J. Cent. South Univ. (2012) 19: 213-221
DOI: 10.1007/s11771-012-0994-5![]()
Effects of different conditions on Pb2+ adsorption from soil by irrigation of sewage in South China
HUANG Guan-xing(黄冠星), ZHANG Ying(张英), SUN Ji-chao(孙继朝),
JING Ji-hong(荆继红), LIU Jing-tao(刘景涛), WANG Ying(王莹)
Institute of Hydrogeology and Environmental Geology, Chinese Academy of Geological Sciences,Shijiazhuang 050061, China
? Central South University Press and Springer-Verlag Berlin Heidelberg 2012
Abstract:
Pb2+ adsorption onto a soil by irrigation of sewage in the Pearl River Delta of South China was examined as a function of the reaction time, solution pH, initial lead concentration, organic matter (humic acid) and competitive ions (Cu2+). The adsorption of Pb2+ onto the soil was investigated on batch equilibrium adsorption experiments. Results show that the Pb2+ adsorption on the soil is relatively rapid in the first 30 min and reaches equilibrium at 2 h, and the kinetics of the adsorption process on the soil is well characterized by the pseudo-second order reaction rate. Langmuir, Freundlich and Temkin isothermal models are fit for the adsorption of Pb2+ onto the soil, and the maximum amount of Pb2+ adsorption (Qm) is 7.47 mg/g. The amount of Pb2+ adsorption increases with increasing the pH at the range of 1.2-4.5 and reaches a plateau at the range of 4.5-12. The presence of humic acid in soil decreases the adsorption of Pb2+ onto the soil at solution pH of 8 since the negatively charged humic acid with Pb2+ is difficult to be adsorbed on the negatively charged soil surface. The adsorption of Pb2+ onto the soil also decreases in the presence of Cu2+ due to the competition adsorption between Pb2+ and Cu2+.
Key words:
adsorption; Pb2+; soil; pH; humic acid;
1 Introduction
Heavy metals are natural constituents of soils. However, in the last decade, significant changes in the global budget of heavy metals at the surface of the earth have occurred. Contamination of soils with heavy metals results from several anthropogenic activities, including irrigation of sewage water, smelting and metal treatment operations, mining, and so on [1-3]. This pollution can pose a serious threat to plants, animals and even human beings because of bioaccumulation, non-biodegradable properties and toxicity of the contaminants [4]. One of the most potentially toxic heavy metals is lead. Classified as soluble and strongly hydrated cation [5], lead can cause debilitating diseases in humans and animals as it causes irreversible changes in the body, especially in the central nervous system, leading to psychotic disorders [6-7]. The distribution of lead between the solid and the solution phases of soil is a key issue in assessing the mobility and availability of lead in soils, and the concentration of lead in the soil solution is most likely to be controlled by adsorption and desorption reactions on the surfaces of soils and oxides [8]. Adsorption of lead by soils is strongly influenced by various soil characteristics. In the last decade, a great number of studies have been focused on the adsorption of lead(II) on different soil materials and minerals [9-12], but few researches have been focused on the adsorption of lead(II) in sewage irrigation soils by taking the background content of lead on soil into account. These works showed that reaction time, ionic strength of the soil solution, organic matter, simultaneous presence of competing metals and the soil pH are known to affect adsorption processes [12-16], especially, the pH is regarded as one of the most important factors in lead adsorption on soils [12, 17-18]. However, previous studies were focused on the adsorption of lead(II) in soils with a few certain values of pH [10, 12, 18] or a narrow range of pH [19]; in contrast, few studies have been focused on the adsorption of lead(II) in soils with a wider range of pH.
The Pearl River Delta is situated in the southern part of Guangdong Province, China. In the last three decades, the region has undergone a rapid transition from a traditionally agricultural-based economy to an increasingly industrial-based economy. The establishment of industrial operations has considerably increased industrial wastewater discharge in this region [20]. Coupled with the lack of pollution controls, the increase of industrial wastewater can affect the local soil quality since heavy metals such as lead may enter and accumulate in agricultural soils through sewage irrigation [21-22], which could enhance the risk of lead contamination of the food chains in the region [23]. Therefore, it is necessary to understand the processes of migration and transformation mechanisms of lead onto sewage irrigation soil, which are closely related to the adsorption mechanism of lead onto sewage irrigation soil. However, the adsorption mechanism of lead onto sewage irrigation soil in the Pearl River Delta is still scarce, especially the wider range of pH impact on the adsorption of lead in sewage irrigation soil has not been reported. The objective of the present work is to determine the effects of pH (wider range 1-12), reaction time, organic matter, initial concentration and competitive ions on the adsorption of lead(II) by the sewage irrigation soil of Pearl River Delta region situated in South China for evaluating the potential of this metal to migrate through this soil. The results of this wok could be greatly beneficial to the prediction of lead transport and to evaluate the impact of lead onto soil quality.
