Trans. Nonferrous Met. Soc. China 26(2016) 1410-1418
Toxicity and bioaccumulation of heavy metals in Phanerochaete chrysosporium
Mei-hua ZHAO1, Chao-sheng ZHANG1, Guang-ming ZENG2, Dan-lian HUANG2, Min CHENG2
1. College of Civil Engineering, Guangzhou University, Guangzhou 510006, China;
2. College of Environmental Science and Engineering, Hunan University, Changsha 410082, China
Received 1 July 2015; accepted 6 January 2016
Abstract:
The responses of the growth and metabolism activity of Phanerochaete chrysosporium (P. chrysosporium) to cadmium (Cd), lead (Pb) and their combined pollution stress, were investigated in plate and liquid culture conditions. The diameter of colony, biomass of P. chrysosporium, ligninolytic enzyme activities and bioaccumulation quantity of heavy metals were detected. The results indicated that Cd was more toxic than Pb to P. chrysosporium and the toxicity of Cd and Pb to P. chrysosporium was further strengthened under Cd+Pb combined pollution in different culture conditions. Heavy metals Cd and Pb had indirect influence on the production of ligninolytic enzymes by directly affecting the fungal growth and metabolic activity, and by another way in liquid culture. In addition, the results provided an evidence of the accumulation of Cd and Pb on the mycelia of P. chrysosporium.
Key words:
Phanerochaete chrysosporium; heavy metal; Cd; Pb; toxicity; bioaccumulation; ligninolytic enzyme ; ; ; ; ; ; ; ; ; ; ; ; ; ; ; ; ; ; ; ; ; ; ; ; ; ; ; ; ; ; ; ; ; ; ; ; ; ; ; ; ; ; ; ; ; ; ; ; ; ; ; ; ; ; ; ; ; ; ; ; ; ; ; ; ; ; ; ; ; ; ; ; ; ; ; ; ; ; ; ; ; ; ; ; ; ; ; ; ; ; ; ; ; ; ; ; ; ; ; ; ; ; ;;
1 Introduction
Heavy metal contamination in surface, ground water and soil becomes a widespread and serious problem, which poses a huge threat to plants, animals and human beings [1-4]. Conventional methods such as chemical precipitation, coagulation, adsorption, membrane processes and ion exchange have been employed for removing heavy metals from contaminated sites, which are inefficient and expensive [5-8]. These shortcomings of conventional physico-chemical methods have led to the investigation of the use of microorganisms in bioremediation for heavy metals, which provides an economic and safe alternative that does not aggravate other environmental problems [9,10]. To date, there are numerous microbe species including bacteria, actinomycetes and fungi, which are known to have the potential to adapt the heavy metals-contaminated environment and remove heavy metals [7,11,12]. Phanerochaete chrysosporium (P. chrysosporium), a well-known white-rot fungus, has been found to be potential in treating heavy metal-contaminated agricultural waste, and wastewater containing heavy metals and toxic organic pollutants, due to its unique degradation to a wide range of xenobiotic and persistent pollutants, and biosorption ability to heavy metals [2,9,13,14].
Commonly, some heavy metals are essential as trace elements for the fungal growth and metabolism including Cu, Fe, Mn, Mo, Zn and Ni, but these metals are toxic when they are present in excess. Nonessential metals commonly including Cr, Cd, Pb, Hg and Ag, which are widely present in the environment, are toxic to most microorganisms [15,16]. Excess heavy metals would cause morphological and physiological changes, and affect the reproduction of microorganism. Cd is one of the most toxic environmental pollutants to all living cells. The symptoms of Cd toxicity include growth inhibition, enzyme inactivation, proteins and DNA oxidation, nucleotide conformation changes and ultrastructural changes [17-20]. Pb has also been recognized as one of the most hazardous heavy metals among environmental pollutants [21], which can cause cell membrane damage and destroy the transport of nutrients [15,22]. However, so far, there has been only scattered information about the physiological effects of Pb or Cd pollution on white-rot fungi. In particular, the responses of white-rot fungi growth to Pb and Cd combined pollution stress have not yet been reported until now.
The objective of the present investigation was to study the toxicity of heavy metals on growth and enzyme production of P. chrysosporium in plate and liquid culture conditions, respectively. In addition, the bioaccumulation of heavy metals by P. chrysosporium was studied under different culture conditions. These results are expected to clarify the heavy metal toxicity, provide useful references on alleviating the environmental impact of metal-contaminated sites by P. chrysosporium, and promote further application of P. chrysosporium for bioremediation.
2 Experimental
2.1 Fungal strain and chemicals
The white-rot basidiomycete, P. chrysosporium strain BKM-F-1767 used in this work was obtained from the China Center for Type Culture Collection (Wuhan, China). Stock cultures were maintained on malt extract agar slants at 4 °C. All the chemicals used in this work were of analytical reagent grade.
