Trans. Nonferrous Met. Soc. China 29(2019) 1353-1364
Individual and synergistic effect of gamma alumina (γ-Al2O3) and strontium on microstructure and mechanical properties of Al-20Si alloy
Mihira ACHARYA, Animesh MANDAL
School of Minerals, Metallurgical and Materials Engineering, Indian Institute of Technology Bhubaneswar, Argul 752050, Odisha, India
Received 14 September 2018; accepted 12 March 2019
Abstract:
An optimized combination of gamma alumina (4 wt.%) and strontium (0.1 wt.%) was incorporated in cast Al-20Si alloy to obtain fine form of silicon. During casting process, the amount of γ-Al2O3 was varied from 0.5-6 wt.% to refine primary Si and Sr was varied from 0.05-0.1 wt.% to modify eutectic Si. The results showed that the average size of primary Si is 24 μm for addition of 4 wt.% γ-Al2O3 to the alloy whereas 0.1 wt.% Sr resulted in sphericity of eutectic Si to ~0.6 and average length of ~1.2 μm. The thermal analysis revealed that γ-Al2O3 can act as potential heterogeneous nucleation sites. Moreover, simultaneous addition of γ-Al2O3 and Sr does not poison γ-Al2O3 particles and inhibit their nucleation efficiency as in the case of combined addition of phosphorous and strontium to Al-20Si alloy. Therefore, it was concluded that enhanced tensile strength, i.e., ultimate tensile strength (increase by 20%) and elongation (increase by 23%) in Al-20Si-4γ-Al2O3-0.1wt.%Sr alloy as compared to as-cast Al-20Si alloy can be attributed to refinement of primary Si, modification of eutectic Si and the presence of α(Al) in the alloy as evident from eutectic shift.
Key words:
Al-Si alloy; γ-Al2O3; refinement; modification; primary Si;
1 Introduction
Hypereutectic Al-Si alloys are the preferred choice for aircraft and automobile industry due to their light weight and high specific strength [1]. These properties led to increased fuel economy and thus better vehicular emission standard. They have specific applications for automobile components subjected to continuous wear environment. Besides, hypereutectic Al-Si alloys exhibit excellent properties such as good corrosion resistance, high thermal conductivity [2] and low thermal coefficient of expansion [3]. These properties are regulated by the microstructure of the alloys. The typical microstructure of hypereutectic Al-Si alloy consists of coarse primary Si and relatively fine eutectic phases of eutectic Si and eutectic α(Al). The primary Si exists in various shapes such as star-like, polygonal, feathery types whereas eutectic Si appears in an acicular structure in the microstructure [4]. It is worth noting that the quantity and distribution of these phases in the microstructure dictate the properties of the alloy. The presence of primary Si with sharp edges leads to stress concentration with the application of load during service conditions [5]. The crack initiates near the edges and further propagates resulting in premature failure of the component. Moreover, the size and distribution of phases in hypereutectic Al-Si alloys influence the wear resistance of the alloy [6]. Hence, the refinement of primary Si, as well as eutectic Si, is crucial in achieving enhanced mechanical and tribological properties in such alloys.
Several investigations have been carried out to alter the morphology of primary Si in hypereutectic Al-Si alloys by friction stir processing [7], rapid solidification processing, electromagnetic stirring [8] and semi-solid processing [9,10]. Powder metallurgy is also another popular route for incorporating ceramic reinforcements in the alloy and composites. However, the inability to produce intricate shapes limits its industrial applications [11]. The size of the product and complex shape are the primary reasons for not implementing it for mass production. As compared to powder metallurgy, better particle matrix bonding and near net shape can be achieved with liquid metallurgy route [12,13]. The liquid state processing is perceived as the better method for mass production as compared to powder metallurgy route. Chemical modification, a part of melt processing, remains the preferred method due to its simplicity and cost-effectiveness [14]. STERNER-RAINER [15] opted the chemical modification route by incorporating phosphorous in hypereutectic Al-Si alloys. He found that phosphorous forms AlP particles which act as nucleation sites and thereby effectively reduce the size of primary Si. Consequently, the mechanical and tribological properties of the alloy are improved [16]. Later, it was reported that rare earth elements like Ce [17], Sc [18], Nd [19] and Er [20] can also refine primary Si in hypereutectic Al-Si alloy. The different size reduction of primary Si for hypereutectic binary alloys reported in prior literatures has been mentioned in Table 1.
Table 1 Effect of refining elements on size reduction of primary Si in hypereutectic Al-Si alloys
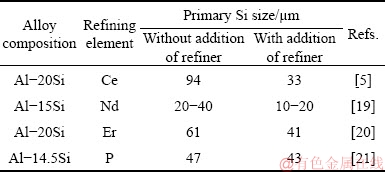
Table 1 shows that the minimum primary silicon size can be obtained by adding different elements to Al-20Si alloy is 33 μm. There has been a lot of research work carried out to further reduce the size of primary Si in hypereutectic alloys. Recently, few studies have been conducted to investigate the effect of γ-Al2O3 particles on Al-Si alloys [22-24]. CHOI and LI [23] found that γ-Al2O3 particles (~50 nm), when dispersed in Al-20Si- 4.5Cu alloy using ultrasonic cavitation technique, refine both primary Si and eutectic Si, though the modification of eutectic Si is not very clear from the microstructures. In one of the study, LI et al [24] investigated the effect of in-situ γ-Al2O3 particles synthesized by decomposition of aluminium ammonium sulphate [NH4Al(SO4)2] powder in molten aluminium alloy. They showed that 0.8 wt.% of in-situ γ-Al2O3 formed by decomposition of NH4Al(SO4)2 in Al-20Si alloy is effective in refining primary Si and eutectic Si alike. However, there is no significant modification of eutectic Si observed as reported in the study. Therefore, it can be seen that there is no clear consensus on the refining ability of γ-Al2O3 particles on Al-Si alloys. Meanwhile, Sr which is conventionally used in the form of Al-Sr master alloy for modification of eutectic Si is well documented in Ref. [25]. Further, attempts were made to modify both eutectic and primary Si using P and Sr to hypereutectic alloy [26]. However, the effective refining efficiency reduces as P poisons the modifying effect of Sr in the alloy [26,27]. Therefore, γ-Al2O3 can be considered as the alternative of P for the present study as it has no reactivity with Sr at high temperature. Based on the existing literatures, two main objectives of the present work are: (1) to investigate the effect of γ-Al2O3 and Sr on the microstructure of Al-20Si alloy by adding them individually and simultaneously; (2) to study the mechanical properties for the optimized amount of γ-Al2O3 and Sr and correlate with the microstructure.