2 Experimental
2.1 Soil sample
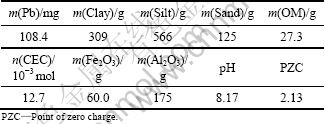
Approximately 28 kg of a bulk soil sample was prepared by collecting about 2 kg of loose surface soil at depth of 0-10 cm by stainless steel sampler digging at 14 sites located in the Pearl River Delta region (South China), in 2008. The soil sample was air-dried and ground with a wooden roller and then passed through 850 μm and 150 μm sieves. Soil particles less than 150 μm were selected and used for adsorption studies. Soil particle size distribution was determined by the sedimentation method. Soil pH was measured with a glass electrode using a suspension with soil-to-water mass ratio of 1:5. Ferric oxide (Fe2O3) and aluminum oxide (Al2O3) were extracted twice with an acid ammonium oxalate solution (0.2 mol/L at pH 3.0) by reciprocal shaking in dark for 1 h at a soil-to-water mass ratio of 1:25 [24], and the contents of them in the extract were determined using a sequential plasma spectrometer [25]. The soil organic matter (OM) was measured by the K2Cr2O7 method [26]. The cation exchange capacity (CEC) of the soil was determined by the ammonium acetate method [26]. The background value (total concentration) of lead in soil was obtained by digesting soil sample with aqua regia [27], and the concentration of extracted Pb was determined by inductively coupled plasma optical emission spectroscopy (ICP-OES) in a Perkin-Elmer 2100DV apparatus. Point of zero charge (PZC) of soil was determined by potentiometric titration [28]. The obtained results of soil sample are given in Table 1.
Table 1 Chemical components and physical properties of 1 kg sewage irrigation soil
2.2 Batch adsorption experiments
Batch adsorption tests were performed to evaluate the lead from the sewage irrigation soil as a function of reaction time, initial concentrations, competition ions, humic acid and pH. A kinetic adsorption test was firstly performed in order to determine the time necessary to reach equilibrium, after which the static tests were performed for the previously determined time. All solutions were prepared with deionized water. The ionic strength was adjusted to 0.1 mol/L with NaNO3, and the ionic strength adjustment was to limit the effect of a change in ion concentrations on the adsorption properties. Soil samples of 1.0 g in duplicates were equilibrated with 40 mL of the solutions in 50 mL polyethylene centrifuge tubes. Firstly, during the adsorption kinetic test for the reaction time, all the experiments were carried out at room temperature (25 °C) under continuous stirring (end-over-end rotation, about 200 r/min) for a known period of time (10, 30, 60, 120, 180, 240, 300, 360, 420, 480, 540, 600 and 1 440 min). The initial concentration of lead in solutions was 17.8 mg/L, and the initial pH of solutions was adjusted using KOH and HNO3 to 8.0 and it was not further modified during these experiments in order to simulate the real environmental conditions. After the corresponding time interval, the solutions were centrifuged at onents lnditions 4 000 r/min for 20 min and the supernatants were filtered through a 0.45 μm pore size membrane. The lead concentrations in the filtered solutions were determined using an inductively coupled plasma optical emission spectrometer (Optima 2100DV, Perkin-Elmer).
The adsorption isotherm as a function of initial lead concentration was conducted in a series of 50 mL polyethylene centrifuge tubes. Each tube was filled with 40 mL of different initial lead concentrations (1.78, 8.9, 17.8, 35.6, 71.2, 106.8, 142.4 and 178 mg/L) and the initial pH was adjusted to 8. After equilibrium, the solutions were separated and analyzed. In addition, in order to study the effect of competition ion and organic matter on the adsorption of lead, a series of 50 mL polyethylene centrifuge tubes containing different initial lead concentrations (1.78, 8.9, 17.8, 35.6, 71.2, 106.8, 142.4 and 178 mg/L) with a certain concentration of copper (1.0 mmol/L) or a certain quality of humic acid (1.0 g) were prepared, similar to the adsorption isotherm as a function of initial lead concentration. After equilibrium, the solutions were separated and analyzed.
To study the effect of pH on the adsorption of lead, a series of the solutions containing the same initial lead concentrations (17.8 mg/L) were prepared with different initial pH from 1 to 12, and the initial pH of solutions was adjusted using KOH and HNO3. After equilibrium, the solutions were centrifuged at 4 000 r/min for 20 min and the supernatants were filtered through a 0.45 μm pore size membrane. The lead concentrations and equilibrium pH values in the filtered solutions were determined by ICP-OES and a pH meter, respectively. Each experiment was repeated twice to check the reproducibility.
According to the mass balance equation, the total amount of lead in the solution at time t=0 plus the amount of lead adsorbed on soil at t=0 is equal to the amount of lead left in the solution plus the amount of lead adsorbed on soil:
m(Qt-Q0)=V(c0-ct) (1)
where m is the air dried mass of soil, g; Qt is the amount of lead adsorbed per unit mass of soil at time t, mg/g; and Q0 is the background value of lead in sewage irrigation soil, mg/g; V is the volume of the solution, L; c0 and ct are the initial lead concentration solution and at the time t, respectively, mg/L.
As sewage irrigation soil at t=0 contained the background value of lead, Q0=0.108 4 mg/g (Table 1).
Therefore,
Qt=V(c0-ct)/m+Q0 (2)
2.3 Theoretical background
2.3.1 Adsorption kinetics
Adsorption processes are influenced by experimental conditions such as solution pH, temperature, ionic strength of the solution and initial metal concentration. There are three most widely used kinetic models in adsorption processes (Elovich, pseudo-first and pseudo- second order models) applied to experimental data [29-30]. The Elovich model is used to express the second-order kinetics, assuming that the adsorption occurs on energetically heterogeneous surfaces:
Qt=(1/β)ln(αβ)+(1/β)lnt (3)
where α and β are the Elovich coefficients and represent the initial adsorption rate and the desorption constant, respectively.