2.2 Plate culture studies
Three sets of experimental plates were prepared and labeled as Pb, Cd and Pb+Cd, and the concentration of heavy metal in each experimental plate is shown in Table 1. The experimental plates without addition of any heavy metal were used as control. Fungal cultures were transferred to potato dextrose agar (PDA) plates at 37 °C for several days. Then, fungi scone was obtained in colony border with a punch (D=10 mm) and inoculated into the center of experimental plate with the mycelium down under aseptic conditions. All experiments were performed in six replicates, and plates of three replicates were put with cellophanes and others without cellophanes. All experimental plates were placed in a constant-temperature (30 °C) incubator and incubated until the colony reached the edge of the plate. The tendency of hypha growth of P. chrysosporium was observed, and the colony diameter, mycelial dry mass of P. chrysosporium and heavy metal accumulation by P. chrysosporium were measured.
2.3 Liquid culture studies
P. chrysosporium spore suspensions were prepared by diluting the spores in ultrapure water and adjusted to a concentration of 2.0×106 CFU/mL. 2 mL of spore suspensions were inoculated to 500 mL Erlenmeyer flasks containing 200 mL liquid medium (pH 4.5) as described by TIEN and KIRK [23]. Each flask was treated with various kinds and concentrations of heavy metals, and the initial concentrations of different heavy metals are shown in Table 1. Then, the liquid culture experiments were performed at 30 °C for 7 d and the rotation speed was fixed at 120 r/min. To make a better comparison, the non-heavy metal control flasks were used. All experiments were performed in three replicates. After 7 d, samples were taken to determine biomass, ligninolytic enzyme activities and concentrations of heavy metals in solution.
2.4 Determination assays
2.4.1 Tendency of hypha growth
The tendency of hypha growth of P. chrysosporium was observed. Comparing the tendency of hypha growth in experimental groups with that in the control group, some words such as sparse, intensive, strong and weak were used to describe different degrees of growth.
2.4.2 Diameter and net growth of colony
Firstly, select colony at any place to measure diameter, then rotate the plate clockwise 60° for the second measure of its diameter, and finally rotate the plate clockwise 60° again for the third measure of its diameter [24]. The diameter of colony of P. chrysosporium was the average of three measures. The net growth was calculated as the difference between diameters of colony and scone.
2.4.3 Dry mass and inhibition rate of growth of P. chrysosporium
In plate culture, plates were put in the microwave oven for heating. After PDA was completely melted, mycelia were obtained and washed five times with ultrapure water, and then put into the oven of 105 °C (12 h) for drying to constant mass.
In liquid culture, mycelia were filtered and washed five times with ultrapure water after culture process, and then put into the oven of 105 °C (12 h) for drying to constant mass. The dry mass was identified as the biomass of P. chrysosporium.
The inhibition rate of growth in both plate and liquid cultures was calculated with the formula as follows:
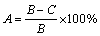 (1)
(1)
where A is the rate inhibition of growth, B is the net growth in blank group, and C is the net growth in experimental group.
2.4.4 Ligninolytic enzyme activity
The supernatant fluid from liquid culture was filtered through 0.45 μm filter papers for ligninolytic peroxidase activity analyses. In this work, two main ligninolytic peroxidases (LiP and MnP) were measured with an ultraviolet spectrophotometer (UV-2250, SHIMADZU, Japan). LiP activity was measured as described by TANAKA et al [25]. One unit (U) of LiP activity was defined as the amount of the enzyme required to produce 1 mol/L veratryl aldehyde from the oxidation of veratryl alcohol per minute. MnP activity was measured as described by LOPEZ et al [26]. MnP unit activity was defined as the amount of enzyme required for producing 1 mol/L Mn3+ from the oxidation of Mn2+ per minute.
2.4.5 Bioaccumulation of heavy metals
In plate culture, mycelia in plates with cellophanes were obtained and washed with utrapure water five times, and then put into the oven of 105 °C for drying to constant mass. Microwave digestion by adding concentrated HNO3 was performed under these conditions (the first process: 140 °C, 5 MPa and 10 min; the second process: 170 °C, 10 MPa and 8 min; the last process: 190 °C, 10 MPa and 5 min). After digestion, the sample solution was analyzed for content of Pb or Cd using an atomic absorption spectrophotometer (AAS, Agilent 3510, USA).
In liquid culture, 1.25 mL of samples from the P. chrysosporium culture were filtered through Whatman paper, and diluted with untrapure water to 50 mL for heavy metal analysis with an atomic absorption spectrophotometer (AAS, Agilent 3510, USA).
2.5 Statistical analysis
The analysis of experimental samples was made three replications, and results were expressed as the mean value with the standard deviation. Statistical analyses were performed using SPSS software (SPSS 18.0, Germany). The level of significance was set at P<5% in all cases.