2 Experimental
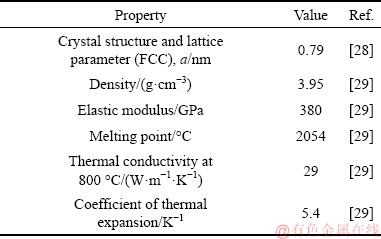
In the present work, Al-20Si hypereutectic alloy was prepared by melting together commercial pure Al (CPAl) and Al-50wt.%Si master alloy in the appropriate ratio. The melt was prepared in a clay graphite crucible heated in a resistance furnace at 800 °C. After complete melting, commercially available γ-Al2O3 particles (white and spherical in shape) of size, 40-60 μm (Fig. 1(a)) was added to Al-20Si alloy. The γ-Al2O3 phase was ensured from the XRD pattern as shown in Fig. 1(b). The melt was held for 20 min after addition of γ-Al2O3 particles with intermittent stirring every 10 minutes. Thereafter, 1 wt.% of C2Cl6 was added to the melt and stirred for 1 min for complete degassing of the melt. It was then cast in a split type graphite mould (2.5 cm in diameter, 4 cm in height) preheated at 200 °C for 2 h. Table 2 shows the properties of γ-Al2O3 particles. Four different addition levels (0.5, 2, 4 and 6 wt.%) of γ-Al2O3 were added to Al-20Si alloy in order to study its effect on refinement of primary Si.
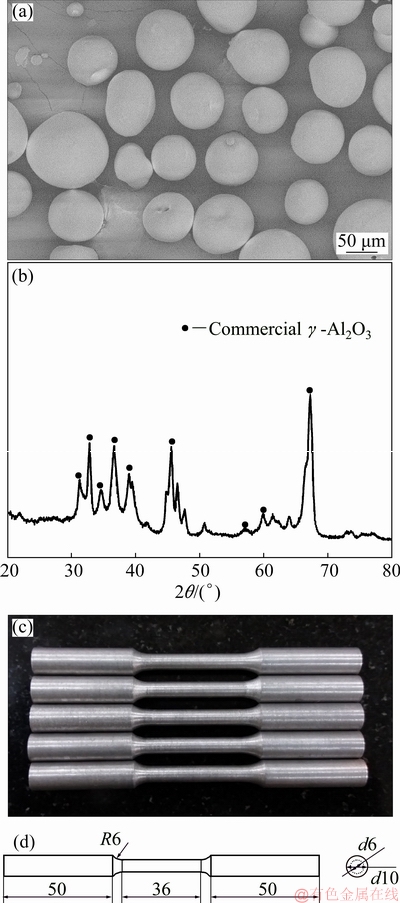
Fig. 1 FESEM micrograph of γ-Al2O3 particles (a), XRD pattern of commercial γ-Al2O3 particles (b), standard tensile sample (c) and dimensions of tensile testing samples (d) (unit: mm)
Table 2 Crystal structure and properties of γ-Al2O3
The castings obtained were then sectioned and hot mounted for metallography. In order to ensure an identical cooling rate of samples, alloys were sectioned from identical locations in all alloys. The samples were polished according to the standard metallographic procedure and then etched with Keller’s reagent (95 mL water, 2.5 mL HNO3, 1.5 mL HCI, 1.0 mL HF) to reveal the microstructure. The microstructural features of Al-20Si-xwt.%γ-Al2O3 alloys (x=0, 0.5, 2, 4, 6) were characterized with particular reference to primary Si and eutectic Si using optical microscope (LEICA, DMI 3000M) and field emission scanning electron microscopy (Zeiss, Merlin Compact) with EDX facility (Oxford instruments, 51-XMX1004). The particle size analysis of primary Si was carried out by ImageJ software (1.46 r) from optical micrographs. The thermal analysis was also carried out for the as-cast alloy and the alloy for which maximum refinement of primary Si was observed. The DSC test was conducted to find the nucleation kinetics at four different heating rates (5, 10, 15 and 20 K/min) in a nitrogen atmosphere. The mass of the samples was kept under 20 mg for each experiment. Later, the activation energies were calculated based on the above mentioned input parameters. Similarly, optimum mass fraction of Sr was obtained by adding different amounts of Sr (0.05, 0.07 and 0.1 wt.%) in the form of Al-10wt.%Sr master alloy. The combined effect of Sr and γ-Al2O3 on the microstructure of Al-20Si was also studied by adding the optimized amount of γ-Al2O3 and Sr. For investigation of mechanical properties, the samples were prepared by casting in a split type mild steel mould with 15 mm in diameter and 150 mm in height. The standard tensile samples were then prepared by machining according to ASTM E8 standard as shown in Fig. 1(c). Tensile properties of all the alloys were measured in a universal testing machine (Tinius Olsen H50KS) at a constant cross-head speed of 0.3 mm/min.
3 Results and discussion
3.1 Microstructure of Al-20Si alloys with addition of γ-Al2O3
Figure 2(a) shows the typical microstructure of as-cast Al-20Si alloy with coarse primary Si particles of varied morphology surrounded by α(Al) dendrites and needle/plate like eutectic Si particles. It is evident that the morphology of the majority of primary Si is coarse polygonal, star-like and irregular for most of the particles. The present results are in agreement with Ref. [30] where it is was reported that at a pouring temperature below 850 °C, irregular primary Si is formed. A comparison between Figs. 2(a) and (b) shows that the addition of 0.5 wt.% γ-Al2O3 results in refinement of primary Si. It can be attributed to the fact that the crystal structures of Si and γ-Al2O3 are similar and the lattice parameter mismatch between Si and γ-Al2O3 is about 3% [23]. Also, according to Ref. [31], effective nucleation occurs for less than 6% lattice mismatch. Thus, γ-Al2O3 acts as favourable heterogeneous nucleation sites for primary Si during solidification of the alloy. When the amount of γ-Al2O3 is increased to 2 wt.%, there is a significant decrease in the size of primary Si and the star-shaped and irregular shaped primary Si particles were transformed to blocky shaped particles (Fig. 2(c)). With further increment to 4 wt.% γ-Al2O3 addition, the size of primary Si seems to decrease significantly with a major fraction of them being blocky shape. Moreover, it can be observed from Fig. 2(a) that the as received alloy has many irregular primary silicon particles. It is worth noting that the addition of γ-Al2O3 results in fine and blocky shape primary silicon distributed in the matrix. Further, increase in γ-Al2O3 results in coarsening of primary Si in the binary alloy which is evident from Fig. 2(e). Therefore, it can be concluded that there seem to be a critical amount of γ-Al2O3 beyond which no further refinement of primary Si is observed. Similarly, results were also obtained for in-situ formed γ-Al2O3 which showed a threshold beyond which there was no further refinement of primary Si [24]. A higher amount of γ-Al2O3 could lead to higher agglomeration probability and thereby significantly reducing the effective number of sites available for nucleation of primary Si. Therefore, the size of the primary Si increases abruptly for 6 wt.% γ-Al2O3 addition to Al-20Si alloy. Moreover, the volume fraction of porosity increases at a higher level of γ-Al2O3 addition which has a detrimental effect on the mechanical properties of the binary Al-20Si alloy. The increase in porosity can be attributed to the nucleation of pores at a higher mass fraction of γ-Al2O3 acting as potent nucleation sites for pore formation [32-34]. This could explain the abrupt coarsening of primary Si as well as porosity in the alloy containing 6 wt.% γ-Al2O3 particles.