The pseudo-first order kinetics model is expressed as follows:
lg(Qe-Qt)=lg Qe-K1t/2.303 (4)
where Qe is the amount of metal adsorbed at equilibrium and K1 is the adsorption rate constant.
The pseudo-second order kinetics model is expressed as follows:
t/Qt=1/(K2Qe2)+t/Qe (5)
where K2 is the second-order reaction rate.
2.3.2 Adsorption isotherms
Lead adsorption by the soils and other minerals was studied commonly using the Langmuir, Freundlich and Temkin adsorption isotherms. The Langmuir isotherm model assumes that monolayer coverage of adsorption of each molecule onto the surface has equal activation energy of adsorption [31]. The Langmuir isotherm model is expressed as follows:
Qe=Qmce/(1/KL+ce) (6)
Or, in its linear form:
ce/Qe=1/KLQm+ce/Qm (7)
where Qm is the maximum adsorbed lead by adsorbent mass; ce is the equilibrium lead concentration; KL is the adsorption equilibrium constant related to adsorption capacity and energy of adsorption.
The Freundlich isotherm theory presents that the ratio of the amount of solute adsorbed onto a given mass of adsorbent to the concentration of the solute in the solution is not constant at different concentrations [32]. It is expressed as follows:
Qe=KFce1/n (8)
where Qe is the adsorbed lead by adsorbent mass at equilibrium; KF is the Freundlich constant related to the relative adsorption capacity of the adsorbent; n is the indicative constant of the intensity of the adsorption. This equation is often used in its logarithmic form for the linear relationship:
lgQe=lgKF+(1/n)lgce (9)
The Temkin isotherm equation assumes that the heat of adsorption of all the molecules in the layer decreases linearly with the coverage due to the adsorbent–adsorbate interactions and the adsorption is characterized by a uniform distribution of the binding energies, up to some maximum binding energy [33]. It is expressed as follows:
Qe= RTln(KTce)/b=B1ln(KTce) (10)
where constant B1=RT/b, is related to the heat of adsorption; R is the ideal gas constant (8.315 J/(mol·K)); T is the temperature; KT is the equilibrium binding constant corresponding to the maximum binding energy; b is the variation of adsorption energy.
3 Results and discussion
3.1 Adsorption kinetics
The result of adsorption kinetic of Pb2+ is shown in Fig. 1. It is found that the adsorption capacity of sewage irrigation soil for Pb2+ increases with the increase of contact time and becomes constant after the equilibrium time is reached. These preliminary kinetic experiments strongly indicate that the adsorption of Pb2+ on the sewage irrigation soil is a two-step process: A rapid kinetic reaction of Pb2+ adsorption by the soil occurs in the first 30 min, which may be due to the large amount of adsorption sites available at the beginning. After that, in the second stage, the reaction rate slows down and the amount of adsorbed Pb2+ slowly increases to a steady- state value, because the adsorption sites are gradually filled up and the adsorption becomes slower. After about 120 min of reaction, the equilibrium is achieved, with adsorption capacity of 819.24 mg/kg. This result is in accordance with the experimental results of other authors [10, 12, 29, 34] where a two-phase reaction with a rapid first step and a slower second phase was observed, and the equilibrium was achieved at about 120 min. This two-stage process can also be due to the fact that adsorption occurs onto two different types of binding sites on the adsorbent particles. Similar results have also been obtained by other researchers [35]. It is worth mentioning that in these preliminary kinetic experiments, the maximum adsorption capacity of 819.44 mg/kg occurs at 420 min. Therefore, the stirring time of all batch adsorption tests (except adsorption kinetic tests) is 420 min.
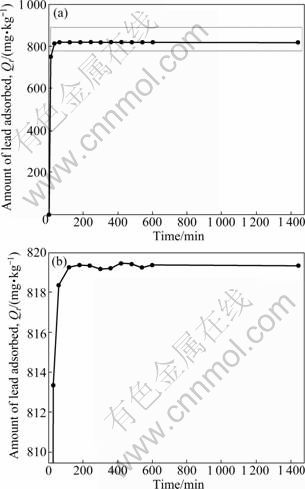
Fig. 1 Kinetics of Pb2+ adsorption in 17.8 mg/L Pb2+ at 25 °C on sewage irrigation soil: (a) Initial image; (b) Enlarged image of (a)
Elovich, pseudo-first order and pseudo-second order rate equations were applied to experimental data for evaluating the adsorption of Pb2+ on sewage irrigation soil at 25 °C. Figure 2 shows the plot of the Elovich equation for adsorption of Pb2+ by the sewage irrigation soil, and the result shows that the Elovich equation is not quite fit for the description of Pb2+ adsorption on sewage irrigation soil. Similar to Elovich equation, as can be seen from Fig. 3, the pseudo-first order equation is also not fit for the description of Pb2+ adsorption on sewage irrigation soil. Compared to the former two equations, as can be seen from Fig. 4, the pseudo-second order equation is more fit for the description of Pb2+ adsorption on sewage irrigation soil. Furthermore, as can be seen in Table 2, the result of regression coefficients (R2) for Elovich, pseudo-first and pseudo-second order kinetic models also confirms that the pseudo-second order equation is most fit for the description of Pb2+ adsorption on sewage irrigation soil due to the highest R2 value. This result is in accordance with the experimental results of other authors [29, 36]. In addition, equation constant and calculated Qe value for pseudo-second order kinetic model are also presented in Table 2. The calculated Qe value of 819.7 mg/kg obtained from pseudo-second order equation agrees well with the maximum experimental value (819.4 mg/kg), and the pseudo-second order reaction rate K2 is 7.405 g/(mg·min).