3 Results and discussion
3.1 Toxicity and bioaccumulation of heavy metals of P. chrysosporium in plate culture
3.1.1 Tendency of hypha growth of P. chrysosporium
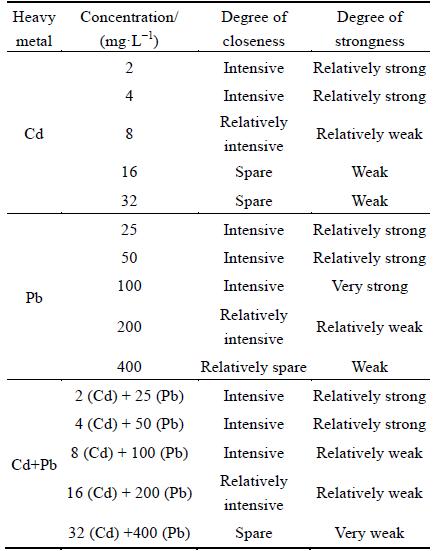
In order to research the effect of heavy metals on the growth of P. chrysosporium, the hypha growth of P. chrysosporium was observed, and the results of tendency of hypha growth with qualitative analysis are shown in Table 1. On the whole, the change trends of hypha growth were the same under Pb, Cd and combined Cd+Pb pollution stress. The hypha growth of P. chrysosporium increased progressively with increasing concentration of the heavy metal firstly, and then decreased with further increasing concentration of the heavy metal. However, there is a great difference of P. chrysosporium among the three conditions. When Pb concentration was 25 mg/L and Pb+Cd concentration was 2 (Cd) + 25 (Pb) mg/L, hypha grew strongly and were characterized by dense. The tendency of hypha growth was further strengthened with the increase of the test Pb concentration. However, the tendency of hypha growth did not change with the increase of the Cd+Pb concentration. The tendency of hypha growth was slowly weaken when Cd concentration was 8 mg/L, and the hypha growth became very weak and had performance in aerial hyphae thinning with the further increase of the Cd concentration. At high concentrations of Pb, Cd and Cd+Pb, the growth of P. chrysosporium was weak, and the hypha was relatively sparse.
Table 1 Effect of Cd, Pb and Cd+Pb on tendency of hypha growth of P. chrysosporium
3.1.2 Diameter and net growth of colony
The diameters of colony of P. chrysosporium under Cd, Pb and combined Cd+Pb pollution stress are shown in Fig. 1. The diameter of colony was 81 mm when Cd concentration was 2 mg/L, which increased by 1 mm on average compared with that of blank group. Meanwhile, the diameter increased by 2 mm and reached 82 mm when the Cd concentration was 4 mg/L. The colony diameter increased slightly under low concentration of Cd and the changes might be due to stimulating the growth of P. chrysosporium by the low concentration of Cd. With further increase of Cd concentration, colony diameter became smaller, while Cd concentration reached 32 mg/L, the colony diameter was only 26 mm. For single Pb pollution, colony diameter of P. chrysosporium increased with the increase of Pb concentration in Pb concentrations of 25-100 mg/L, while it decreased gradually with the increase of Pb concentration in the range of 200-400 mg/L. This suggested that the low concentration of Pb pollution could promote the growth of colonies scattered, and also showed that P. chrysosporium had a certain tolerance for low concentration of Pb (≤100 mg/L). For combined pollution of Cd+Pb, the colony diameter was larger than that of the blank group when Cd+Pb concentration in the range from 2 (Cd) +25 (Pb) to 8 (Cd) + 100 (Pb) mg/L, and colony diameter was smaller than that of the blank group when the concentration was greater than 16 (Cd) + 200 (Pb) mg/L, especially under 32 (Cd) + 400 (Pb) mg/L, the colony was only 18 mm in diameter and the net growth was 8 mm.

Fig. 1 Effect of Cd, Pb and Cd+Pb concentrations on diameter of colony of P. chrysosporium
3.1.3 Dry mass of P. chrysosporium
Measuring the colony diameter in fungal plate culture is often adopted to evaluate the impact of exogenous substances or condition on fungal mycelium growth, but the disadvantage of this method is that, the density, thickness and material accumulation inner of mycelium are not to be considered. Therefore, besides the observation of hypha growth and determination of colony diameter, the mycelial dry mass was also measured, and inhibition rate of dry mass was calculated in this experiment.
