
Microstructure of sepiolite and its adsorbing properties to dodecanol
WANG Fei(王 菲), LIANG Jin-sheng(梁金生), TANG Qing-guo(汤庆国),
MENG Jun-ping(孟军平), WU Zi-zhao(吴子钊), LI Guo-sheng(李国胜)
Institute of Power Source and Ecomaterials Science,
Hebei University of Technology, Tianjin 300130, China
Received 10 April 2006; accepted 25 April 2006
Abstract:
The acid treatment process, testing methods, microstrcture of sepiolite mineral materials and their adsorbing properties to dodecanol were studied respectively. The results show that by acid treatment to raw sepiolite thinner fibre clusters and single fibres turn up, the pore volume and the number of micropore and mesopore in sepiolite all increase, and adsorbing properties of modified sepiolite to dodecanol are improved significantly. In the combined materials of dodecanol and sepiolite prepared under the best condition, the proportion of dodecanol is 67.96%, and then it is much higher than the result calculated from traditional BET method.
Key words:
nanoporous materials; physical adsorption; mineral materials; sepiolite; dodecanol;
1 Introduction
Along with the rapid development of industry, environmental pollution has already involved each field. The gas, water and microbe pollution are all in the key scope of the pollution control. Sepiolite has many advantages, such as abundant, low-price, good-effect and reusable, so it has a brilliant applicative prospect in the field of environmental control. In recent years, many scientific workers used sepiolite in governing envir -onmental pollution and took certain effects in the field of water pollution prevention [1-3], gas purification [4-6] and microbial contamination control [7-8].
Sepiolite is a natural fibrous morphology clay mineral with a unit cell formula of Si12O30Mg8(OH)4-
(H2O)4?8H2O[9]. It has fine microporous channels of dimensions 0.37 nm×1.06 nm running parallel to the length of the fibres[10]. It also has a specific surface area of 300 m2/g, about 50 % of which within the parallepedic pores and the other half in the mesoporosity[11]. The peculiar pore structure with interior channels allows organic molecules and ions to penetrate into the structure of sepiolite, and then it makes sepiolite a powerful adsorbent for organic compounds. Meanwhile the desorption of liquid adsorbed to interior channels is prevented from going out by nanopores of sepiolite.
Dodecanol is a typical phase change temperature- self-adjusting material. It has a good applicative prospect in the field of constructive energy conservation[12]. In order to make full use of phase change temperature -self-adjusting properties of sepiolite composites, the proportion of dodecanol in the combined materials should be increased to enhance the efficiency of phase change temperature-self-adjusting units.
The objective of the present work is to study acid treatment process, the microstrctural changes of modified sepiolite and its adsorbing properties to dodecanol.
2 Experimental
2.1 Materials
The sepiolite (in mass fraction, SiO2 72.19%, MgO 25.73%, CaO 0.89%, Fe2O3 1.20%) was purchased from Hebei Province, China. The dodecanol was from Indonesia, containing 99.6% pure dodecanol content with melting point of 23.22 ℃ and latent heat of 185.2 J/g. In addition, absolute alcohol was used as solvent for dodecanol dissolving to form solution where sepiolite was immerged.
2.2 Preparation of sepiolite samples
Sepiolite was mixed with the certain density H2SO4 in proportion of 1∶20 by mass then the solutions were stirred for 6-12 h at the room temperature, and prepared the compound slurry of H2SO4 and sepiolite; then the sepiolite solid was separated from the compound slurry by vacuum filtering and washed some times with distilled water until SO42- was not tested with Ba(NO3)2; then the solid was baked at 106 ℃ in oven until the mass was not changed; after shivered, the modified sepiolite samples were produced.
2.3 Testing methods of sepiolite samples
The microstructure of the raw sepiolite and modified sepiolite was observed by Philips XL30 scanning electron microscope. The specific surface area, pore volume and average pore diameter of sepiolite were determined by the instrument of ASAP 2010.
2.4 Adsorbing properties of sepiolite to dodecanol
Dodecanol was mixed with absolute alcohol in a certain proportion to form organic solution, and then some sepiolite powder was slowly put into the solution of each sample respectively; then the compound slurry was baked, after keeping warm in different times and temperatures, and the combined materials of sepiolite and dodecanol were produced. The adsorbing properties of sepiolite to dodecanol were expressed as Eqn.1 by proportion of dodecanol in the combined materials.
![]() (1)
(1)
which η means the proportion of dodecanol in the combined materials; wd means the mass of adsorbing dodecanol; ws means the mass of sepiolite.
