Trans. Nonferrous Met. Soc. China 28(2018) 2553-2565
Synthesis of novel silica-supported chelating resin containing tert-butyl 2-picolyamino-N-acetate and its properties for selective adsorption of copper from simulated nickel electrolyte
Cai-xia WANG1,2, Hui-ping HU1,2, Xue-jing QIU1,2, Ze-ying CHENG1,2, Lu-jia MENG1, Li ZHU1
1. School of Chemistry and Chemical Engineering, Central South University, Changsha 410083, China;
2. Hunan Provincial Key Laboratory of Efficient and Clean Utilization of Manganese Resources, Central South University, Changsha 410083, China
Received 4 November 2017; accepted 7 May 2018
Abstract:
A novel silica-supported tert-butyl 2-picolyamino-N-acetate chelating resin (Si-AMPY-1) was successfully synthesized and characterized by elemental analysis, FT-IR, SEM and 13C CP/MAS NMR. The adsorption behaviors of the Si-AMPY-1 resin for Cu(II) and Ni(II) were studied with batch and column methods. The batch experiments indicated that the Si-AMPY-1 resin adsorbed Ni(II) mainly via physisorption, while adsorbed Cu(II) via chemisorption. The column dynamic breakthrough curves revealed that the Si-AMPY-1 resin can efficiently separate Cu(II) from the simulated nickel electrolyte before the breakthrough point. Moreover, the concentration of Cu(II) in the column effluent was decreased to be less than 3 mg/L within the first 43 BV (bed volumes), and the mass ratio of Cu/Ni was 21:1 in the saturated resin, which completely satisfied the industrial requirements of the nickel electrorefining process. Therefore, it was concluded that the Si-AMPY-1 resin can be a promising candidate for the deep removal of Cu(II) from the nickel electrolyte.
Key words:
chelating resin; selective adsorption; copper removal; simulated nickel electrolyte; synthesis;
1 Introduction
In the electrolytic refining of nickel, impurities such as copper, cobalt and iron must be reduced to very low levels prior to nickel electro-deposition in the nickel electrolyte [1]. As a major impurity element in the nickel electrolyte, copper can be more easily deposited on the cathode than nickel due to its higher positive potential, which affects the quality of the electrodeposited nickel [2]. Compared with traditional copper removal methods like chemical precipitation [3-5], electro- deposition [6], solvent extraction [7] and ion-exchange method [8], the adsorption with chelating resins can solve the problems such as organic contamination of streams and generation of toxic sludge [9,10]. Chelating resins can be synthesized by immobilizing appropriate chelating organic molecules on the insoluble substrate surfaces.
In addition, as a substrate material, silica gel has been studied extensively due to its high surface areas, uniform and controllable pore size and shape as well as good mechanical, chemical and thermal stabilities [11-14]. The active H atom in the surface silanol groups dispersed on the surface of silica gel can react with organosilyl groups, which makes it possible for organic molecules to be loaded on the inorganic matrix of silica gel [15]. Consequently, a number of silica-supported chelating resins (see Fig. 1) were synthesized and applied in the separation of Cu(II) from hydrometallurgical electrolyte solutions [2,16-20]. For instance, CuWRAM, one of the silica-supported chelating resins, was synthesized by a pre-grafting method as follows: the active silica gel was grafted with polyamine, followed by the reaction with 2-aminomethylpyridine (AMP). Nevertheless, this pre-grafting method could cause a number of undesirable terminal functional groups grafted on the silica gel such as primary amine groups, which results in the poor adsorption selectivity towards Cu(II) over Ni(II) using the CuWRAM resin [20]. Then, our research group proposed a post-grafting method which was involved in the reaction of 2-aminomethylpyridine (AMP) with γ-chloropropyltrimethoxysilane (CPTS) prior to immobilization on the silica surface to synthesize a chelating resin Si-AMP-M-H [2]. Although the purity of functional groups anchored on the silica surface of the Si-AMP-M-H resin was predominantly improved, the level of the adsorption selectivity for Cu(II) versus Ni(II) was still undesirable [21]. Therefore, more attentions should be paid to the improvement of the adsorption selectivity for specific metal ions using these chelating resins.
In our previous work, a novel organic compound, tert-butyl 2-(N-octyl-2-picolyamino) acetate (AMPA), was successfully prepared as Scheme 1, and AMPA can not be coordinated with Ni(II), but with Cu(II) [22]. Therefore, we can suppose that a novel chelating resin immobilized with similar functional groups as those in the AMPA can be a promising candidate for the deep removal of copper(II) from the simulated nickel electrolyte. This novel chelating resin, denoted as Si-AMPY-1, was obtained by immobilization of a 2-aminomethylpyridine derivative, tert-butyl 2-picoly- amino-N-acetate (AMPY-1), onto the silica gel. The adsorption behavior of metal ions onto the self-synthesized resin was systematically investigated by carrying out batch adsorption for single and binary metal solution systems, respectively. Furthermore, column experiments have been carried out to verify the potential application of the novel chelating resin for the selective separation of Cu(II) from the simulated nickel electrolyte.
2 Experimental
2.1 Reagents and apparatus
The silica gel (0.125-0.150 mm in size) was purchased from Qingdao Haiyang Chemical Co., Ltd., China. Organic reagents 2-aminomethylpyridine (AMP) (Leqi Chemical Factory, China), tert-butyl bromoacetate (Taishan Chemical Factory, China), γ-chloro- propyltrimethoxysilane (CPTS) (Xiya Chemical Factory, China) of chemically pure grade and meglumine (Aladdin Co., Ltd., China) of analytical grade were used as received. Analytical reagents, triethylamine and N,N-dimethylformamide (DMF), were purchased from Sinopharm Group Chemical Reagent Co., Ltd. and purified according to the published procedures [23]. CuCl2·2H2O, NiCl2·6H2O, CuSO4·5H2O and NiSO4·6H2O of analytical grade were purchased from Sinopharm Group Chemical Reagent Co., Ltd., and used without further purification. Single or binary metal working aqueous solutions at various concentrations were prepared by dissolving single or binary metal salts in ultrapure water, respectively. The pH values of the working aqueous solutions were adjusted to the desired values with dilute sodium hydroxide or hydrochloric acid.