The dry masses of P. chrysosporium under Cd, Pb and Cd+Pb combined pollution stress are shown in Fig. 2. The results showed that mycelial dry mass under Cd+Pb combined pollution had different degrees of reduction compared with that of the blank group, and the mycelial dry mass gradually reduced with the increase of concentration of heavy metals. At the Cd+Pd concentration of 32 (Cd) + 400 (Pb) mg/L, mycelial dry mass was reduced to 15 mg and the inhibition rate was as high as 90.51%, which may be attributed to synergistic inhibition to the growth of P. chrysosporium by a high concentration of Cd+Pb combined pollution. Under Cd single pollution, the mycelial dry mass at low Cd concentration (2 mg/L) was higher than that of the blank group. Mycelial dry mass was inhibited when the concentrations of Cd were 8, 16 and 32 mg/L, and the inhibition rates were 11.39%, 51.90% and 75.32%,respectively. For single Pb pollution, the change of mycelial dry mass showed an increase trend followed by a decrease with the increase of Pb concentration. The dry mass of mycelium reached the maximum of 172 mg at Pb concentration of 100 mg/L. When the concentrations of Pb were 200 and 400 mg/L, the inhibition rates of dry mass were 13.29% and 48.10%, respectively.
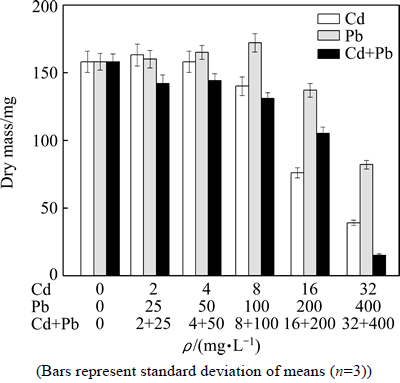
Fig. 2 Effect of Cd, Pb and Cd+Pb on dry mass of P. chrysosporium
The change tendency of the dry mass of mycelium was in line with that of tendency of hypha growth of P. chrysosporium, and presumably dry mass of mycelium dip may be caused by gradually weakened aerial hypha growth. On the other hand, it may be one reason that substrate mycelium growth was restrained by increasing concentration of heavy metals.
3.1.4 Bioaccumulation of heavy metals by P. chrysosporium
Figure 3 shows the bioaccumulation ability of heavy metals (Cd and Pb) by P. chrysosporium. The bioaccumulation content of heavy metals in vivo P. chrysosporium increased gradually with the increase of concentration of heavy metals. This was coinciding with Cd or Pb content in fruiting body of macrofungi which increased with increasing concentration of Cd or Pb [27,28]. However, the content of heavy metals in mycelium showed no linear correlation with heavy metal concentration. This may be due to the difference in fungi growth at different concentrations of heavy metals. Because the bioaccumulation of heavy metals by fungi was not only related to the basic concentrations of heavy metals, but also related to the quantity of fungi. The bioaccumulation quantity of Cd by P. chrysosporium under Cd single pollution was greater than that under the corresponding Cd+Pb combined pollution. The bioaccumulation quantity of Pb by P. chrysosporium under single Pb pollution was less than that under the corresponding concentrations of Cd+Pb combined pollution. This showed that the combined pollution stress promoted P. chrysosporium’ ability for Pb bioaccumulation and slightly dampened the Cd bioaccumulation capability.

Fig. 3 Accumulation of Cd (a) and Pb (b) on P. chrysosporium
3.2 Toxicity and removal of heavy metals of P. chrysosporium in liquid culture
3.2.1 Biomass of P. chrysosporium
Trace metal elements required for some metabolic functions play an important role in nature decomposition, transformation and recycling, but when the concentration of metals in the environment increases to a certain extent, they will affect or inhibit the growth of microorganism and metabolic activity. Some heavy metals such as Cd and Pb have certain toxicity to microorganisms and serve no biological function [29]. The determination of microbial biomass in the process of pure culture is widely applied in life sciences research, industry, agriculture, medical and health care. The growth of microorganism is one of the biological indicators of heavy metals toxicity. Therefore, the detection of the growth situation of P. chrysosporium in the liquid culture will help for the intuitive understanding of the toxic effects of Cd and Pb on P. chrysosporium.
The biomasses of P. chrysosporium under Cd, Pb and Cd+Pb combined pollution stress are shown in Fig. 4. The change trends of biomass of P. chrysosporium in all experimental groups were the same. The biomass of P. chrysosporium decreased with the increase of heavy metal concentration, and was smaller than that of blank group, suggesting that when P. chrysosporium was exposed to heavy metals in liquid environment, Cd and Pb would have negative effect on its growth, and the greater the concentrations of Cd and Pb are, the greater the negative effect is. Cd had mutagenic effect, which led to the DNA chain rupture, thereby changing the microbial growth and metabolism. Pb can cause cell membrane damage and destroy nutrient transport, which affects the growth of microorganisms. By comparison of single Cd and Pb pollution, it was found that Cd had much more toxic effect on P. chrysosporium than Pb, and the biomass of P. chrysosporium was very little under a very low concentration of Cd pollution. When the concentration of Cd was 32 mg/L, the biomass dry mass of P. chrysosporium (120 mg) was far less than that in the blank group (450 mg). Under Cd+Pb combined pollution, the toxicity of Cd and Pb to P. chrysosporium was further strengthened, and the biomass decreased dramatically. This may be attributed to that the environment polluted by heavy metals is so complex for P. chrysosporium to adapt to, and the exposure to Cd and Pb will lead to DNA damage of P. chrysosporium, so as to greatly limit the growth of P. chrysosporium.