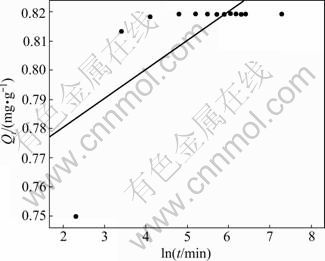
Fig. 2 Plot of Elovich equation for Pb2+ adsorption by soil
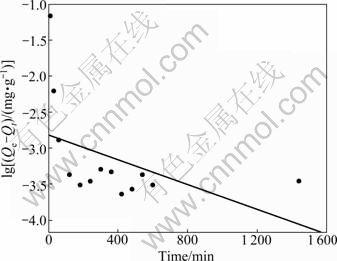
Fig. 3 Plot of pseudo-first order equation for Pb2+ adsorption by soil
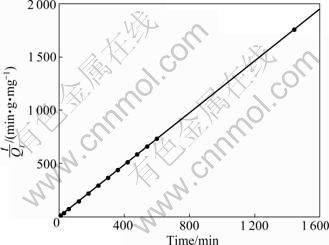
Fig. 4 Plot of pseudo-second order equation for Pb2+ adsorption by sewage irrigation soil
Table 2 Kinetic model verification for Pb2+ adsorption at 17.8 mg/L in 24 h

3.2 Adsorption isotherms
The analysis of equilibrium data is essential to understand the adsorption process and to be able to compare different adsorbents under different operational conditions. In this work, three kinds of isotherm models (Langmuir, Freundlich and Temkin) were applied to the experimental data. The results of these three isotherm models applied to the Pb2+ adsorption on sewage irrigation soil are shown in Fig. 5. The isotherm constants and correlation coefficients (R2) for these models are presented in Table 3. As can be seen in Fig. 5 and Table 3, all of the three isotherm models give a good fit for the adsorption of Pb2+ with similar accuracy (P<0.001). However, by comparing the basis of the correlation coefficients, a little difference among the fitting degree of these three isotherm models for Pb2+ adsorption can be discovered. Freundlich isotherm model is more fit for the adsorption of Pb2+ on sewage irrigation soil than other models due to the highest R2 value. This result is in accordance with experiments of other authors [29, 37]. The value of the Freundlich model constant KF is 18.03 mg/g, which is a measure of relative adsorption capacity and is higher for Pb2+ adsorption than some agricultural soils of India [37] and lower for Pb2+ adsorption than some paddy soils of southern China [11]. This shows that the adsorption capacity of sewage irrigation soil to Pb2+ may be higher than some agricultural soils of India and lower than some paddy soils of southern China. In addition, Langmuir isotherm model is more fit for the adsorption of Pb2+ on sewage irrigation soil than Temkin model, and the maximum adsorption of Pb2+ (Qm) is 7.47 mg/g, like the result of Freundlich model constant KF, which is also higher than those of some agricultural soils of India [37].
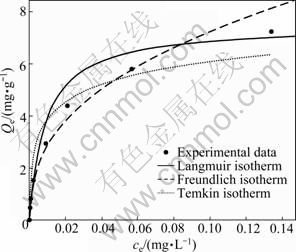
Fig. 5 Different adsorption isotherm models applied to Pb2+ adsorption by sewage irrigation soil
Table 3 Parameters for Langmuir, Freundlich and Temkin isotherms as function of Pb2+ concentration at pH 8

3.3 Effect of pH
Soil solution pH plays an important role in the adsorption of lead as it directly controls the solubilities of lead hydroxides and organic matter, and the surface charge of iron and aluminum oxides [19, 38-39]. Increasing soil solution pH can increase Pb2+ retention to soil surfaces via adsorption [5]. This phenomenon has been demonstrated by many researchers in a variety of soils and minerals in batch study [38, 40-42]. Batch equilibrium experiments were performed in initial pH range of 1-12 to determine the effect of pH on the adsorption of Pb2+ by sewage irrigation soil (Fig. 6). As can be seen in Fig. 6, the adsorption of Pb2+ increases from about -8% to 100% at equilibrium pH range of 1.2-4.5. It increases quickly at equilibrium pH<2, more quickly at equilibrium pH range of 2-3, then slowly at equilibrium pH range of 3-4.5 and reaches a plateau at equilibrium pH range of 4.5-12. There is a special phenomenon that the rate of Pb2+ adsorption by sewage irrigation soil is about -8% (<0) while the equilibrium pH of solution is 1.2, and the corresponding amount of Pb2+ adsorption by sewage irrigation soil is 50.8 mg/kg, which is lower than the background value (108.4 mg/kg, Table 1). This may be caused by the following reason. Since the surface of sewage irrigation soil contains a large number of adsorption sites and may become positively charged at solution pH
PZC [38], which increases the competition between H+ and Pb2+ for surface adsorption sites, solution H+ can replace the exchangeable fraction of Pb adsorbed by sewage irrigation soil due to competition while the concentration of H+ in solution is very high (or low pH). That is why the amount of Pb2+ adsorption by sewage irrigation soil is lower than the background value while equilibrium pH of solution is 1.2. Then, as solution pH increases, the competition between H+ and Pb2+ becomes weaker and the amount of Pb2+ adsorption by sewage irrigation soil increases obviously, especially after solution equilibrium pH>pHPZC (2.13, Table 1). The surface of sewage irrigation soil becomes negatively charged, which enhances the rate of Pb2+ adsorption by sewage irrigation soil, which may be the main reason why the amount of Pb2+ adsorption by sewage irrigation soil increases quickly at equilibrium pH range of 2-3. Then, as solution pH increases further, the speed of increasing for negative charge on the surface of sewage irrigation soil is low due to the constant surface adsorption sites of sewage irrigation soil, which would decrease the rate of Pb2+ adsorption by sewage irrigation soil, and it may be the main reason why the amount of Pb2+ adsorption increases slowly at equilibrium pH range of 3-4.5 compared with that at equilibrium pH range of 2-3.