Fig. 1 Schematic structure of silica-supported 2-aminomethylpyridine

Scheme 1 Synthesis of tert-butyl 2-(N-octyl-2-picolyamino) acetate, AMPA [22]
A TAS-990F flame atomic adsorption spectrophotometer (FAAS, Beijing Purkinje General Instrument Co., China) was used to determine the concentration of metal ions in aqueous solutions. The pH measurements were done using a Mettler Toledo FE20 digital pH meter (Mettler Toledo Shanghai Co., China). The 1H-NMR spectrum was measured on a Bruker Avance 500MHz NMR spectrometer (Bruker Co., Switzerland). The 13C CP/MAS NMR spectrum of the Si-AMPY-1 resin was recorded on a Bruker Avance 400 MHz NMR spectrometer (Bruker Co., Switzerland). The Fourier transform infrared (FT-IR) spectra were recorded in the range of 4000-400 cm-1 on a Nicolet 6700 Fourier transform infrared spectrophotometer (Thermo Scientific Co., USA), and using KBr pellets. Elemental analyses (C, H and N) were carried out by a flash EA 1112 element analyzer (Thermo Scientific Co., USA). X-ray photoelectron spectroscopy measurements were carried out on an ESCALAB 250Xi XPS spectrometer (Thermo Scientific Co., USA). The XPS analysis used an Al Kα X-ray at source (1486.6 eV of protons) and a high vacuum of 10-8 Pa was maintained in the XPS chamber. All the XPS spectra were corrected for charging effect by referencing the C1s peak of hydrocarbons to 284.8 eV and quoted with a precision of ±0.2 eV. High resolution spectra were analyzed to identify various chemical species present. Thermo avantage 5.52 software was used for data analysis.
2.2 Synthesis of tert-butyl 2-picolyamino-N-acetate (AMPY-1)
The synthesis of tert-butyl 2-picolyamino-N- acetate (AMPY-1) was performed according to the protocol reported in Ref. [24]. A mixture of 2-aminomethyl pyridine (0.1 mol) and purified triethylamine (0.12 mol) in ethanol (100 mL) was stirred under highly pure N2 atmosphere, followed by the addition of tert-butyl bromoacetate (0.1 mol) dropwise over a period of 2 h. The reaction mixture was refluxed with stirring for a further 48 h at ambient temperature. Then the precipitate of triethylamine hydrobromide was filtered off, and the solvent was evaporated under reduced pressure. The residue was purified with silica gel column chromatography (eluent: petroleum ether/EtOAc=1:1 and then CH3OH/CHCl3=3:97, v/v) and yielded a brown oil of the AMPY-1 (55%). 1H-NMR (500 MHz, CDCl3): δ 8.57×10-6 (d, J = 4.5 Hz, 1H), 7.66 (t, J=7.6 Hz, 1H), 7.33 (t, J=17.5 Hz, 1H), 7.25-6.98 (m, 1H), 3.95 (s, 2H), 3.39 (s, 2H), 2.39 (s, 1H), 1.48 (s, 9H). FT-IR (KBr, ν/cm-1): N—H, 3336; C=O, 1735; C—O—C, 1150.
2.3 Synthesis of CPTS-AMPY-1
The AMPY-1 was subsequently functionalized with CPTS. The synthesis was carried out in a 250 mL three- necked round-bottomed flask equipped with a magnetic stirrer, a reflux condenser and a nitrogen inlet. A mixture of CPTS (20 mmol), purified triethylamine (24 mmol) and purified DMF (50 mL) were first added into the flask, stirred and heated to 90 °C under highly pure N2 atmosphere. Then, a solution of AMPY-1 (24 mmol) in 30 mL of purified DMF was slowly and continuously added into the flask, and the mixture in the flask was stirred for a further period of 36 h at 90 °C. Finally, the mixture in the flask was cooled down slowly to the ambient temperature and the precipitate of triethylamine hydrochloride was filtered off. The solvent was then removed from the filtrate in vacuo, and the residue CPTS-AMPY-1 was obtained for further use.
2.4 Synthesis of silica-supported tert-butyl 2-picoly- amino-N-acetate chelating resin (Si-AMPY-1)