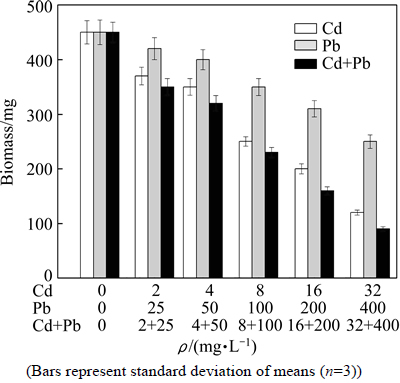
Fig. 4 Effect of Cd, Pb and Cd+Pb on biomass of P. chrysosporium
3.2.2 Production of ligninolytic enzymes
In liquid culture conditions, two ligninolytic enzymes including LiP and MnP were produced by P. chrysosporium in the process of secondary metabolism. As shown in Fig. 5, the ability of P. chrysosporium for producing LiP and MnP under the Cd single pollution stress was weaker than that in the blank group. When the Cd concentration reached 16 mg/L, the amount of LiP and MnP activity were only 14 and 96 U/L, and reduced by 76.67% and 61.06% than that in the blank group, respectively. The results suggested that single Cd could inhibit the production of MnP and LiP by P. chrysosporium. Low concentrations (25 and 50 mg/L) of

Fig. 5 Effect of Cd, Pb and Cd+Pb on LiP (a) and MnP (b) activity of P. chrysosporium
Pb stress could induce the production of MnP and LiP by P. chrysosporium, while the biomass of P. chrysosporium was reduced. This may be because P. chrysosporium would produce antioxidant mechanism to resist Pb pollution when exposed to low concentration of Pb [30]. The production of some antioxidants in antioxidant mechanism might change metabolic environment of P. chrysosporium, and this kind of environment was more conducive to the production of MnP and LiP by P. chrysosporium. Under high concentrations Pb (>50 mg/L) pollution stress, the production of MnP and LiP by P. chrysosporium was less than that in the blank group, this may be because Pb could damage P. chrysosporium in turn and the secretion of these two kinds of extracellular enzymes was decreased. The results confirmed that heavy metals had indirect influence on the production of ligninolytic enzymes by directly affecting the growth and metabolic activity of microorganisms [31]. In addition, other ways of heavy metal influence on ligninolytic enzymes may be: 1) heavy metals could occupy the active center of ligninolytic enzymes, or combine with amido or sulfydryl of enzyme molecules, thereby reducing enzyme activity of inhibition [32]; 2) heavy metal ions as a prosthetic group to join enzyme protein could promote the coordination between enzyme activity center and the substrate, and the enzyme and its active center could keep certain obligatory structure, and thereby transform the surface charge of the enzyme protein and equilibrium properties of enzyme catalytic reaction, leading to enhanced enzyme activity, namely performance for activation [15].
There was a difference between the production of MnP and LiP by P. chrysosporium under Cd+Pb combined pollution. The concentration of Cd+Pb (2 (Cd) + 25 (Pb) mg/L) pollution promoted more LiP generation, while any combination of concentration of Cd+Pb pollution limited the generation of MnP to different degrees. This result suggested that the production mechanism of MnP and LiP by P. chrysosporium was different, and had different requirements on the environment. In addition, high concentrations of combined pollution led to the synergistic inhibition of production of ligninolytic enzymes. At a concentration of 32 (Cd) + 400 (Pb) mg/L, the inhibition rates of MnP and LiP were as high as 82.4% and 78.33%, respectively. This result could also be speculated that under high concentration of combined pollution, the degradation ability of lignin by P. chrysosporium would be greatly restricted.
3.2.3 Concentrations of heavy metals in liquid culture
As shown in Fig. 6, P. chrysosporium showed removal ability of Cd and Pb in liquid culture, which further confirmed that the fungus had prospect of application in heavy metal removal from wastewater [7,8]. In the tested range of Cd concentration, the adsorption removal rate of Cd by P. chrysosporium was as high as 89.5% (2 mg/L), and had the minimum of 51.5% (16 mg/L). As a result, adsorption capability for low concentration of Cd was strong because adsorption capacity offered by the surface of P. chrysosporium was limited, thus its surface adsorption sites tended to be saturated when Cd concentration was increased. On the other hand, the increase of Cd concentration would stimulate the resistance system of P. chrysosporium to heavy metals and facilitated efflux of heavy metals. However, the removal rate of Cd increased when the Cd concentration was up to 32 mg/L, and the mechanism underlying this phenomenon remained to be clarified. The current speculation may be that the enrichment of large number of heavy metal on the surface would increase the pressure of transportation directly to cells in vivo, and more Cd was transported to inside the fungus. Residues of Pb concentration in liquid medium in all experimental groups were very low, which showed that adsorption removal ability of Pb by P. chrysosporium was very strong, and removal rate was all higher than 90%. So, the biosorption ability of Pb by P. chrysosporium was better than that of Cd. Compared with single Cd pollution, the removal ability of Cd was weakened and the maximum removal rate of Cd was 84% (2 mg/L) under Cd+Pb combined pollution, while the removal ability of Pb was slightly increased. The results suggested that there was competition adsorption in adsorption process of Cd and Pb by P. chrysosporium, and the fungi in the competitive adsorption had stronger affinity for Pb, thus the adsorption of Pb played a more dominant position [33]. The bioaccumulation effect of Cd and Pb by P. chrysosporium in liquid culture was similar with that in plate culture.