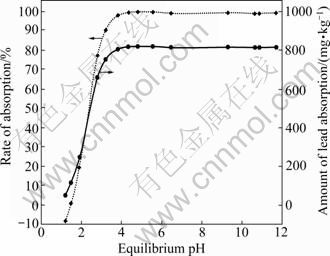
Fig. 6 Amount and rate of adsorption of Pb2+ by sewage irrigation soil at 17.8 mg/L Pb2+ in different solution pH
3.4 Effect of organic matter
Organic matter also plays an important role in the adsorption of lead on soil. The presence of some organic matters such as citrate and EDTA was found to decrease the adsorption of Pb to soil minerals [43-44]. In this work, the effect of humic acid for the adsorption of Pb2+ onto sewage irrigation soil was investigated. As can be seen in Fig. 7, at solution pH 8, the presence of humic acid is found to decrease the adsorption of Pb onto sewage irrigation soil. This result is in accordance with experiments of other authors [41, 45]. It is reported that the complexation between Pb2+ and humic acid is more stable than the complexation between Pb2+ and other soil minerals such as Na-bentonite [46]. Therefore, at high solution pH (>7), the negatively charged humic acid may be difficult to be adsorbed on the negatively charged soil surface, so that the weak complexation ability of soil surface adsorbing humic acid with Pb2+ should result in the decrease of the adsorption of Pb2+ on sewage irrigation soil surface at solution pH 8. For example, ABATE and MASINI [47] studied the Pb2+ adsorption onto vermiculite and also found that the presence of humic acid decreased the adsorption at pH 7.0 as a consequence of the formation of stable complexes between humic acid and Pb2+ in solution.
Compared with the absence of humic acid, the adsorption mechanism of Pb2+ on sewage irrigation soil may be changed in the presence of humic acid. In this work, three kinds of isotherm models (Langmuir, Freundlich and Temkin) were also applied to these experimental data. The results of these three isotherm models applied to Pb2+ adsorption on sewage irrigation soil are shown in Table 4. As can be seen in Table 4, the results indicate that the Langmuir isotherm model poorly describes the adsorption of Pb2+ onto sewage irrigation soil in the presence of humic acid (R2= 0.228) while the Freundlich and Temkin isotherm models fit well the adsorption of Pb2+ onto sewage irrigation soil in the presence of humic acid (R2=0.843, 0.798, P<0.01). These results are in accordance with experiments of other authors [41]. But these results are different from the adsorption mechanism of Pb2+ on sewage irrigation soil in the absence of humic acid, that is, the Langmuir isotherm model fits well the adsorption of Pb2+ onto sewage irrigation soil in the absence of humic acid but poorly describes the adsorption of Pb2+ in the presence of humic acid. It is worth mentioning that Freundlich isotherm model is more fit for the adsorption of Pb2+ onto sewage irrigation soil in the absence and presence of humic acid than Langmuir and Temkin isotherm models due to the highest R2 value.
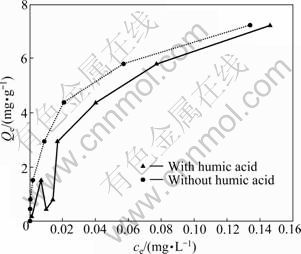
Fig. 7 Effect of humic acid on adsorption of Pb2+ onto sewage irrigation soil at pH 8
3.5 Effect of competitive ions
Generally, the adsorption of Pb onto soil is not only controlled by soil solution pH, organic matter, etc, but also affected by the competitive ions in soil due to the similar existence forms in soil. In this work, batch equilibrium experiments were performed in different initial Pb2+ concentrations on the adsorption of Pb2+ by sewage irrigation soil at the certain concentration (6.35 mg/L) of Cu2+. As can be seen in Fig. 8, in comparison with the absence of Cu2+, the amount of Pb2+ adsorbed by sewage irrigation soil decreases in the presence of constant Cu2+, and the adsorption rate for Pb2+ decreases more quickly in the presence of Cu2+. When the initial Pb2+ is from 1.78 mg/L to 178 mg/L, the corresponding adsorption rate of Pb2+ in the absence of Cu2+ decreases from 100% to 99.92% while the adsorption rate of Pb2+ in the presence of Cu2+ decreases from 100% to 96.5%. The competitive adsorption of Pb2+ and Cu2+ onto sewage irrigation soil is also analyzed by the Langmuir, Freundlich and Temkin isotherm models (Table 5). The values of correlation coefficient (R2) indicate that these three models are fit for the Pb2+ adsorption in competitive system, and the Langmuir isotherm model is more fit for the adsorption of Pb2+ onto sewage irrigation soil in competitive adsorption of Pb2+ and Cu2+ than Freundlich and Temkin isotherm models due to the highest R2 value. In comparison with single system (Table 3), the Qm value for competitive adsorption is reduced due to competition (from 7.47 mg/g to 6.99 mg/g).