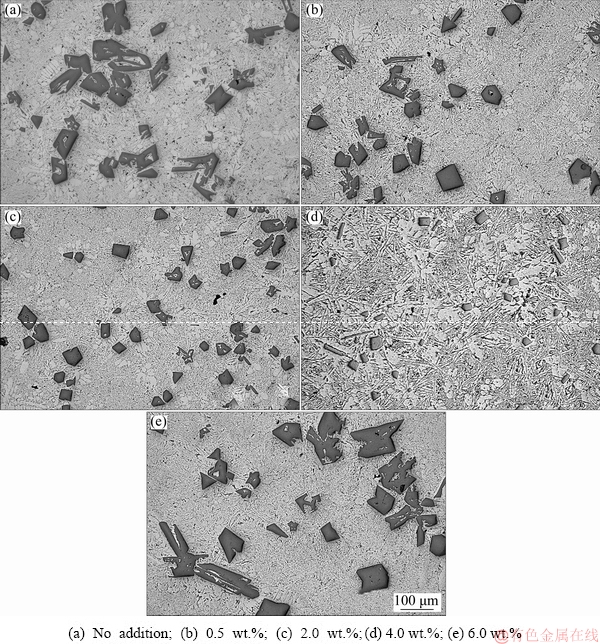
Fig. 2 Optical microstructures of Al-20wt.%Si alloys with different γ-Al2O3
EDS was also carried out on the particle in the vicinity of primary Si as shown in Fig. 3. The peaks of elements Al and O in Fig. 3(b) ensure the presence of γ-Al2O3 in the alloy. The result shows that γ-Al2O3 particles having smaller lattice mismatch could act as heterogeneous nucleation sites for the primary Si, which is in good agreement with Ref. [24].
3.2 Particle size of primary Si in alloys
Figure 4 depicts the size distribution of primary Si and the average size of primary Si in Al-20Si alloy as a function of different γ-Al2O3 additions. In Al-20Si alloy, the majority of primary Si lies in the range of 60-100 μm (Fig. 4(a)). Addition of 0.5 wt.% γ-Al2O3 particles narrows down the size distribution of primary Si to 20-60 μm (Fig. 4(b)). Further, increase in γ-Al2O3 to 2 and 4 wt.% narrows down the size of the primary Si particles (Figs. 4(c) and (d)). The wide particle size distribution of primary Si in Fig. 4(e) corroborates well with the microstructure in Fig. 2(e).
The average particle size of the primary Si in Al-20Si alloy was found to be ~83 μm. The addition of 0.5 wt.% γ-Al2O3 reduces the size of primary Si to~42 μm. Increase in amount of γ-Al2O3 results in significant reduction in the size of primary Si with the minimum size being ~24 μm for 4 wt.% γ-Al2O3. Further, an increase in the amount to 6 wt.% γ-Al2O3 results in an increase in average size of primary Si to ~54 μm due to the reasons explained in the previous section.
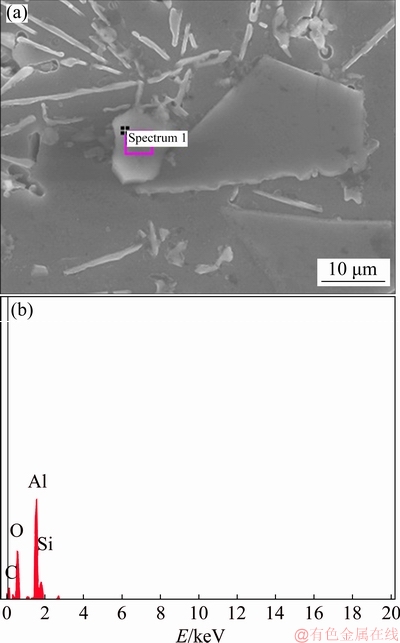
Fig. 3 FESEM micrograph of Al-20Si-4γ-Al2O3 (a) and corresponding EDS spectrum (b)
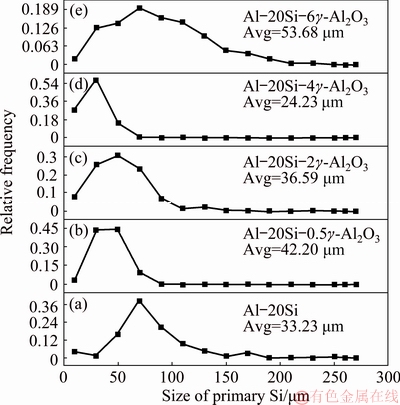
Fig. 4 Particle size distribution of primary Si in Al-20wt.%Si alloys with different γ-Al2O3
3.3 Shifting of eutectic point with γ-Al2O3 addition
The Al-Si system exhibits eutectic point at 12.6 wt.% Si under the equilibrium condition. The possible explanation for the appearance of α(Al) dendrites shown in Fig. 2(d) can be explained by quantifying the shift in eutectic point in hypereutectic Al-Si alloy as per the empirical relationship established by JIANG et al [35] in non-equilibrium solidification for Al-20Si alloy with Y2O3 addition. The eutectic shift under non-equilibrium solidification condition (△CE) can be calculated from the following formula [35]:
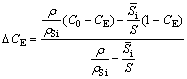 (1)
(1)
where ρ is the density of hypereutectic Al-20Si alloy (2627 kg/m3); ρSi is the density of silicon (2330 kg/m3); C0 is the Si content of hypereutectic Al-Si alloy (20 wt.%); CE is the Si content at the eutectic point (12.6 wt.%); 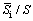 is the area fraction determined using image analysis. The number in parenthesis indicates value used in the present study.
is the area fraction determined using image analysis. The number in parenthesis indicates value used in the present study.
The eutectic point shifts according to varying mass fractions of γ-Al2O3 in Al-20Si alloy and is summarized in Table 3.