Fig. 6 Changes of heavy metal concentration in medium under Cd, Pb and Cd+Pb exposure
4 Conclusions
1) Heavy metals would affect the growth of P. chrysosporium in plate culture, and the effect was positive or negative depending on type and concentration of heavy metals.
2) Cd and Pb would have negative effect on growth of P. chrysosporium in liquid culture, and the greater the concentrations of Cd and Pb are, the greater the negative effect is. In addition, single Cd could inhibit the production of MnP and LiP by P. chrysosporium under all tested concentrations, and low concentration (25 and 50 mg/L) of single Pb stress could induce the production of MnP and LiP by P. chrysosporium. While the concentration of Cd+Pb (2 (Cd)+25 (Pb) mg/L) pollution promoted more LiP generation, all tested concentrations of Cd+Pb pollution limited the generation of MnP.
3) P. chrysosporium showed biological enrichment for both Cd and Pb in plate and liquid cultures, which suggested that P. chrysosporium could be applied to removing Cd and Pb from wastes polluted by heavy metals.
References
[1] ZHU Jian-yu, ZHANG Jing-xia, LI Qian, HAN Tao, HU Yue-hua, LIU Xue-duan, QIN Wen-qing, CHAI Li-yuan, QIU Guan-zhou. Bioleaching of heavy metals from contaminated alkaline sediment by auto- and heterotrophic bacteria in stirred tank reactor [J]. Transactions of Nonferrous Metals Society of China, 2014, 24(9): 2969-2975.
[2] HUANG Dan-lian, ZENG Guang-ming, FENG Chong-ling, HU Shuang, JIANG Xiao-yun, TANG Lin, SU Feng-feng, ZHANG Yu, ZENG Wei, LIU Hong-liang. Degradation of lead-contaminated lignocellulosic waste by Phanerochaete chrysosporium and the reduction of lead toxicity [J]. Environmental Science and Technology, 2008, 42: 4946-4951.
[3] XU Piao, ZENG Guang-ming, HUANG Dan-lian, FENG Chong-ling, ZHAO Mei-hua, LAI Cui, WEI Zhen, HUANG Chao, XIE Geng-xin.Use of iron oxide nanomaterials in wastewater treatment: A review [J]. Science of the Total Environment, 2012, 24: 1-10.
[4] ZENG Guang-ming, HUANG Dan-lian, HUANG Guo-he, HU Tian-jue, JIANG Xiao-yue, FENG Chong-ling, CHEN Yao-ning, TANG Lin, LIU Hong-liang. Composting of lead-contaminated solid waste with inocula of white-rot fungus [J]. Bioresource Technology, 2007, 98: 320-326.
[5] AHLUWALIA S S, GOYAL D. Microbial and plant derived biomass for removal of heavy metals from wastewater [J]. Bioresource Technology, 2007, 98: 2243-2257.
[6] GONG Ji-lai, WANG Bin, ZENG Guang-ming, YANG Chun-ping, NIU Cheng-gang, QIU Qiu-ya, ZHOU Wen-jin, LIANG Yi. Removal of cationic dyes from aqueous solution using magnetic multi-wall carbon nanotube nanocomposite as adsorbent [J]. Journal of Hazardous Materials, 2009, 16: 1517-1522.
[7] GOPAL M, PAKSHIRAJAN K, SWAMINATHAN T. Heavy metal removal by biosorption using Phanerochaete chrysosporium [J]. Applied Biochemistry and Biotechnology, 2002, 102: 227-237.
[8] SAY R, DENIZLI A, ARICA M Y. Biosorption of cadmium (II), lead (II) and copper (II) with the filamentous fungus Phanerochaete chrysosporium [J]. Bioresource Technoloy, 2001, 76: 67-70.
[9] HUANG Dan-lian, ZENG Guang-ming, JIANG Xiao-yun, FENG Chong-ling, YU Hong-yan, HUANG Guo-he, LIU Hong-liang. Bioremediation of Pb-contaminated soil by incubating with Phanerochaete chrysosporium and straw [J]. Journal of Hazardous Materials, 2006, 134: 268-276.