Scheme 2 Synthesis of Si-AMPY-1 resin in this study
Commercially-available silica gel was first activated by refluxing with 1 mol/L nitric acid for 6 h, filtered off, and washed with ultrapure water until free from acid, and air dried at room temperature for 24 h to obtain activated silica gel. The synthesis of the Si-AMPY-1 resin was performed according to Scheme 2, and the procedure was described as follows: meglumine (5.0 g) was immersed into 30 mL of DMF, and a specified volume of ultrapure water was added into the three-necked round-bottomed flask until the meglumine dissolved completely. After activated silica gel (50 g) was dispersed into the meglumine-DMF aqueous solution with mechanical stirring at 90 °C for 1 h, a solution of CPTS-AMPY-1 (5 mL) in 20 mL of purified DMF was added dropwise into the flask. While half the solution of CPTS-AMPY-1 in purified DMF was added, a solution of 5% hydrochloric acid (2 mL) in 8 mL of ethanol was also added dropwise into the flask with continuous stirring. After the addition finished, the reaction was kept at 90 °C for 40 h. Then the suspension was allowed to cool to room temperature, and filtered to obtain a solid product. The solid product was washed with methanol and transferred to Soxhlet extraction apparatus for reflux-extraction in methanol for 8 h. Finally, the solid product was dried under vacuum at 50 °C over 48 h and later used as the novel resin in the adsorption study.
2.5 Adsorption experiments
2.5.1 Batch experiments
All the batch experiments were carried out in a Techne TE-10D thermostat (Techne Co., UK) with temperature stability up to ±0.01 °C. Considering abundant chloride and sulfate anions are often presented in the nickel electrolyte [3], it is important to examine the adsorption properties of the novel chelating resin for aqueous metal solutions with high ionic strength, high contents of chloride and sulfate anions. The ionic strength of all working aqueous solutions was adjusted with proper amount of NaCl and Na2SO4, and the concentrations of Cl- and 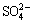 in the working aqueous solutions were correspondingly kept at about 75 and 100 g/L.
in the working aqueous solutions were correspondingly kept at about 75 and 100 g/L.
The influence of the solution pH was studied at the initial pH ranging from 1.0 to 5.0. Several conical flasks, each containing 50 mL of single metal solution with an initial metal concentration of 1 mmol/L of Cu(II) or Ni(II) at different initial pH values, were prepared. A dosage of 0.05 g Si-AMPY-1 resin was added into each flask. Then the flask was immersed in the thermostatted water bath, and the suspension in each flask was stirred at (40±0.01) °C for 90 min.
Kinetic adsorption experiments were performed by mixing 0.05 g Si-AMPY-1 resin to each conical flask containing 50 mL of single metal solution with an initial metal concentration of 1 mmol/L of Cu(II) or Ni(II) at initial pH of 4.0. A series of such conical flasks were kept in the thermostatted water bath. All suspensions in the flasks were stirred at (40±0.01) °C for 5, 10, 15, 30, 45, 60, 90, 120, 150, 180 and 210 min, respectively.
In the adsorption isotherm tests, 0.05 g Si-AMPY-1 resin and 50 mL of the single metal solution with an initial concentration varied in the range of 0.5-5.0 mmol/L at initial pH of 4.0 were added into a series of conical flasks. All flasks were kept in the thermostatted water bath. All suspensions in the flasks were stirred for 90 min at (25±0.01), (40±0.01) and (55±0.01) °C, respectively.
Adsorption selectivity was evaluated based on the competitive adsorption performances in binary metal solutions. For the binary metal solution, the initial concentration of the Cu(II) remained constant at 0.04 mmol/L, whereas the concentration of Ni(II) varied from 0.04 to 200 mmol/L at initial pH of 4.0 (to provide different initial molar concentration ratios of Ni(II) to Cu(II) from 1:1 to 5000:1). Then, a dosage of 0.05 g Si-AMPY-1 resin was added into each conical flask with 50 mL of the binary metal solution. All the flasks were kept in the thermostatted water bath. All the suspensions in the flasks were stirred at (40±0.01) °C for 90 min.
The initial and residual concentrations of the studied metal ions in the working solutions were determined by FAAS. The amount of metal ion adsorbed per unit mass of the resin (Qt), distribution coefficient (Kd), and equilibrium selectivity coefficient α(Cu/Ni) between copper and nickel could be calculated according to following equations:
 (1)
(1)
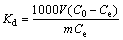 (2)
(2)
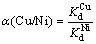 (3)
(3)
where Qt (mmol/g) is the adsorption capacity of the Si-AMPY-1 resin at time t, C0 (mmol/L) is the initial metal ion concentration of the working solution, Ct (mmol/L) is the metal ion concentration of the working solution at time t, V (L) is the volume of the working solution, and the m (g) is the dry mass of the Si-AMPY-1 resin. After the adsorption reached equilibrium, the equilibrium concentration of the metal ion in the working solution was described as Ce. The equilibrium value, Qe, was obtained when Ce is used instead of Ct in Eq. (1). Kd (mL/g) is the distribution coefficient in working solutions. α(Cu/Ni) is the equilibrium selectivity coefficient between copper and nickel.
2.5.2 Column experiments
In the column experiment, 3.85 g of the Si-AMPY-1 resin was immersed in ultrapure water and then placed in a glass column (10 mm in diameter) with a bed volume (BV) of 4 mL. A small amount of cotton wool was placed to retain the packing resin. A simulated nickel electrolyte as the influent was prepared according to the composition of the nickel electrolyte from Jinchuan Group Co. Ltd. in China (see Table 1), and passed through the column at room temperature using a peristaltic pump with an optimum flow rate of 5 BV/h (bed volume per hour) until the breakthrough curve was completed. The samples of the column effluent were collected at different time intervals, and the concentration of metal ion was determined by FAAS. The effluent concentration of copper at specific bed volume is indicated as Cv and the initial concentration of copper in the simulated nickel electrolyte is indicated as Ci.
Table 1 Composition of simulated nickel electrolyte at pH~4