Table 3 Area fraction of primary Si and eutectic shift in Al-20Si alloy with different addition levels of γ-Al2O3
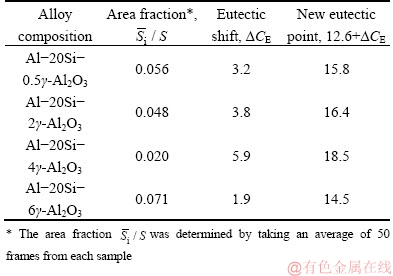
For non-equilibrium solidification of Al-20Si alloy, the eutectic point shifts by △CE towards the right of the equilibrium phase diagram. The eutectic shift can be correlated with the quantitative analysis of micrographs in Fig. 2. Microstructure shows slight coarsening of α(Al) and increase in its fraction is observed as compared to 2 wt.% γ-Al2O3 addition (Fig. 2(d)). It can be attributed to the maximum eutectic shift which occurs with 4 wt.% γ-Al2O3 addition because of the increase in solubility of Si in Al. The eutectic shift and area fraction of primary Si have an inverse relationship with each other [35]. The area fraction for maximum eutectic shift occurs for 4 wt.% addition level of γ-Al2O3 to the alloy. The new eutectic point is 18.5 wt.% of Si which is evident from Table 3. The maximum shift in eutectic point for the binary alloy also explains the coarsening of α(Al) dendrites as evident from Fig. 2(d). However, the eutectic shift is the least for the incorporation of 6 wt.% γ-Al2O3 into Al-20Si alloy and as a consequence coarse primary Si particles are observed in Fig. 2(e).
3.4 Thermal analysis
The thermal analysis was performed using differential scanning calorimetry (DSC) to get further insight into the impact of γ-Al2O3 on activation energy and heterogeneous nucleation in Al-20Si alloy. The activation energy of Al-20Si alloy and γ-Al2O3 treated alloys were evaluated from DSC curves. Temperature scan was carried out from 30 to 800 °C during the heating of samples. The plots were obtained for four different constant heating rates, i.e., 5, 10, 15, 20 K/min and Kissinger equation was applied thereafter at peak temperatures with the following equation [36].
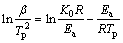 (2)
(2)
where β is the heating rate, Tp is the peak temperature in DSC curve, R is the gas constant, Ea is activation energy and K0 is the coefficient. The peak temperatures at all heating rates were found out from Fig. 5. The plot between ln(β/Tp2) and 1/Tp is presented in Fig. 6. It shows the variation of peaks at different heating rates.
Linear relationships were established for both Al-20Si and g-Al2O3 treated alloy. After fitting the four points onto a straight line, the slope (-Ea/R) of the plot was determined for both alloys and activation energies for the plots were calculated. The gradients of the straight lines were obtained for both the lines as shown in Fig. 6. The activation energies of the alloys are calculated as 672.09 and 609.90 kJ/mol respectively by taking R=8.314 J/(mol·K).
It is evident that there is a decrease in the activation energy with 4 wt.% γ-Al2O3 addition to Al-20Si alloy. As the activation energy is less for γ-Al2O3 treated alloy, the nucleation frequency of the primary Si is more as compared to as-cast Al-20Si alloy. This indicates that the refinement of primary silicon is possible due to g-Al2O3 particles which act as potent heterogeneous nucleation sites during the solidification process.
3.5 Eutectic silicon growth with different addition levels of Sr
The addition level of Sr plays an important role in the modification of eutectic Si in Al-20Si hypereutectic alloy. While the optimum amount of Sr required for complete modification of eutectic Si in hypoeutectic Al-Si alloys is well documented (~0.025 wt.% Sr) in Refs. [37,38], there exist very few literatures mentioning the optimum amount of Sr needed to modify the eutectic Si in high silicon-containing hypereutectic Al-Si alloys. When 0.025 wt.% Sr was incorporated into Al-20Si alloy, no significant modification of eutectic Si is observed in Fig. 7.
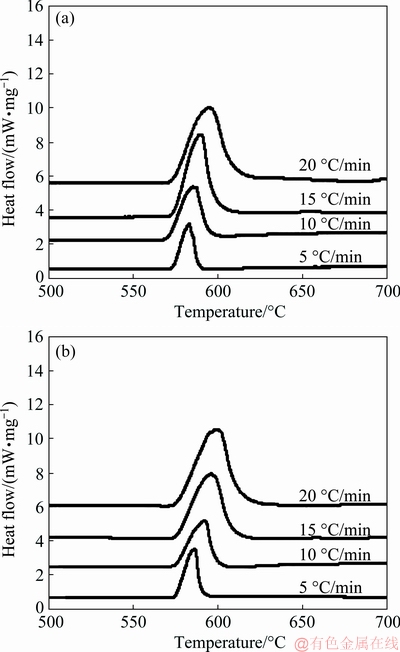
Fig. 5 DSC curves at different heating rates for Al-20Si (a) and Al-20 Si-4γ-Al2O3 (b)
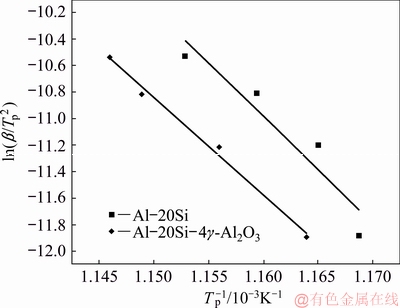
Fig. 6 ln (β/Tp2) vs 1/Tp plot using peak temperature and heating rate
Therefore, higher levels of Sr (i.e., 0.05, 0.07 and 0.1 wt.%) were incorporated into the alloy in order to achieve optimum modification of eutectic Si in Al-20Si alloy. When 0.05 wt.% Sr is added to Al-20Si alloy Fig. 8(a), coarse plate-like structure of eutectic Si persists in the microstructure as can be seen in the inset. However, as the addition level of Sr is increased to 0.07 wt.%, eutectic Si is partially modified in certain regions which can be observed in the microstructure (Fig. 8(b)). It has been reported that Sr effectively modifies eutectic Si by twin plane re-entrant edge (TPRE) mechanism in which the growth of eutectic Si occurs by twinning in {111} planes during solidification [39]. The external angle at re-entrant edges favours the nucleation and growth of Si in <112> direction. When Sr is added, it is adsorbed at the re-entrant edges and hinders the growth in <112> direction [40]. The isotropic growth in different <112> directions enables the eutectic Si to appear in fibrous form instead of plate-like structure attributed to anisotropic growth as mentioned in the previous work [41].