[10] KALPANA D, SHIM J H, OH B T, SENTHIL K, LEE Y S. Bioremediation of the heavy metal complex dye Isolan Dark Blue 2SGL-01 by white rot fungus Irpex lacteus [J]. Journal of Hazardous Materials, 2011, 19: 198-205.
[11] NIES D H. Heavy metal-resistant bacteria as extremophiles: Molecular physiology and biotechnological use of Ralstonia sp. CH34 [J]. Extremophiles, 2000, 4: 77-82.
[12] SCHMIDT A, HAFERBURG G, SCHMIDT A, LISCHKE U, MERTEN D, GHERGEL F,  G, KOTHE E. Heavy metal resistance to the extreme: Streptomyces strains from a former uranium mining area [J]. C1hemie der Erde-Geochemistry, 2009, 69: 35-44.
G, KOTHE E. Heavy metal resistance to the extreme: Streptomyces strains from a former uranium mining area [J]. C1hemie der Erde-Geochemistry, 2009, 69: 35-44.
[13] HUANG Dan-lian, ZENG Guang-ming, FENG Chong-ling, HU Shuang, ZHAO Mei-hua, LAI Cui, ZHANG Yu, JIANG Xiao-yun, LIU Hong-liang. Mycelial growth and solid-state fermentation of lignocellulosic waste by white-rot fungus Phanerochaete chrysosporium under lead stress [J]. Chemosphere, 2010, 81: 1091-1097.
[14] LIU Qian, YANG Hong-ying, TONG Lin-lin. Influence of Phanerochaete chrysosporiumon degradation and preg-robbing capacity of activated carbon [J]. Transactions of Nonferrous Metals Society of China, 2014, 24(6): 1905-1911.
[15] BALDRIAN P. Interactions of heavy metals with white-rot fungi [J]. Enzyme and Microbial Technology, 2003, 32: 78-91.
[16] HATVANI N,  I. Effects of certain heavy metals on the growth, dye decolorization, and enzyme activity of Lentinula edodes [J]. Ecotoxicology and Environmental Safety, 2003, 55: 199-203.
I. Effects of certain heavy metals on the growth, dye decolorization, and enzyme activity of Lentinula edodes [J]. Ecotoxicology and Environmental Safety, 2003, 55: 199-203.
[17] GALLEGO S M, PENA L B, BARCIA RA, AZPILICUETA C E, IANNONE M F, ROSALES E P, ZAWOZNIK M S, GROPPA M D, BENAVIDES M P. Unravelling cadmium toxicity and tolerance in plants: Insight into regulatory mechanisms [J]. Environmental and Experimental Botany, 2012, 83: 33-46.
[18] KIYONO M, MIYAHARA K, SONE Y, PAN-HOU H, URAGUCHI S, NAKAMURA R, SAKABE K. Engineering expression of the heavy metal transporter MerC in Saccharomyces cerevisiae for increased cadmium accumulation [J]. Applied Microbiology and Biotechnology, 2010, 86: 753-759.
[19] XU Qing-song, MIN Hai-li, CAI San-juan, FU Yong-yang, SHA Sha, XIE Kai-bin, DU Kai-he. Subcellular distribution and toxicity of cadmium in Potamogeton crispus L [J]. Chemosphere, 2012, 89: 114-120.
[20] WANG Chun-lin, LIU Yun-guo, ZENG Guang-ming, HU Xin-jiang, YING Yi-cheng, HU Xi, ZHOU Lu, WANG Ya-qin, LI Hua-ying. Mechanism of exogenous selenium alleviates cadmium induced toxicity in Bechmeria nivea (L.) Gaud (Ramie) [J]. Transactions of Nonferrous Metals Society of China, 2014, 24(12): 3964-3970.
[21] CAI Z P, DOREN J, FANG Z Q, LI W S. Improvement in electrokinetic remediation of Pb-contaminated soil near lead acid battery factory [J]. Transactions of Nonferrous Metals Society of China, 2015, 25(9): 3088-3095.
[22] GILLER K E, WITTER E, MCGATH S P. Toxicity of heavy metals to microorganisms and microbial processes in agricultural soils: A review [J]. Soil Biology & Biochemistry, 1998, 30: 1389-1414.
[23] TIEN M, KIRK T K. Lignin-degrading enzyme from the hymenomycete Phanerochaete chrysosporium Burds [J]. Science, 1983, 221: 661-662.
[24] WANG Hai-jun, JIA Xing-huan, SHENG Lian-xi, LI Wei-guo, FU Wei-jie. The effect of environmental factors to mycelia growing of Tricholoma matsutake [J]. Journal of Northeast Normal University, 2005, 37(3): 85-89. (in Chinese)
[25] TANAKA H, KOIKE K, ITAKURA S, ENOKI A. Degradation of wood and enzyme production by Ceriporiopsis subvermispora [J]. Enzyme and Microbial Technology, 2009, 45: 384-390.