The saturated resin was collected after Cv/Ci equals to 0.95. After washing with abundant ultrapure water and drying at room temperature, the saturated resin was desorbed with 2 mol/L sulfuric acid to obtain a desorption solution, and the contents of Cu and Ni in the desorption solution were also analyzed by FAAS. By calculation, the mass ratio of Cu/Ni in the desorption solution was regarded as the mass ratio of Cu/Ni in the saturated resin.
2.5.3 Possible adsorption mechanism
In order to interpret the possible adsorption mechanism of Cu(II) or Ni(II) on the Si-AMPY-1 resin, the XPS analyses for the Si-AMPY-1 resin, Cu(II)- and Ni(II)-loaded Si-AMPY-1 resins (abbreviated as Si-AMPY-1+Cu and Si-AMPY-1+Ni, respectively) were carried out. The Cu(II)- and Ni(II)-loaded Si-AMPY-1 resins were prepared as follows: 0.05 g Si-AMPY-1 resin was added into each conical flask containing 50 mL of sole metal chloride solution with an initial metal concentration of 1 g/L of Cu(II) or Ni(II) at initial pH of 4.0. The suspension in each flask was stirred at (40±0.01) °C for 90 min. The metal-loaded resin was separated by filtration and washed several times with ultrapure water to remove unadsorbed metal ions on the surface of the resin. Then the metal-loaded resin was dried under vacuum at 50 °C over 48 h for subsequent XPS studies.
3 Results and discussion
3.1 Characterization of Si-AMPY-1 resin
3.1.1 Elemental analysis
The degree of immobilization on the silica gel surface was determined by considering the elemental analysis results. From the amount of N (0.99%), the mole amount of AMPY-1 functional groups in per gram resin was calculated to be 0.35 mmol.
3.1.2 FT-IR spectra
The FT-IR spectra of activated silica gel and the Si-AMPY-1 resin were shown in Fig. 2. For activated silica gel (Fig. 2(a)), a large broad band between 3600 and 3200 cm-1 was attributed to the presence of the O—H stretching of the silanol groups. The intensity peaks at about 1100 and 468 cm-1 were correspondingly attributed to Si—O—Si stretching and bending vibrations, and a band was assigned to Si—O stretching frequency for silanol groups at ~900 cm-1 [25]. The Si-AMPY-1 resin presents two new bands at 2983 and 2950 cm-1 (see Fig. 2(b)) due to the C—H stretching of the tetrahedral carbon and the peak at 688 cm-1 was aliphatic C—H bending vibrations [26]. Moreover, the characteristic peak of C=O was observed at 1732 cm-1. The weak adsorption at 1479 cm-1 in Si-AMPY-1 was ascribed to C—N stretching vibration of aliphatic tertiary amine. From all these evidences, it was clear that the AMPY-1 had been grafted to the silica gel matrix successfully.
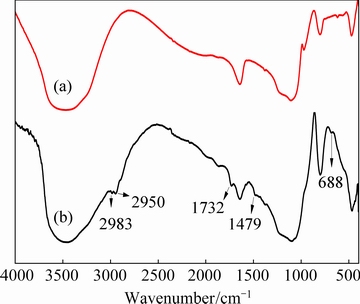
Fig. 2 FT-IR spectra of activated silica gel (a) and Si-AMPY-1 resin (b)
3.1.3 SEM images
The surface topography of activated silica gel and the Si-AMPY-1 resin was further observed by SEM, and micrographs of SEM were shown in Fig. 3. As shown in Fig. 3, there was no drastic difference between the morphology of activated silica gel in comparison with the Si-AMPY-1 resin. Specifically, more wrinkled and rough topography was presented in the Si-AMPY-1 resin, whereas more homogeneous surfaces was presented in activated silica gel. This was mainly because of the AMPY-1 grafted onto the silica gel surface, which effectively increased the specific surface area of the silica gel during the synthesis of the Si-AMPY-1 resin.
3.1.4 13C CP/MAS NMR spectra
The identity of the Si-AMPY-1 resin was also confirmed by solid-state 13C CP/MAS NMR spectroscopy. Three signals observed at δ=9.3×10-6, 20.9×10-6 and 53.5×10-6 may be correspondingly assigned to the carbon atoms of the pendant groups labeled 1, 2 and 3 [26,27], as indicated in Fig. 4. The five aromatic carbons labeled 9-13 were assigned in the range of (120-160)×10-6, due to the structural symmetry of the pyridine [19]. All these observations supported the success of the immobilization of the AMPY-1 onto the silica gel matrix through a —O3Si—(CH2)3— spacer.