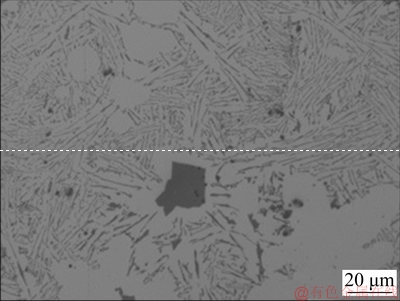
Fig. 7 Optical micrograph of Al-20Si-0.025Sr alloy
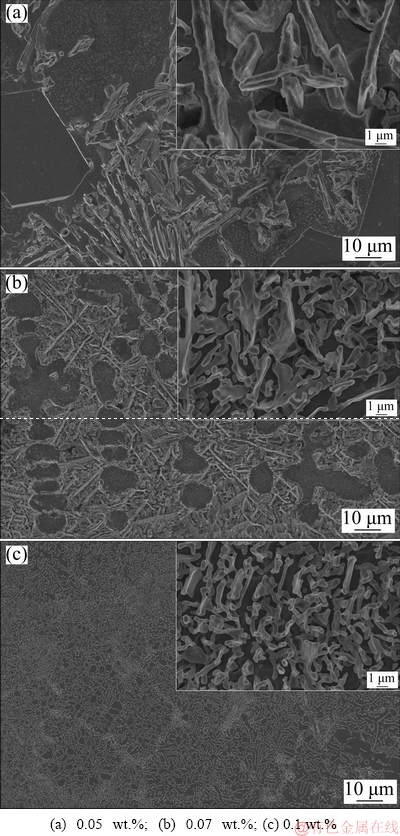
Fig. 8 FESEM micrographs of Al-20Si alloy with different additions of Sr
Figure 9 shows the three-dimensional morphology of eutectic Si with the addition of Sr in the Al-20Si alloy. It can be noted that the reentrant corner which forms 141° angle has been transformed into the ridge. The bounding planes make an angle of 219° externally to form the ridge as shown in Fig. 9. Consequently, the growth of eutectic silicon along <112> direction is stopped. A stepped boundary can be observed at the centre of the eutectic silicon particle indicating multiple twinning [42] and thus restricting the growth of {111}Si. On further increasing the Sr content to 0.1 wt.% Sr, the eutectic modification of Si was found to be uniform throughout the microstructure. The presence of coral-like structure in the inset is quite evident from Fig. 8(c). Hence, no further increase in Sr addition to Al-20Si alloy and 0.1 wt.% Sr was chosen for further investigation. In addition, it was observed that there is no notable change in the size and morphology of primary Si with the addition of Sr in all of the above cases.
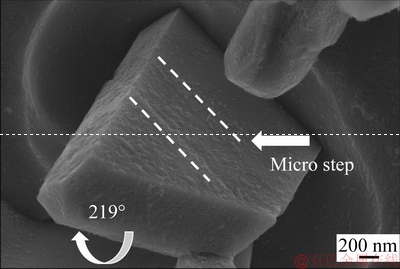
Fig. 9 Eutectic Si morphology with addition of Sr
3.6 Quantitative estimation of modification of eutectic Si
The extent of modification has been estimated by determining the change in length, width and sphericity/shape factor ( SF=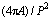 ) [43] of eutectic Si for three different addition levels of Sr as shown in Table 4. It is evident that the average length of eutectic Si decreases from 8.26 μm in the alloy with 0.05 wt.% Sr to 1.18 μm in the alloy containing 0.1 wt.% Sr. A corresponding reduction in width of eutectic Si is observed with addition of Sr. Also, the sphericity is found to be increased up to 0.59, which is beneficial for achieving better mechanical properties. Therefore, it can be deduced that the incorporation of 0.1 wt.% Sr leads to providing optimum modification effect in Al-20Si alloy.
) [43] of eutectic Si for three different addition levels of Sr as shown in Table 4. It is evident that the average length of eutectic Si decreases from 8.26 μm in the alloy with 0.05 wt.% Sr to 1.18 μm in the alloy containing 0.1 wt.% Sr. A corresponding reduction in width of eutectic Si is observed with addition of Sr. Also, the sphericity is found to be increased up to 0.59, which is beneficial for achieving better mechanical properties. Therefore, it can be deduced that the incorporation of 0.1 wt.% Sr leads to providing optimum modification effect in Al-20Si alloy.
Table 4 Variation in size and sphericity of eutectic Si with Sr addition to Al-20Si alloy

3.7 Microstructure analysis with optimized addition of γ-Al2O3 and Sr to Al-20Si alloy
Figure 10 shows that the simultaneous addition of γ-Al2O3 and Sr refines primary Si to polyhedral shape and modifies eutectic Si to coral-like morphology respectively. This is possible only when there is no or minimum interaction of γ-Al2O3 and Sr with each other to nullify the effect. As mentioned, the low lattice mismatch of 3% between primary Si and γ-Al2O3 promotes heterogeneous nucleation of primary Si on γ-Al2O3 particles and thereby refining them [44].
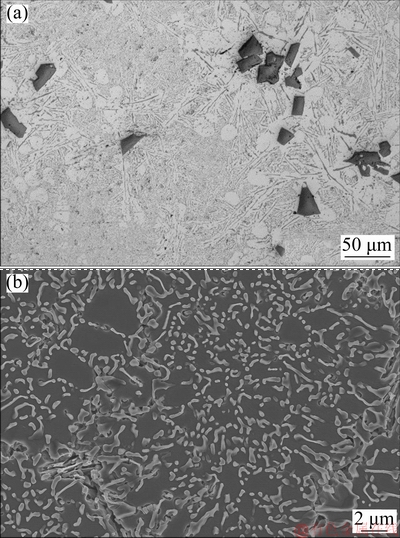
Fig. 10 Optical micrograph (a) and FESEM micrograph (b) of Al-20Si-4γ-Al2O3-0.1Sr alloy
From the previous sections, the optimized amount of γ-Al2O3 (4 wt.%) and Sr (0.1 wt.%) was chosen to refine primary Si and modify eutectic Si respectively in Al-20Si alloy. In this regard, it would be worth mentioning that when P and Sr are added to hypereutectic Al-Si alloys simultaneously, Sr3P2 may be formed leading to reduction in effective modification efficiency as well as refinement as reported earlier [26]. It is expected that simultaneous addition of γ-Al2O3 and Sr would not interfere with each other in negating the effectiveness towards the refinement of the microstructure. It has been reported that in a smaller quantity of melts, the rate of oxidation of Sr is very high resulting in a detrimental effect on the modification of eutectic Si [45]. In the case of a larger quantity of melts, oxidation of Sr does not occur even after longer holding time with 0.1 wt.% Sr addition to the melt due to the lower surface area to volume ratio. In the present study, approximately 1 kg of melt (large quantity melt) was prepared for each casting, which ensures the better chemical stability of Sr and no deleterious interaction between γ-Al2O3 and Sr.
3.8 Tensile properties and fractography of Al-20Si alloys
The tensile properties of Al-20Si alloy were studied. Also, the effects of individual and combined addition of γ-Al2O3 and Sr on Al-20Si alloy were studied. Table 5 summarizes the tensile properties of the alloys studied.