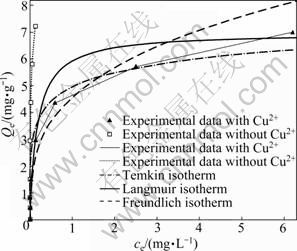
Fig. 8 Effect of competitive ion (Cu2+, 6.35 mg/L) on adsorption of Pb2+ onto sewage irrigation soil at pH 8
Table 4 Parameters for Langmuir, Freundlich and Temkin isotherms in presence of humic acid at pH 8

Table 5 Parameters of Langmuir, Freundlich and Temkin isotherms for Pb with competitive ion (Cu2+, 6.35 mg/L)

4 Conclusions
1) The Pb2+ adsorption onto the soil is relatively rapid and reaches equilibrium at about 2 h, and the pseudo-second order equation is fit for the adsorption of Pb2+ onto sewage irrigation soil. The adsorption isotherm of Pb2+ onto sewage irrigation soil can be well characterized by the Langmuir, Freundlich and Temkin isotherm models.
2) The adsorption of Pb2+ onto soil increases from about -8% to 100% at equilibrium pH range of 1.2-4.5, increases quickly at equilibrium pH<2, more quickly at equilibrium pH range of 2-3, then slowly at equilibrium pH range of 3-4.5 and reaches a plateau at equilibrium pH range of 4.5-12.
3) The presence of humic acid in soil decreases the adsorption of Pb2+ onto the soil at solution pH 8, and the adsorption of Pb2+ onto sewage irrigation soil is also influenced by the presence of Cu2+ mainly due to the competition adsorption between Pb2+ and Cu2+.
References
[1] KARCZEWSKA A. Metal species distribution in top- and sub-soil in an area affected by copper smelter emissions [J]. Applied Geochemistry, 1996, 11(1/2): 35-42.
[2] LIU Wen-hua, ZHAO Jing-zhu, OUYANG Zhi-yun, S?DERLUND L, LIU Guo-hua. Impacts of sewage irrigation on heavy metal distribution and contamination in Beijing, China [J]. Environment International, 2005, 31(6): 805-812.
[3] CHEN Zong-hui, HE Ming, SAKURAI K, KANG Y, IWASAKI K. Concentrations and chemical forms of heavy metals in urban soils of Shanghai, China [J]. Soil Science and Plant Nutrition, 2007, 53(4): 517-529.
[4] BONANNO G, GIUDICE R L. Heavy metal bioaccumulation by the organs of Phragmites australis (common reed) and their potential use as contamination indicators [J]. Ecological Indicators, 2010, 10(3): 639-645.
[5] MCBRIDE M B. Environmental chemistry in soil [M]. New York: Oxford University Press, 1994: 406.
[6] COEN N, MOTHERSILL C, KADHIM M, WRIGHT E G. Heavy metals of relevance to human health induce genomic instability [J]. Journal of Pathology, 2001, 195(3): 293-299.
[7] DOLK H, VRIJHEID M. The impact of environmental pollution on congenital anomalies [J]. British Medical Bulletin, 2003, 68(1): 25- 45.
[8] AINSWORTH C C, PILON J L, GASSMAN P L, VANDERSLUYS W G. Cobalt, cadmium, and lead sorption to hydrous iron oxide: Residence time effect [J]. Soil Science Society of America Journal, 1994, 58(6): 1615-1623.
[9] TRIVEDI P, DYER J A, SPARKS D L. Lead sorption onto ferrihydrite: 1. A macroscopic and spectroscopic assessment [J]. Environmental Science Technology, 2003, 37(5): 908-914.
[10] AZIZ H M A. Sorption equilibria of lead(II) on some Palestinian soils-the natural ion exchangers [J]. Colloids and Surfaces A: Physicochemical Engineering Aspects, 2005, 264(1/2/3): 1-5.
[11] MA Liang, XU Ren-kou, JIANG Jun. Adsorption and desorption of Cu(II) and Pb(II) in paddy soils cultivated for various years in the subtropical China [J]. Journal of Environmental Sciences, 2010, 22(5): 689-695.
[12] MOUNI L, MERABET D, ROBERT D, BOUZAZA A. Batch studies for the investigation of the sorption of the heavy metals Pb2+ and Zn2+ onto Amizour soil (Algeria) [J]. Geoderma, 2009, 154(1/2): 30-35.