Fig. 3 SEM micrographs of activated silica gel (a) and Si-AMPY-1 resin (b)

Fig. 4 13C CP/MAS NMR spectra of Si-AMPY-1 resin
3.2 Effect of initial pH
The effect of initial pH on the adsorption in a pH range from 1.0 to 5.0 was presented in Fig. 5. As shown in Fig. 5, the adsorption capacities of Cu(II) and Ni(II) ions increased with the increase of the pH values. It can be explained with competitive adsorption of H3O+ ions and metal ions for the same active adsorption site [28]. At low pH values, the competition of H3O+ ions for active adsorption sites increases and the chelating ability of resin is greatly reduced. An increase in pH decreases the competition of H3O+ ions for the active adsorption sites, and the adsorption of the metal ions is favored. On the other hand, during the purification process with adsorption method, the pH value of nickel electrolyte was usually kept at 4-4.5. In the light of these results, pH 4.0 was chosen as optimum pH for further experiments.

Fig. 5 Effect of pH on adsorption of Cu(II) and Ni(II) on Si-AMPY-1 resin
3.3 Adsorption kinetics
The effects of contact time on Cu(II) and Ni(II) adsorption were investigated and the results were shown in Fig. 6(a). It was evident that the adsorption of Cu(II) or Ni(II) was initially rapid, and thereafter proceeded at a slower rate, and finally reached equilibrium within a contact time of 30 min. Probably the fast adsorption of Cu(II) and Ni(II) on the Si-AMPY-1 resin can be ascribed to the good accessibility of the binding sites on the surface of the Si-AMPY-1 resin [29]. The equilibrium was reached within 90 min and the equlibrium adsorption capacities for Cu(II) and Ni(II) on the Si-AMPY-1 resin with the batch adsorption method were correspondingly 0.44 mmol/g and 0.05 mmol/g in the range of the given test conditions. The contact time of 90 min was chosen in the subsequent experiments.
Both the pseudo first- and second-order models were used to fit the experimental data, which were presented as Eqs. (4) and (5) [30].
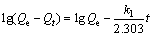 (4)
(4)
 (5)
(5)
where k1 (min-1) and k2 (g·mmol-1·min-1) are the pseudo-first-order and pseudo-second-order rate constant, respectively.
The plots of t/Qt versus t were depicted in Fig. 6(b) and the parameters of the pseudo-first-order and pseudo-second-order models were summarized in Table 2. As listed in Table 2, the pseudo second-order model correlated better (R2>0.99) than the pseudo first-order model with the experimental data. Meanwhile, the theoretical  estimated by the pseudo second-order kinetic model fitted well with all experimental data
estimated by the pseudo second-order kinetic model fitted well with all experimental data 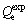 . This means that the adsorption process of Cu(II) and Ni(II) on the Si-AMPY-1 resin could be best described by the pseudo second-order kinetic model.
. This means that the adsorption process of Cu(II) and Ni(II) on the Si-AMPY-1 resin could be best described by the pseudo second-order kinetic model.
3.4 Adsorption isotherms
The adsorption isotherms described the relationship between the concentration of metal ion in solution and the amount of metal ion adsorbed on the adsorbent at a constant temperature [31]. The results of adsorption equilibrium of Cu(II) and Ni(II) on the Si-AMPY-1 resin at (25±0.01), (40±0.01) and (55±0.01) °C were shown in Fig. 7, respectively. The isotherm adsorption data were analyzed by fitting Langmuir and Freundlich adsorption isotherm models, respectively. The equations were expressed by Eqs. (6) and (7) [32].
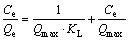 (6)
(6)
Table 2 Kinetic parameters for Cu(II) and Ni(II) on Si-AMPY-1 resin
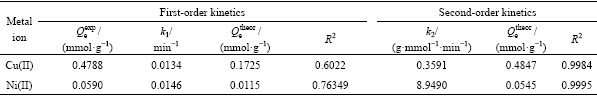

Fig. 6 Adsorption kinetics of Cu(II) and Ni(II) on Si-AMPY-1 resin (a), pseudo second-order plots for Cu(II) and Ni(II) on Si-AMPY-1 resin (b)
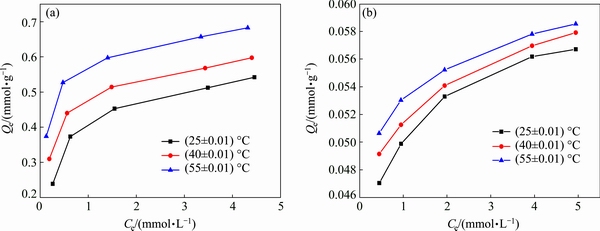
Fig. 7 Adsorption isotherms of Si-AMPY-1 resin for Cu(II) (a) and Ni(II) (b) at different temperatures
 (7)
(7)
where Qmax (mmol/g) is the maximum adsorption capacity of Langmuir, KL (L/mmol) is the Langmuir constant related to the energy of adsorption, KF (mmol/g) is the Freundlich constant related to the adsorption capacity and n is the heterogeneity factor which represents the bond distribution.
In this study, the results of the adsorption isotherms of Cu(II) and Ni(II) at different temperatures were shown in Figs. 8 and 9, and the corresponding adsorption parameters along with correlation coefficients were summarized in Table 3. It was found that the adsorption of Cu(II) and Ni(II) on the Si-AMPY-1 resin increased along with the increase of initial metal concentration at all the studied temperatures. As shown in Table 3, the Langmuir isotherm model correlated better (R2>0.998) than the Freundlich isotherm model with the experimental data from adsorption equilibrium of Cu(II) ions by the Si-AMPY-1 resin, suggesting a monolayer adsorption. For the investigated Ni(II) concentration range, the correlation coefficient values for the Langmuir and Freundlich isotherm models were relatively high (above 0.989). It can be seen that the Langmuir and Freundlich isotherm models were both fitted for Ni(II) adsorption on the Si-AMPY-1 resin.