Table 5 Tensile properties of as-cast Al-20Si alloy with addition of γ-Al2O3 and Sr
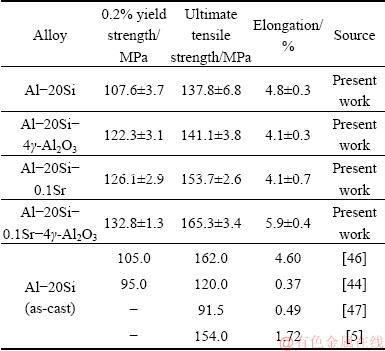
Addition of 4 wt.% γ-Al2O3 leads to almost 14% in yield strength with a slight drop in ductility. Likewise, the addition of 0.1 wt.% Sr to Al-20Si alloy results in 17% increase in YS and 12% increase in UTS and YS with a slight drop in ductility. The increase in strength can be attributed to modification of eutectic Si. While the drop in ductility could be possibly due to an increase of porosity in the castings at higher Sr contents. It is well known that Al-Si alloys treated with high amounts of Sr result in porosity of the castings [48-52]. The enhancement in porosity decreases the effective load bearing area during application of tensile load. As a result, it induces stress concentration nearby the pore which in turn reduces the UTS of the material. It is interesting to note that the simultaneous addition of γ-Al2O3 and Sr optimized previously results in significant increase in UTS and YS as compared to Al-20Si alloy. This is due to the refinement of primary Si and modification of eutectic silicon and due to dispersion hardening by γ-Al2O3 particles. The significant increase in ductility of the alloy (5.9%) can be attributed to the higher fraction of soft and ductile α(Al) dendrites (Fig. 10 (a)) in the microstructure coupled with finer Si (both primary and eutectic). A similar increase in ductility was also reported in Al-20Si-4.5Cu treated with 0.5 wt.% of 50 nm γ-Al2O3 particles [23].
Figure 11 shows the FESEM fractographs of tensile fracture surfaces. The presence of numerous cleavage planes and cracks in Figs. 11(a, b) is suggestive of brittle fracture in Al-20Si alloy. The initiation of cracks generally occurs on blocky and brittle primary Si particles and propagates through the weak α(Al)-Si interface causing failure by linking up with other cracks. Figure 11(d) shows that primary Si particles have been de-cohered from the matrix. Particle fracture and particle decohesion seem to play a significant role in the failure of the alloy as suggested by XU et al [53]. The tensile strength largely depends on the morphology and distribution of primary Si and eutectic Si in the alloy. The size of the primary Si governing the properties can be explained with the help of Griffith’s criterion [54]. The fracture stress (σf) and the length of an internal defect in primary Si are related as follows:
σf =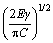 (3)
(3)
where γ, E and C are fracture surface energy, elastic modulus of the particle and crack length, respectively.
Coarser primary Si is likely to have more internal defects as compared to finer primary Si. Thus, there is a decrease in the fracture stress which helps in crack initiation and propagation leading to premature fracture of Si particles in Al-20Si alloy (Fig. 11(d)). Secondly, the load transfer to primary Si plays an important role in altering the strength of the alloy. It is more in the case of coarse primary Si due to high aspect ratio leading to initiation of a crack. Thus, the primary Si fractures at lower load resulting in a tensile fracture. Addition of γ-Al2O3 and Sr results in reducing the size of primary Si and eutectic Si which are more effectively bound by the matrix and thus, reducing the possibility of formation of microcracks.
Moreover, the propagation of cracks through the rounded eutectic Si and smoothened edges of primary Si are relatively difficult as compared to as received Al-20Si alloy. The increase in tensile strength and ductility is attributed to the refinement and uniform distribution of primary Si and eutectic Si in the entire alloy.

Fig. 11 FESEM micrographs of fractured surface of Al-20Si (a, b) and Al-20Si-4γ-Al2O3-0.1Sr (c, d)
4 Conclusions
(1) The maximum refinement of primary Si occurs with 4 wt.% γ-Al2O3, while the best modification of eutectic Si that can be achieved in Al-20Si alloy occurs with 0.1 wt.% Sr. The minimum average size of primary Si is ~24 μm with 4 wt.% γ-Al2O3.
(2) Simultaneous addition of γ-Al2O3 and Sr to Al-20Si alloy does not show the formation of new compounds producing poisoning effect which could inhibit the nucleation efficiency of the particles for the formation of fine primary Si and eutectic Si.
(3) The calculation using area fraction of primary Si in the alloys shows a significant shift in eutectic point. The maximum eutectic shift of 18.5 wt.% i.e. to higher Si content was observed when 4 wt.% γ-Al2O3 was added to Al-20Si alloy.
(4) The alloy with 4 wt.% γ-Al2O3 and 0.1 wt.% Sr shows the maximum UTS, YS, and ductility. The improvements in UTS and ductility are found to be 20% and 23%, respectively. The enhanced ductility obtained in these alloys could be due to two reasons: the refinement of primary silicon and modification of eutectic silicon, and the presence of α(Al) in the microstructure as a consequence of eutectic shift and the alloy behaving like a near-eutectic alloy.
Acknowledgments
The authors are grateful to Central Research Facility at IIT Kharagpur for providing the necessary facilities to carry out tensile testing.
References
[1] GE L L, LIU R P, LI G, MA M Z, WANG W K. Solidification of Al-50at.%Si alloy in a drop tube [J]. Materials Science and Engineering A, 2004, 385(1-2): 128-132.
[2] CUI C, SCHULZ A, SCHIMANSKI K, ZOCH H W. Spray forming of hypereutectic Al-Si alloys [J]. Journal of Materials Processing Technology, 2009, 209(11): 5220-5228.
[3] HSU Chang-chuan, WANG Jian-yih, HUANG Jian-jia, LEE Shyong. Refinement of the primary Si particles in hypereutectic aluminum alloy [J]. Metals and Materials International, 2012, 18(4): 567-571.
[4] WU F F, LI S T, ZHANG G A, JIANG F. Microstructural evolution and mechanical properties of hypereutectic Al-Si alloy processed by liquid die forging [J]. Bulletin of Materials Science, 2014, 37(5): 1153-1157.
[5] LI Q, XIA T, LAN Y, ZHAO W, FAN L, LI P. Effect of rare earth cerium addition on the microstructure and tensile properties of hypereutectic Al-20%Si alloy [J]. Journal of Alloys and Compounds, 2013, 562: 25-32.
[6] LI Q L, XIA T D, LAN Y F, LI P F. Effects of melt superheat treatment on microstructure and wear behaviours of hypereutectic Al-20Si alloy [J]. Materials Science and Technology, 2014, 30(7): 835-841.
[7] RAO A G, RAO B R K, DESHMUKH V P, SHAH A K, KASHYAP B P. Microstructural refinement of a cast hypereutectic Al-30Si alloy by friction stir processing [J]. Materials Letters, 2009, 63(30): 2628-2630.
[8] LU D, JIANG Y, GUAN G, ZHOU R, LI Z, ZHOU R. Refinement of primary Si in hypereutectic Al-Si alloy by electromagnetic stirring [J]. Journal of Materials Processing Technology, 2007, 189(1-3): 13-18.
[9] CAI Z, WANG R, ZHANG C, PENG C, XIE L, WANG L. Characterization of rapidly solidified Al-27Si hypereutectic alloy: Effect of solidification condition [J]. Journal of Materials Engineering and Performance, 2015, 24(3): 1226-1236.
[10] CUI C, SCHULZ A, EPP J, ZOCH H W. Deformation behavior of spray-formed hypereutectic Al-Si alloys [J]. Journal of Materials Science, 2010, 45(10): 2798-2807.