[13] PONIZOVSKY A A, TSADILAS C D. Lead(II) retention by Alfisol and clinoptilolite: Cation balance and pH effect [J]. Geoderma, 2003, 115(3/4): 303-312.
[14] SCHWAB A P, HE Y H, BANKS M K. The influence of organic ligands on the retention of lead in soil [J]. Chemosphere, 2005, 61(6): 856-866.
[15] GUO Xue-yan, ZHANG Shu-zhen, SHAN Xiao-quan, LUO Lei, PEI Zhi-guo, ZHU Yong-guan, LIU Tao, XIE Ya-ning, GAULT A. Characterization of Pb, Cu and Cd adsorption on particulate organic matter in soil [J]. Environmental Toxicology and Chemistry, 2006, 25(9): 2366-2373.
[16] COVELO E F, VEGA F A, ANDRADE M L. Competitive sorption and desorption of heavy metals by individual soil components [J]. Journal of Hazardous Materials, 2007, 140(1/2): 308-315.
[17] LEE Suen-zone, CHANG Li-zone, YANG His-hsien, CHEN Chien-min, LIU Ming-chou. Adsorption characteristics of lead onto soils [J]. Journal of Hazardous Materials A, 1998, 63(1): 37-49.
[18] MART?NEZ-VILLEGAS N, FLORESl-V?LEZ L M, DOM?NGUEZ O. Sorption of lead in soil as a function of pH: A study case in México [J]. Chemosphere, 2004, 57(10): 1537-1542.
[19] SAUV? S, HENDERSHOT W, ALLEN H E. Solid-solution partitioning of metals in contaminated soils: Dependence on pH, total metal burden, and organic matter [J]. Environmental Science and Technology, 2000, 34(7): 1125-1131.
[20] HILLS P, ZHANG Lei, LIU Jian-hua. Transboundary pollution between Guangdong Province and Hong Kong: Threats to water quality in the Pearl River Estuary and their implications for environmental policy and planning [J]. Journal of Environmental Planning and Management, 1998, 41(3): 375-396.
[21] WONG S C, LI X D, ZHANG G, QI S H, MIN Y S. Heavy metals in agricultural soils of the Pearl River Delta, South China [J]. Environmental Pollution, 2002, 119(1): 33-44.
[22] LI Pei-jun, WANG Xin, ALLINSON G, LI Xiao-jun, XIONG Xian-zhe. Risk assessment of heavy metals in soil previously irrigated with industrial wastewater in Shenyang, China [J]. Journal of Hazardous Materials, 2009, 161(1): 516-521.
[23] CHEN Hua-main, ZHENG Chun-rong, TU Cong, ZHU Yong-guan. Heavy metal pollution in soils in China: Status and countermeasures [J]. Ambio, 1999, 28(2): 130-134.
[24] MCKEAGUE J A, DAY J H. Dithionite and oxalate-extractable Fe and Al as aids in differentiating various classes [J]. Canadian Journal of Soil Science, 1966, 46(1): 13-22.
[25] LI Jin-ling, HE Ming, SUN Shou-qin, HAN Wei, ZHANG You-chi, MAO Xiao-hui, GUO Yi-fan. Effect of the behavior and availability of heavy metals on the characteristics of the coastal soils developed from alluvial deposits [J]. Environmental Monitoring and Assessment, 2009, 156(1-4): 91-98.
[26] PANSU M, GAUTHEYROU J. Handbook of soil analysis- mineralogical, organic and inorganic methods [M]. Berlin: Springer- Verlag, Heidelberg, 2006: 993.
[27] GASPARATOS D, HAIDOUTI C. A comparison of wet oxidation methods for determination of total phosphorus in soils [J]. Journal of Plant Nutrition and Soil Science, 2001, 164(4): 435-439.
[28] RAIJ G V, PEECH M. Electrochemical properties of some Oxisols and Alfisols of the tropics [J]. Soil Science Society of America Proceedings, 1972, 36(4): 587-593.
[29] KALUDJEROVIC-RADOICIC T, RAICEVIC S. Aqueous Pb sorption by synthetic and natural apatite: Kinetics, equilibrium and thermodynamic studies [J]. Chemical Engineering Journal, 2010, 160: 503-510.
[30] PLANTE B, BENZAAZOUA M, BUSSI?RE B, BIESINGER M C, PRATT A R. Study of Ni sorption onto Tio mine waste rock surfaces [J]. Applied Geochemistry, 2010, 25(12): 1830-1844.
[31] CHEN Zhen, MA Wei, HAN Mei. Biosorption of nickel and copper onto treated alga (Undaria pinnatifida): Application of isotherm and kinetic models [J]. Journal of Hazardous Materials, 2008, 155(1/2): 327-333.
[32] HO Y S, PORTER J F, MCKAY G. Equilibrium isotherm studies for the sorption of divalent metal ions onto peat: Copper, nickel and lead single component systems [J]. Water Air Soil Pollution, 2002, 141(1-4): 1-33.
[33] KUNDU S, GUPTA A K. Arsenic adsorption onto iron oxide-coated cement (IOCC): Regression analysis of equilibrium data with several isotherm models and their optimization [J]. Chemical Engineering Journal, 2006, 122(1/2): 93-106.
