applied Energy, 2015, 155: 660-670. DOI: 10.1016/j.apenergy.2015. 06.062.
[24] DAS S K, NANDY A S, PAUL A, SAHOO B K, CHAKRABORTY B, DAS A. Coal blend moisture—A boon or bane in cokemaking? [J]. Coke and Chemistry, 2013, 56(4): 126-136. DOI: 10.3103/S1068364X13040030.
[25] QIN Lin-bo, HAN Jun, ZHAO Bo, CHEN Wang-sheng, XING Fu-tang. The kinetics of typical medical waste pyrolysis based on gaseous evolution behaviour in a micro-fluidised bed reactor [J]. Waste Management & Research, 2018, 36: 1073-1082. DOI: 10.1177/0734242x 18790357.
[26] QIN Lin-bo, XING Fu-tang, ZHAO Bo, CHEN Wang-sheng, HAN Jun. Reducing polycyclic aromatic hydrocarbon and its mechanism by porous alumina bed material during medical waste incineration [J]. Chemosphere, 2018, 212: 200-208. DOI: 10.1016/j.chemosphere.2018.08.093.
[27] ZHAO Bo, HAN Jun, QIN Lin-bo, CHEN Wang-sheng, ZHOU Zi-jian, XING Fu-tang. Impact of individual flue gas components on mercury oxidation over a V2O5–MoO3/TiO2 catalyst [J]. New Journal of Chemistry, 2018, 42(24): 20190-20196. DOI:10.1039/C8NJ05084H.
[28] TIWARI H P, SHANKAR U, GUPTA R, SRIRAMOJU S K, DUTTA S, MISHRA P. Assessment of thermal efficiency of recovery stamp charge cokemaking [J]. Energy & Fuels, 2018, 32(6): 7017-7024. DOI: 10.1021/acs.energyfuels. 8b01199.
(Edited by FANG Jing-hua)
中文导读
炼焦过程中配合煤水分对焦炭质量和产物产率的影响
摘要:低水分煤炼焦不仅可以提高焦炭产率,而且可以提高焦炭质量、降低能耗。本论文研究了炼焦过程中不同水分的配合煤(1.8%~10.13%)对焦化产物产率和焦炭质量的影响。结果表明,小型固定床干馏实验中配合煤水分由10.13%降至1.80%时,焦炭产率由75.90%提高到77.16%,焦炭与CO2反应后强度(CSR)、焦炭反应性指数(CRI)、破碎指数(M25)和磨损指数(M10)等焦炭品质也得到改善。同时,随着配合煤水分的降低,焦油轻质化程度变高。为了进一步证实上述结果,在6 m高的工业焦炉中对比研究了配合煤水分为1.8%和9%~10%炼焦现场试验,焦炭CRI分别为23.4%和27.3%,CRS分别为67.1%和62.2%,说明降低配合煤水分有利于改善焦炭质量。此外,降低配合煤水分对焦炉上升管和装煤车的粉尘排放量影响较小。
关键词:配合煤;低水分;干馏;焦炭;焦炭质量
Foundation item: Project(51706160) supported by the National Natural Science Foundation of China; Project(T201906) supported by the Foundation for Outstanding Youth Innovative Research Groups of Higher Education Institution in Hubei Province, China
Received date: 2018-09-30; Accepted date: 2019-03-14
Corresponding author: ZHANG Hong-jie, PhD, Associate Professor; Tel: +86-27-68862880; E-mail: zhanghongjie@wust.edu.cn; ORCID: 0000-0001-8434-2304; QIN Lin-bo, PhD, Associate Professor; Tel: +86-27-68862880; E-mail: qinlinbo@wust.edu.cn; ORCID: 0000-0002-3855-0580
Abstract: The coal with low moisture during carbonization could not only increase the yield of coke, but also promote the coke quality and reduce the energy consumption. In this paper, the influence of the moisture in the blend coal (1.8%-10.13%) on the product yields and coke quality during coal carbonization were investigated. The results show that the coke yield is increased from 75.90% to 77.16%, and the coke qualities such as coke strength after reaction with CO2 (CSR), coke reactivity index (CRI), fragmentation index (M25) and abrasion index (M10)) are also improved when the moisture of the blend coal decreases from 10.13% to 1.80 % in a bench scale reactor. Due to the secondary reaction, tar become lighter when the moisture is decreased. In order to further prove the above results, the blend coal with 1.8% and 9%-10% (common moisture used in coke plant) moisture is carbonized in a coke oven with 6 m height, the results show that CRI are 23.4% and 27.3%, CRS are 67.1% and 62.2% under 1.8% and 9%-10% moisture of blend coal. Moreover, the variation of the moisture in blend coal has a limited influence on dust emission at the ascension pipe and the charging car.