[11] üNLü B S. Investigation of tribological and mechanical properties Al2O3-SiC reinforced Al composites manufactured by casting or P/M method [J]. Materials and Design, 2008, 29(10): 2002-2008.
[12] KOK M. Production and mechanical properties of Al2O3 particle-reinforced 2024 aluminium alloy composites [J]. Journal of Materials Processing Technology, 2005, 161(3): 381-387.
[13] TAHA M A, EL-MAHALLAWY N A. Metal-matrix composites fabricated by pressure-assisted infiltration of loose ceramic powder [J]. Journal of Materials Processing Technology, 1998, 73: 139-146.
[14] CHEN Chong, LIU Zhong-xia, REN Bo, WANG Ming-xing, WENG Yong-gang, LIU Zhi-yong. Influences of complex modification of P and RE on microstructure and mechanical properties of hypereutectic Al-20Si alloy [J]. Transactions of Nonferrous Metals Society of China, 2007, 17(2): 301-306.
[15] STERNER-RAINER R. Aluminium silicon alloy with a phosphorus content of 0.001 to 0.1%: U.S. Patent, 1940922 [P]. 1933-12-26.
[16] VIJEESH V, PRABHU K N. Review of microstructure evolution in hypereutectic Al-Si alloys and its effect on wear properties [J]. Transactions of the Indian Institute of Metals, 2014, 67(1): 1-18.
[17] CHANG J, MOON I, CHOI C. Refinement of cast microstructure of hypereutectic Al-Si alloys through the addition of rare earth metals [J]. Journal of Materials Science, 1998, 3: 5015-5023.
[18] RAGHUKIRAN N, KUMAR R. A Effect of scandium addition on the microstructure, mechanical and wear properties of the spray formed hypereutectic aluminum–silicon alloys[J]. Materials Science and Engineering A, 2015, 641: 138-147.
[19] SHI W, GAO B, TU G, LI S, HAO Y, YU F. Effect of neodymium on primary silicon and mechanical properties of hypereutectic Al-15Si alloy [J]. Journal of Rare Earths, 2010, 28(Suppl 1): 367-370.
[20] LI Q, XIA T, LAN Y, LI P, FAN L. Effects of rare earth Er addition on microstructure and mechanical properties of hypereutectic Al-20%Si alloy [J]. Materials Science and Engineering A, 2013, 588: 97-102.
[21] ZHANG Heng-hua, DUAN Hai-li, SHAO Guang-jie, XU Luo-ping. Microstructure and mechanical properties of hypereutectic A1-Si alloy modified with Cu-P [J]. Rare Metals, 2008, 27(1): 59-63.
[22] JIANG L, JIANG B, WANG Z, WANG Y, LIU J. In situ prepared Al-Si alloy matrix composites reinforced by γ-Al2O3p [J]. Journal of University of Science and Technology Beijing, 2007, 14(3): 276-279.
[23] CHOI H, LI X. Refinement of primary Si and modification of eutectic Si for enhanced ductility of hypereutectic Al-20Si-4.5Cu alloy with addition of Al2O3 nanoparticles [J]. Journal of Materials Science, 2012, 47(7): 3096-3102.
[24] LI Q, XIA T, LAN Y, ZHAO W, FAN L, LI P. Effect of in situ γ-Al2O3 particles on the microstructure of hypereutectic Al-20%Si alloy [J]. Journal of Alloys and Compounds, 2013, 577: 232-236.
[25] MAKHLOUF M M, GUTHY H V. The aluminum-silicon eutectic reaction: Mechanisms and crystallography [J]. Journal of Light Metals, 2001, 1(4): 199-218.
[26] ZUO M, ZHAO D, TENG X, GENG H, ZHANG Z. Effect of P and Sr complex modification on Si phase in hypereutectic Al-30Si alloys [J]. Materials and Design, 2013, 47: 857-864.
[27] DAI H, LIU X. Effects of individual and combined additions of phosphorus, boron and cerium on primary and eutectic silicon in an Al-30Si alloy [J]. Rare Metals, 2009, 28(6): 651-655.
[28] KUEMMEL M, GROSSO D, BOISSIERE C, SMARSHLY B, BREZESINSKI T, ALBOUY P A, AMENITSCH H, SANCHEZ C. Thermally stable nanocrystalline γ-alumina layers with highly ordered 3D mesoporosity [J]. Angewandte Chemie Int Ed, 2005, 44: 4589-4592.
[29] EL-MAHALLAWI I, SHASH A Y, AMER A E. Nanoreinforced cast Al-Si alloys with Al2O3, TiO2 and ZrO2 nanoparticles [J]. Metals, 2015, 5(2): 802-821.
[30] XU C L, JIANG Q C. Morphologies of primary silicon in hypereutectic Al-Si alloys with melt overheating temperature and cooling rate [J]. Materials Science and Engineering A, 2006, 437(2): 451-455.
[31] BRAMFITT B L. The effect of carbide and nitride additions on the heterogeneous nucleation behavior of liquid iron [J]. Metallurgical Transactions, 1970, 1: 1987-1995.
[32] SAJJADI S A, EZATPOUR H R, TORABI PARIZI M. Comparison of microstructure and mechanical properties of A356 aluminum alloy/Al2O3 composites fabricated by stir and compo-casting processes [J]. Materials and Design, 2012, 34: 106-111.
[33] MAZAHERY A, ABDIZADEH H, BAHARVANDI H R. Development of high-performance A356/nano-Al2O3 composites [J]. Materials Science and Engineering A, 2009, 518(1-2): 61-64.
[34] SAJJADI S A, TORABI PARIZI M, EZATPOUR H R, SEDGHI A. Fabrication of A356 composite reinforced with micro and nano Al2O3 particles by a developed compocasting method and study of its properties [J]. Journal of Alloys and Compounds, 2012, 511(1): 226-231.
[35] JIANG Q C, XU C L, WANG H Y, WANG J G, YANG Y F. Estimation of the shifting distance of the eutectic point in hypereutectic Al-Si alloys by the lever rule [J]. Scripta Materialia, 2007, 56(5): 329-332.
[36] KISSINGER H E. Reaction kinetics in differential thermal analysis [J]. Analytical Chemistry, 1957, 29(11): 1702-1706.
[37] NOGITA K, YASUDA H, YOSHIDA K, UESUGI K, TAKEUCHI A, SUZUKI Y, DAHLE A K. Determination of strontium segregation in modified hypoeutectic Al-Si alloy by micro X-ray fluorescence analysis [J]. Scripta Materialia, 2006, 55(9): 787-790.
[38] SUN Yu, PANG Shao-ping, LIU Xue-ran, YANG Zi-run, SUN Guo-xiong. Nucleation and growth of eutectic cell in hypoeutectic Al-Si alloy [J]. Transactions of Nonferrous Metals Society of China, 2011, 21(10): 2186-2191.