[26] LOPEZ M J,  M D C,
M D C, F, NICHOLS N N, DIEN B S, MORENO J. Lignocellulose-degrading enzymes produced by the ascomycete Coniochaeta ligniaria and related species: Application for a lignocellulosic substrate treatment [J]. Enzyme and Microbial Technology, 2007, 40: 794-800.
F, NICHOLS N N, DIEN B S, MORENO J. Lignocellulose-degrading enzymes produced by the ascomycete Coniochaeta ligniaria and related species: Application for a lignocellulosic substrate treatment [J]. Enzyme and Microbial Technology, 2007, 40: 794-800.
[27]  A. Metal ion uptake by mushrooms from natural and artificially enriched soils [J]. Food Chemistry, 2002, 78: 89-93.
A. Metal ion uptake by mushrooms from natural and artificially enriched soils [J]. Food Chemistry, 2002, 78: 89-93.
[28] TUZEN M, SESLI E, SOYLAK M. Trace element levels of mushroom species from east black sea region of turkey [J]. Food Control, 2007, 18: 806-810.
[29] FALIH A M. Influence of heavy-metals toxicity on the growth of Phanerochaete chrysosporium [J]. Bioresource Technology, 1997, 60: 87-90.
[30] JOMOVA K, VALKO M. Advances in metal-induced oxidative stress and human disease [J]. Toxicology, 2011, 283: 65-87.
[31] MORA A P D, ORTEGA-CALVO J J, CABRERA F, . Changes in enzyme activities and microbial biomass after “in situ” remediation of a heavy metal-contaminated soil [J]. Applied Soil Ecology, 2005, 28: 125-137.
. Changes in enzyme activities and microbial biomass after “in situ” remediation of a heavy metal-contaminated soil [J]. Applied Soil Ecology, 2005, 28: 125-137.
[32] STOHS S, BAGCHI D. Oxidative mechanisms in the toxicity of metal ions [J]. Free Radical Biology and Medicine, 1995, 18: 321-336.
[33] LI Qing-biao, WU Song-tao, LIU Gang, LIAO Xin-kai, DENG Xu, SUN Dao-hua, HU Yue-lin, HUANG Yi-li. Simultaneous biosorption of cadmium (II) and lead (II) ions by pretreated biomass of Phanerochaete chrysosporium [J]. Separation and Purification Technology, 2004, 34: 135-142.
重金属对黄孢原毛平革菌的毒性及其生物积累
赵美花1,张朝升1,曾光明2,黄丹莲2,程 敏2
1. 广州大学 土木工程学院,广州 510006;
2. 湖南大学 环境科学与工程学院,长沙 410082
摘 要:研究平板和液态培养过程中黄孢原毛平革菌的生长代谢活动对镉、铅单一污染及其复合污染胁迫毒性的响应。测定黄孢原毛平革菌的菌落直径、微生物量、木质素降解酶活性及其对重金属的生物积累量。结果显示,在这两种培养条件下镉比铅对黄孢原毛平革菌的毒性大,且镉-铅复合污染会加强这种毒性。在液态培养条件下,重金属镉和铅通过直接影响真菌生长、代谢或其他方式间接影响黄孢原毛平革菌产木质素降解酶的能力。此外,结果证明黄孢原毛平革菌菌丝体能够积累去除部分镉和铅。
关键词:黄孢原毛平革菌;重金属;镉;铅;毒性;生物积累;木质素降解酶
(Edited by Wei-ping CHEN)
Foundation item: Projects (21477027, 51278176) supported by the National Natural Science Foundation of China; Project (2014A020216048) supported by the Science and Technology Planning Project of Guangdong Province, China; Project (2015M582363) supported by the China Postdoctoral Science Foundation
Corresponding author: Chao-sheng ZHANG; Tel: +86-20-39366961; E-mail: gdzcs@gzhu.edu.cn
DOI: 10.1016/S1003-6326(16)64245-0
Abstract: The responses of the growth and metabolism activity of Phanerochaete chrysosporium (P. chrysosporium) to cadmium (Cd), lead (Pb) and their combined pollution stress, were investigated in plate and liquid culture conditions. The diameter of colony, biomass of P. chrysosporium, ligninolytic enzyme activities and bioaccumulation quantity of heavy metals were detected. The results indicated that Cd was more toxic than Pb to P. chrysosporium and the toxicity of Cd and Pb to P. chrysosporium was further strengthened under Cd+Pb combined pollution in different culture conditions. Heavy metals Cd and Pb had indirect influence on the production of ligninolytic enzymes by directly affecting the fungal growth and metabolic activity, and by another way in liquid culture. In addition, the results provided an evidence of the accumulation of Cd and Pb on the mycelia of P. chrysosporium.