[34] HASHIMOTO Y, SATO T. Removal of aqueous lead by poorly-crystalline hydroxyapatites [J]. Chemosphere, 2007, 69(11): 1775-1782.
[35] SERRANO S, GARRIDO F, CAMPBELL C G, GARC?A- GONZ?LEZ M T. Competitive sorption of cadmium and lead in acid soils of Central Spain [J]. Geoderma, 2005, 124(1/2): 91-104.
[36] GHAEDI M, GHEZELBASH G R, MARAHEL F. Equilibrium, thermodynamic, and kinetic studies on lead(II) biosorption from aqueous solution by saccharomyces cerevisiae biomass [J]. Clean-Soil, Air, Water, 2010, 38(9): 877-885.
[37] ADHIKARI T, SINGH M V. Sorption characteristics of lead and cadmium in some soils of India [J]. Geoderma, 2003, 114(1/2): 81- 92.
[38] WENG C H. Modeling Pb(II) adsorption onto sandy loam soil [J]. Journal of Colloid and Interface Science, 2004, 272(2): 262-270.
[39] XU D, TAN X L, CHEN C L, WANG X K. Adsorption of Pb(II) from aqueous solution to MX-80 bentonite: Effect of pH, ionic strength, foreign ions and temperature [J]. Applied Clay Science, 2008, 41(1/2): 37-46.
[40] FAN Qiao-hui, LI Zhan, ZHAO Hao-gui, JIA Ze-hong, XU Jun-zheng WU Wang-suo. Adsorption of Pb(II) on palygorskite from aqueous solution: Effects of pH, ionic strength and temperature [J]. Applied Clay Science, 2009, 45(3): 111-116.
[41] WANG X, XU D, CHEN C, TAN X, ZHOU X, REN A, CHEN C. Sorption and complexation of Eu(III) on alumina: Effects of pH, ionic strength, humic acid and chelating resin on kinetic dissociation study [J]. Applied Radiation Isotopes, 2006, 64(4): 414-421.
[42] DONG Li-jing, ZHU Zhi-liang, MA Hong-mei, QIU Yan-ling, ZHAO Jian-fu. Simultaneous adsorption of lead and cadmium on MnO2-loaded resin [J]. Journal of Environmental Sciences, 2010, 22(2): 225-229.
[43] CHEN Y X, LIN Q, LUO Y M, HE Y F, ZHEN S J, YU Y L, TIAN G M, WONG M H. The role of citric acid on the phytoremediation of heavy metal contaminated soil [J]. Chemosphere, 2003, 50(6): 807-811.
[44] WU L H, LUO Y M, CHRISTIE P, WONG M H. Effects of EDTA and low molecular weight organic acids on soil solution properties of a heavy meal polluted soil [J]. Chemosphere, 2003, 50(6): 819-822.
[45] TAKAHASHI Y, MINAI Y, AMBE S, MAKIDE Y, AMBE F. Comparison of adsorption behavior of multiple inorganic ions on kaolinite and silica in the presence of humic acid using the multitracer technique [J]. Geochimca et Cosmochimica Acta, 1999, 63(6): 815-836.
[46] WANG Suo-wei, HU Jun, LI Jia-xing, DONG Yun-hui. Influence of pH, soil humic/fulvic acid, ionic strength, foreign ions and addition sequences on adsorption of Pb(II) onto GMZ bentonite [J]. Journal of Hazardous Materials, 2009, 167(1/2/3): 44-51.
[47] ABATE G, MASINI J C. Influence of pH, ionic strength and humic acid on adsorption of Cd(II) and Pb(II) onto vermiculite [J]. Colloids and Surfaces A, 2005, 262(1/2/3): 33-39.
(Edited by HE Yun-bin)
Foundation item: Project(SK201109) supported by the Basic Scientific Study Funding from Institute of Hydrogeology and Environmental Geology, Chinese Academy of Geological Sciences; Project(2010CB428806-2) supported by the National Basic Research Program of China
Received date: 2011-01-14; Accepted date: 2011-03-29
Corresponding author: HUANG Guan-xing, PhD; Tel: +86-13833398747; E-mail: huangguanxing2004@126.com
Abstract: Pb2+ adsorption onto a soil by irrigation of sewage in the Pearl River Delta of South China was examined as a function of the reaction time, solution pH, initial lead concentration, organic matter (humic acid) and competitive ions (Cu2+). The adsorption of Pb2+ onto the soil was investigated on batch equilibrium adsorption experiments. Results show that the Pb2+ adsorption on the soil is relatively rapid in the first 30 min and reaches equilibrium at 2 h, and the kinetics of the adsorption process on the soil is well characterized by the pseudo-second order reaction rate. Langmuir, Freundlich and Temkin isothermal models are fit for the adsorption of Pb2+ onto the soil, and the maximum amount of Pb2+ adsorption (Qm) is 7.47 mg/g. The amount of Pb2+ adsorption increases with increasing the pH at the range of 1.2-4.5 and reaches a plateau at the range of 4.5-12. The presence of humic acid in soil decreases the adsorption of Pb2+ onto the soil at solution pH of 8 since the negatively charged humic acid with Pb2+ is difficult to be adsorbed on the negatively charged soil surface. The adsorption of Pb2+ onto the soil also decreases in the presence of Cu2+ due to the competition adsorption between Pb2+ and Cu2+.