[39] TIMPEL M, WANDERKA N, SCHLESIGER R, YAMAMOTO T, LAZARV N, ISHEIM D, SCHMITZ G, MATSUMURA S, BANHART J. The role of strontium in modifying aluminium-silicon alloys [J]. Acta Materialia, 2012, 60(9): 3920-3928.
[40] KOBAYASHI K F, HOGAN L M. The crystal growth of silicon in Al-Si alloys [J]. Journal of Materials Science, 1985, 20(6): 1961-1975.
[41] LIU X, ZHANG Y, BEAUSIR B, LIU F, ESLING C, YU F, ZHAO X, ZUO L. Acta Materialia Twin-controlled growth of eutectic Si in unmodified and Sr-modified Al-12.7%Si alloys investigated by SEM/EBSD [J]. Acta Materialia, 2015, 97: 338-347.
[42] LU Shu-zu, HELLAWELL A. The mechanism of silicon modification in aluminum-silicon alloys: Impurity induced twinning [J]. Metallurgical Transactions A, 1987, 18(10): 1721-1733.
[43] ACHARYA M, DEEPAK KUMAR S, MANDAL A. Effect of cooling slope angle on microstructure of Al-7Si alloy [J]. Transactions of the Indian Institute of Metals, 2015, 68(6): 1095-1099.
[44] CHOI H, KONISHI H, LI X. Al2O3 nanoparticles induced simultaneous refinement and modification of primary and eutectic Si particles in hypereutectic Al-20Si alloy [J]. Materials Science and Engineering A, 2012, 541: 159-165.
[45] MIRESMAEILI S M. Effect of strontium on the oxidation behavior of liquid Al-7Si alloys [J]. Oxidation of Metals, 2009, 71(1-2): 107-123.
[46] YEH Jien-wei, YUAN Shi-ying, PENG Chao-hung. A reciprocating extrusion process for producing hypereutectic Al-20wt.% Si wrought alloys [J]. Materials Science and Engineering A, 1998, 252(2): 212-221.
[47] MA P, PRASHANTH K G, SCUDINO S, JIA Y, WANG H, ZOU C, WEI Z, ECKERT J. Influence of annealing on mechanical properties of Al-20Si processed by selective laser melting [J]. Metals, 2014, 4(1): 28-36.
[48] LIU L, SAMUEL A M, SAMUEL F H, DOTY H W, VALTIERRA S. Influence of oxides on porosity formation in Sr-treated Al-Si casting alloys [J]. Journal of Materials Science, 2003, 38(6): 1255-1267.
[49] LEE P D, SRIDHAR S. Direct observation of the effect of strontium on porosity formation during the solidification of aluminium-silicon alloys [J]. International Journal of Cast Metals Research, 2000, 13(4): 185-198.
[50] SHAFYEI A, ANIJDAN S H M, BAHRAMI A. Prediction of porosity percent in Al-Si casting alloys using ANN [J]. Materials Science and Engineering A, 2006, 431(1-2): 206-210.
[51] KNUUTINEN A, NOGITA K, MCDONALD S D, DAHLE A K. Porosity formation in aluminium alloy A356 modified with Ba, Ca, Y and Yb [J]. Journal of Light Metals, 2001, 1(4): 241-249.
[52] MCDONALD S D, DAHLE A K, TAYLOR J A, STJOHN D H. Modification-related porosity formation in hypoeutectic aluminum- silicon alloys [J]. Metallurgical and Materials Transactions B, 2004, 35: 1097-1106.
[53] XU C L, WANG H Y, YANG Y F, JIANG Q C. Effect of Al-P-Ti-TiC-Nd2O3 modifier on the microstructure and mechanical properties of hypereutectic Al-20 wt.%Si alloy [J]. Materials Science and Engineering A, 2007, 452-453: 341-346.
[54] TONG X C, GHOSH A K. Fabrication of in situ TiC reinforced aluminum matrix composites [J]. Journal of Materials Science, 2001, 36(16): 4059-4069.
氧化铝(γ-Al2O3)和锶对Al-20Si合金显微组织和力学性能的影响
Mihira ACHARYA, Animesh MANDAL
School of Minerals, Metallurgical and Materials Engineering, Indian Institute of Technology Bhubaneswar, Argul 752050, Odisha, India
摘 要:为得到细晶硅,向铸造Al-20Si合金中加入4%(质量分数,下同) γ-Al2O3和0.1% Sr。在铸造过程中,γ-Al2O3的作用是细化初晶硅,其添加量为0.5%~6%,而锶的作用是改性共晶硅,其添加量为0.05%~0.1%。结果表明,当添加4% γ-Al2O3时,初晶硅的平均尺寸为24 μm。当添加0.1% Sr时,共晶硅的形状因子约为0.6,平均长度约为1.2 μm。热分析发现γ-Al2O3可以作为潜在的异质形核点。而且,与在Al-20Si合金中同时添加P和Sr一样,同时添加γ-Al2O3和Sr不会污染γ-Al2O3颗粒和抑制他们的形核效率。与铸态Al-20Si合金相比,Al-20Si-4γ-Al2O3- 0.1%Sr合金的极限抗拉强度提高了20%,伸长率提高了23%。抗拉强度的提高可归因于初晶硅的细化、共晶硅的改性,以及合金中由于共晶转变而析出的α(Al)。
关键词:铝-硅合金;γ-Al2O3;细化;改性;初晶硅
(Edited by Xiang-qun LI)
Corresponding author: Mihira ACHARYA; Tel : +91-943392133; E-mail: ma10@iitbbs.ac.in
DOI: 10.1016/S1003-6326(19)65042-9
Abstract: An optimized combination of gamma alumina (4 wt.%) and strontium (0.1 wt.%) was incorporated in cast Al-20Si alloy to obtain fine form of silicon. During casting process, the amount of γ-Al2O3 was varied from 0.5-6 wt.% to refine primary Si and Sr was varied from 0.05-0.1 wt.% to modify eutectic Si. The results showed that the average size of primary Si is 24 μm for addition of 4 wt.% γ-Al2O3 to the alloy whereas 0.1 wt.% Sr resulted in sphericity of eutectic Si to ~0.6 and average length of ~1.2 μm. The thermal analysis revealed that γ-Al2O3 can act as potential heterogeneous nucleation sites. Moreover, simultaneous addition of γ-Al2O3 and Sr does not poison γ-Al2O3 particles and inhibit their nucleation efficiency as in the case of combined addition of phosphorous and strontium to Al-20Si alloy. Therefore, it was concluded that enhanced tensile strength, i.e., ultimate tensile strength (increase by 20%) and elongation (increase by 23%) in Al-20Si-4γ-Al2O3-0.1wt.%Sr alloy as compared to as-cast Al-20Si alloy can be attributed to refinement of primary Si, modification of eutectic Si and the presence of α(Al) in the alloy as evident from eutectic shift.