3 Results and discussion
3.1 Microstructure of sepiolite mineral materials
Fig.1 shows the microstructure of the raw and the acid modified sepiolite. As shown in Fig.1, the majority fibres of raw sepiolite appear in the form of clusters, with the diameter about 0.3 μm and the length over 10 μm, while the acid modified sepiolite clusters are obviously thinner and shorter than the raw ones, and the majority fibres of modified sepiolite assemble loosely with many single fibres, with the diameter about 0.2 μm and the length about 2.5-10 μm respectively. In fact, modified sepiolite fibre clusters become thinner ones and single fibres because the connected sections are etched. The reason of this phenomenon is that H+ can displace Mg2+ and H2O combined with Mg2+ under the acid circumstances; at the same time, silicon hydride bunches on the surface of sepiolite also can transform into siloxanes by the acid treatment, so the structure of sepiolite has changed.
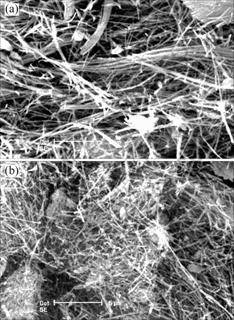
Fig.1 SEM photographs of raw and acid modified sepiolite: (a) Raw sepoiolite; (b) Acid modified sepiolite
Fig.2 shows the above progress. As shown in Fig.2, the Si—O—Mg—O—Si bond in sepiolite turns into two Si—O—H bonds after acid treatment. So the internal channels are connected, and the specific surface area becomes larger than the start.
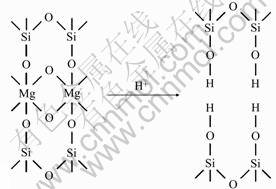
Fig.2 Sketch map of ion exchange in sepiolite acid treatment
Fig.3 shows the channel extension mechanism of sepiolite during the acid treatment. As shown in Fig.3, the crystal interior channel of sepiolite is connected because of the removal of some magnesium, so part of micropores turn into mesopores. When most magnesium is been removed, part of micropores and mesopores expand to maxipores[13].
Fig.4 shows the pore distribution of raw sepiolite and acid modifieded sepiolite. As shown in Fig.4, the number relation of different-size pores in the sepiolite
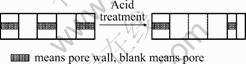
Fig3 Extension mechanism of sepiolite
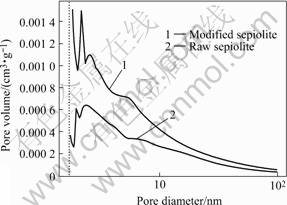
Fig.4 Pore distribution of raw and acid modified sepiolite
is as follows: micropores>mesopores>maxipores. The smooth zones are located in the different positions about the raw and modified sepiolites, as shown in Fig.4. This means that acid etching is not equal. It can be seen from ordinate that the pore volume corresponded different pore diameter all increase in various extent, so the acid treatment can broaden and open up a path for the channels of sepiolite. During the above, many closed channels in the raw sepiolite can be opened. It also can expound the increase of pore volume.
3.2 Adsorbing properties of sepiolite to dodecanol
3.2.1 Influence of acid treatment process on adsorbing properties of sepiolite to dodecanol
Fig.5 shows adsorbing properties of acid modified sepiolite to dodecanol, comparing with the raw one in different stirring times when the original dodecanol solution concentration is 70 %. As shown in Fig.5, the proportions of dodecanol adsorbed by the raw and modified sepiolite all increase during the stirring, meanwhile adsorbing property of modified sepiolite to dodecanol is much better than that of the raw one, and the difference is up to 14.43% when the stirring time reaches 5 h. The main reason is that the pore volume of sepiolite becomes much larger.
3.2.2 Influence of original dodecanol solution concen-
tration on adsorbing properties of sepiolite to dodecanol
From Ref.[14], it can be seen that the dodecanol can be dissolved in alcohol in order to reduce the viscosity and make dodecanol easier to be adsorbed by sepiolite at relatively low temperature. To study the influence of
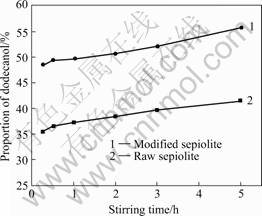
Fig.5 Adsorbing properties of raw and acid modified sepiolite to dodecanol
original dodecanol solution concentration on adsorbing properties of sepiolite to dodecanol, original dodecanol solution concentration is set as 10 %, 20 %, 50 %, 70 % and 100 % respectively when stirring time is 0.5, 2 and 5 h. The results are shown in Fig.6. As shown in Fig.6, the proportions of dodecanol in the combined materials, prepared in 0.5, 2 and 5 h, all increase with the rise of original dodecanol solution concentration. The proportions of dodecanol increase sharply when original dodecanol solution concentration is lower than 50%; the proportions of dodecanol increase slowly when original dodecanol solution concentration is between 50% and 70%; the proportions of dodecanol increase much more slowly when original dodecanol solution concentration is between 70% and 100%.