Fig. 8 Langmuir isotherms of Si-AMPY-1 resin for Cu(II) (a) and Ni(II) (b) at different temperatures
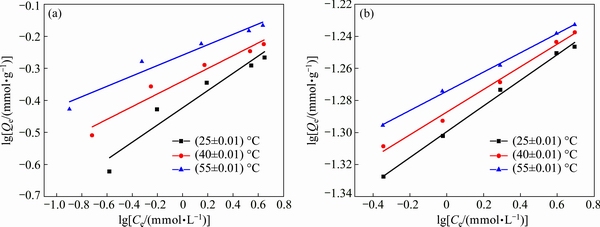
Fig. 9 Freundlich isotherms of Si-AMPY-1 resin for Cu(II) (a) and Ni(II) (b) at different temperatures
Table 3 Isotherm parameters for adsorption of Cu(II) and Ni(II) using Si-AMPY-1 resin

3.5 Thermodynamic studies
Figure 7 illustrated that the adsorption capacities significantly increase along with the increase of temperature, which indicates that higher temperature is in favor of the adsorption. Initial concentrations of Cu(II) and Ni(II) were taken as 1 mmol/L, respectively. The thermodynamic parameters for the adsorption of Cu(II) and Ni(II) on the Si-AMPY-1 resin can be calculated from Eqs. (8) and (9).
 (8)
(8)
ΔG=-RTln Kd (9)
where R is the mole gas constant, T (K) is the temperature, Kd (mL/g) is the distribution coefficient, ΔS (J/(mol·K-1) is the entropy change, ΔH (kJ/mol) is the enthalpy change and ΔG (kJ/mol) is the Gibbs free energy change in a given process. The results of ΔS, ΔH and ΔG for the adsorption process were listed in Table 4.
Table 4 Thermodynamic parameters for adsorption of Cu(II) and Ni(II) on Si-AMPY-1 resin

From the thermodynamics point of view, the magnitude of ΔG decreased slightly with a rise in temperature. The negative values of ΔG indicated that the adsorption of Cu(II) and Ni(II) on the Si-AMPY-1 resin was a spontaneous process. The positive values of ΔH confirmed that the adsorption was an endothermic process and the adsorption efficiency can increase with the increase of temperature. It was shown that the ΔH value for Ni(II) was 1.85 kJ/mol and that for Cu(II) was 16.93 kJ/mol, suggesting that the Si-AMPY-1 resin adsorbed Ni(II) mainly via physisorption, while adsorbed Cu(II) via chemisorption [33]. In addition, the positive values of ΔS suggested an increase in the randomness at the solid-liquid interface, which may be attributed to the liberation of water molecules from hydrated metal ions during the adsorption process [34].
3.6 Adsorption selectivity
The adsorption selectivity coefficients α(Cu/Ni) of the Si-AMPY-1 resin in binary metal solutions were calculated and presented in Fig. 10. In Fig. 10, it was interesting to note that the Kd values of the two metal ions all decreased with the elevated initial concentration ratio of Ni(II) to Cu(II), but the Kd of Ni(II) dropped sharply in contrast with Cu(II). Moreover, as indicated by the increase of α(Cu/Ni) from 0.35 to 3238.26 when an initial molar concentration ratio of Ni(II) to Cu(II) varied from 1:1 to 5000:1, higher concentration of Ni(II) rendered the separation more efficiently. As commercial Purolite S984 resin, the adsorption selectivity coefficient α(Cu/Ni) increased from 1.2 to 370 while the initial molar concentration ratio of Ni(II) to Cu(II) varied from 1:1 to 2000:1 [35]. When the initial molar concentration ratio of Ni(II) to Cu(II) was 2000:1, the adsorption selectivity coefficients α(Cu/Ni) of the Si-AMPY-1 resin and commercial Purolite S984 resin were 1566.55 and 370 [35], respectively. It was shown that the Si-AMPY-1 resin displayed an excellent selectivity in the adsorption of copper over nickel and the maximum selectivity coefficient α(Cu/Ni) was 3238.26. Therefore, the Si-AMPY-1 resin offered excellent copper removal ability in the presence of a high concentration of competing Ni(II).