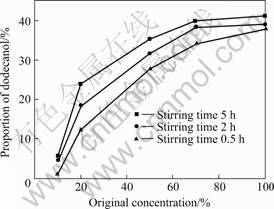
Fig.6 Influence of original dodecanol solution concentration on adsorbing properties of sepiolite to dodecanol
The reason of the above is that the principal proponent is alcohol when original dodecanol solution concentration is relatively low, then alcohol vapors during the baking of the compound slurry, thus there is very few dodecanol in the organic solution, so the organic solution adsorbed by sepiolite is very few; dodecanol increases and alcohol decreases along with rising of original dodecanol solution concentration, and remanent dodecanol increases significantly by vaporing, so the organic solution adsorbed by sepiolite increases sharply; when original dodecanol solution concentration is higher than 50 %, alcohol has lost much during the vaporing, therefore the adsorption by sepiolite increases slowly when the concentration is between 50 % and 70 % and increases more slowly when the concentration is between 70 % and 100 %. Considering the above and the fluidity of dodecanol organic solution, the best original dodecanol solution concentration is 70%.
3.2.3 Influence of stirring time on adsorbing properties of sepiolite to dodecanol
From Ref.[15] it can be seen that the stirring time has great influence on the proportions of dodecanol adsorbed in the combined materials. To study the influence of stirring time on adsorbing properties of sepiolite to dodecanol, the stirring time is set as 0.25, 0.5, 1, 2, 3, 4, 5, 6, 7, 8, 9 and 10 h respectively when the original dodecanol solution concentrations are 50 % and 70 %. The results are shown in Fig.7. As shown in Fig.7, it can be seen that the proportions of dodecanol in combined materials, prepared with the original dodecanol solution concentrations of 50% and 70%, all increase up to the maximum till 5 h, and then decrease along with stirring time prolonging.
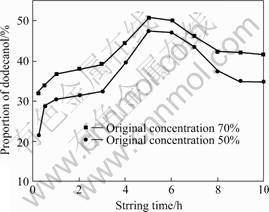
Fig.7 Influence of stirring time on adsorbing properties of sepiolite to dodecanol
The main reason for this phenomenon lies in that from the very first to 5 h the alcohol vapors so that the concentration of dodecanol becomes much higher than the original one, so the proportions of dodecanol in combined materials increase along with stirring time prolonging. When stirring time is more than 5 h, the adsorbing properties to dodecanol are restricted because organic solution reaches internal of micropores, so the proportions of dodecanol in combined materials start to reduce. According to above analysis, the best stirring time is 5 h.
3.2.4 Influence of temperature on adsorbing properties of sepiolite to dodecanol
Adsorption from solid to solution is an exothermic progress, so low temperature is beneficial for adsorption. But adsorbate in the research is dodecanol, it may become solid at very low temperature, therefore the adsorption cannot continue. In addition, temperature also has great influence on viscosity and fluidity of dodecanol. To study the influence of temperature on adsorbing properties of sepiolite to dodecanol, temperature is set as 35, 40, 45, 50, 55, 60 and 65 ℃ respectively with the original dodecanol solution concentration of 70% when the stirring time is 5 h. The results are shown in Fig.8. As shown in Fig.8, the proportion of dodecanol in combined materials, prepared with the original dodecanol solution concentration of 70% when stirring time is 5 h, increases slowly in the range from 35 ℃ to 45 ℃, then it increases sharply in the range from 45 ℃ to 55 ℃, and it is up to maximum at 55 ℃, after that it decreases relatively fast.
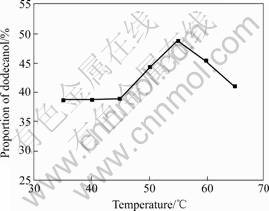
Fig.8 Influence of temperature on adsorbing properties of sepiolite to dodecanol
The main reason of the phenomenon is discussed as follows. When the temperature is lower than 45 ℃, although low temperature is beneficial for adsorption, the fluidity of dodecanol is not good enough because the viscosity of dodecanol is so high, therefore the adsorbing properties to dodecanol are not good. When the temperature is between 45 ℃ and 55 ℃, firstly, low temperature is beneficial for adsorption; secondly, the fluidity of dodecanol is improved because the viscosity of dodecanol becomes more lower than the former, so the adsorbing properties to dodecanol are improved. When the temperature is up to 55 ℃, the temperature and fluidity are perfect for the adsorption, so the adsorbing properties to dodecanol are the best. When the temperature is between 55 ℃ and 65 ℃, the adsorbing properties to dodecanol are not good because of desorption. According to above analysis, the best temperature is 55 ℃.
In the combined materials of dodecanol and sepiolite, prepared under the best condition of original dodecanol solution concentration, stirring time and temperature, the proportion of dodecanol is 67.96% calculated from Eqn.1. The pore volume of modified sepiolite is 0.248 050 cm3/g which is tested by BET method. Supposing dodecanol is adsorbed totally into the pores, 1 g sepiolite can adsorb 0.206 130 g dodecanol with the volume of 0.248 050 cm3, so the proportion of dodecanol in the combined materials is 17.09 % calculated from BET method. It can be obviously seen that the experimental result is much higher than the result calculated from BET method. The further research about the above mentioned phenomenon is under investigation.
4 Conclusions
Sepiolite fibre clusters become thinner ones and single fibres by acid treatment process. The function of acid treatment process is that it may increase the pore volume and the number of micropore and mesopore. Compared with the raw sepiolite, the adsorbing properties of modified sepiolite to dodecanol is improved significantly. In the combined materials of dodecanol and sepiolite prepared in the best condition, the proportion of dodecanol is much higher than the result calculated from traditional BET method.
References[1] NIHAL B, AKMAN A B, SERDAR K. Kinetic and equilibrium studies in removing lead ions from aqueous solutions by natural sepiolite[J]. Journal of Hazardous Materials, 2004, 112(1-2): 115-122.
[2] SAFA O A, SERIFE T, ADNAN O. Adsorption of acid dyes from aqueous solutions onto sepiolite[J]. Separation Science and Technology, 2004, 39(2): 301-320.
[3] NESE O, DUYGU K. Boron removal from aqueous solutions by adsorption on waste sepiolite and activated waste sepiolite using full factorial design[J]. Adsorption, 2004, 10(3): 245-257.
[4] TOKIYOSHI M, MOTOJI I. Thermal stability of SO2 and NO2 adsorbed on the surface of sepiolite, a porous clay mineral[J]. Japanese Journal of Applied Physics, Part 1: Regular Papers and Short Notes and Review Papers, 2002, 41(5A): 2916-2919.
[5] SAGRARIO M, ISABEL G M, BERMEJO P J, VICENTA M. Mercury retrieval from flue gas by monolithic adsorbents based on sulfurized sepiolite[J]. Environmental Science and Technology, 1999, 33(10): 1697-1702.
[6] WATANABE Y, BANNO K, SUGIURA M. Calcined sepiolite-supported Pt/Fe catalyst[J]. Applied Clay Science, 2000, 16(1-2): 59-71.
[7] SOLEDAD A M, SONIA R C M, JESUS S M M, MARIA S C. Effect of the modification of natural clay minerals with hexadecylpyridinium cation on the adsorption-desorption of fungicides[J]. International Journal of Environmental Analytical Chemistry, 2004, 84(1-3): 133-141.
[8] PEREZ-RODRIGUEZ J L, MAQUEDA C, CARRETERO M I. Effect of some clay minerals on the growing of sulphate-reducing bacteria in anaerobic reactors[J]. Applied Clay Science, 1989, 4(5-6): 449-459.
[9] RYTWO G, TROPP D, SERBAN C. Adsorption of diquat, paraquat and methyl green on sepiolite: experimental results and model calculations[J]. Applied Clay Science, 2002, 20(6): 273-282.
[10] MOLINA M, CZTURLA F, RODRIGUEZ F, KHARITONOVA G. Porous structure of a sepiolite as deduced from the adsorption of N2, CO2, NH3 and H2O[J]. Microporous and Mesopoporous Materials, 2001, 47(2-3): 389-396.
[11] SUN A, D’ESPINOSE DE LA CAILLERIE J B, FRIPIAT JOSE J. A new microprous material: aluminated sepiolite[J]. Microporous Materials, 1995, 5(3): 135-142.
[12] LIANG Jin-sheng, LIANG Guang-chuan, WU Zi-zhao, LIANG Xiu-hong, REN Bao-shan. Preparation and Use of Temperature-self-adjusting Combined Units [P]. CN1473905A, 2004-02-11.
[13] LI Song-jun, LUO Lai-tao. Studies on the modification of sepiolite[J]. JIA-NGXI Science, 2001, 19(1): 61-66.
[14] KAASINEN H. The absorption of phase change substances into commonly used building materials[J]. Solar Energy Materials and Solar Cells, 1992, 27(2): 173-179.
[15] HAWES D W, FELDMAN D. Absorption of phase change materials in concrete[J]. Solar Energy Materials and Solar Cells, 1992, 27(2): 91-101.
Foundation item: Project (2001AA322040) supported by the High-Tech Research and Development Program of China
Corresponding author: LIANG Jin-sheng; Tel: +86-22-26582575; Fax: +86-22-26564850; E-mail: liang_jinsheng@sina.com