Fig. 10 Distribution coefficients and selectivity coefficients for Cu(II) and Ni(II) under different initial concentration ratios of Ni(II) to Cu(II)
3.7 Column adsorption
Compared with the batch experiments, the mini-column adsorption experiment is more meaningful for judging whether the adsorbents can be applied in practical field application or not. It is possible to estimate the breakthrough volume of the Si-AMPY-1 resin towards Ni(II) and Cu(II) under column adsorption. The breakthrough curves for the removal of Cu(II) from the simulated nickel electrolyte were displayed in Fig. 11. It was obvious that the breakthrough of Ni(II) occurred immediately just after the start of flow, which indicates that the Si-AMPY-1 resin has weak affinity to adsorb Ni(II). As expected, the breakthrough of Cu(II) took place at 44 BV (the point at which Cv/Ci=0.05) and reached saturation at 87 BV (the point at which Cv/Ci =0.95). It was shown that the Si-AMPY-1 resin can efficiently separate Cu(II) from Ni(II) in the simulated nickel electrolyte before breakthrough point. Moreover, the concentration of Cu(II) in the column effluent was reduced to less than 3 mg/L within the first 43 BVs, and the mass ratio of Cu to Ni was 21:1 in the saturated resin, which completely satisfied the industrial requirements of the nickel electrorefining process [36]. This indicates that the novel chelating resin can be a promising candidate for the deep removal of Cu(II) from the simulated nickel electrolyte.

Fig. 11 Breakthrough curves for adsorption of Cu(II) and Ni(II) on Si-AMPY-1 resin in simulated nickel electrolyte
3.8 Possible adsorption mechanism
From the wide-scan XPS spectra in Fig. 12, the predominant peaks observed in Si-AMPY-1 were Si 2p, C 1s, N 1s and O 1s, which suggests the successful modification of the silica gel surface. Compared with the XPS spectra of pure Si-AMPY-1, no other significant peaks appear in Si-AMPY-1+Ni. Obviously, the simultaneous presence of the peaks of Cu2p and Cl2P in Si-AMPY-1+Cu verified the fact that Cu(II) is adsorbed on the Si-AMPY-1 resin accompanied with Cl-, i.e., the copper atom is coordinated by the chloro anion ligands.

Fig. 12 X-ray photoelectron spectra of Si-AMPY-1 (a), Si-AMPY-1+Cu (b) and Si-AMPY-1+Ni (c)

Fig. 13 N 1s and O 1s spectra of Si-AMPY-1 and Si-AMPY-1+Cu
The N1s and O1s spectra of Si-AMPY-1 before and after the adsorption of Cu(II) were shown in Fig. 13. The N 1s spectra of Si-AMPY-1 were deconvoluted into two peaks at 400.42 and 398.63 eV, which corresponded to the nitrogen atoms in the tertiary amine group and pyridine group, respectively [37,38]. Compared with the pure Si-AMPY-1, the binding energies of the tertiary amine and pyridine N1s for Si-AMPY-1+Cu were correspondingly increased by 0.77 and 0.3 eV. The increase of the binding energies of the N 1s could be interpreted that rather amount of feedback of d electrons existing in the coordination bond of N→Me2+ leads to the decrease of the electron cloud density of nitrogen atoms. The O 1s spectra of Si-AMPY-1 were deconvoluted into three different component peaks. The O 1s spectra of Si-AMPY-1 were deconvoluted into three peaks at 533.47, 532.30 and 531.56 eV, which corresponded to the characteristic peaks of the C—O single bond of the ester group, the Si—O single bond of the siloxane group and the C=O double bond of the ester group, and Si—O single bond of the silanol group, respectively [39,40]. Interestingly, compared to Si-AMPY-1, Si-AMPY-1+Cu presented a new peak at the binding energy of 533.36 eV which may be the characteristic peak of the Cu—O single bond of H2O—Cu [41]. It was verified that Cu(II) may be chemically adsorbed onto the Si-AMPY-1 resin accompanied with coordinated water molecule. Therefore, the possible chelating forms between Cu(II) and the Si-AMPY-1 resin may be represented in Fig. 14.
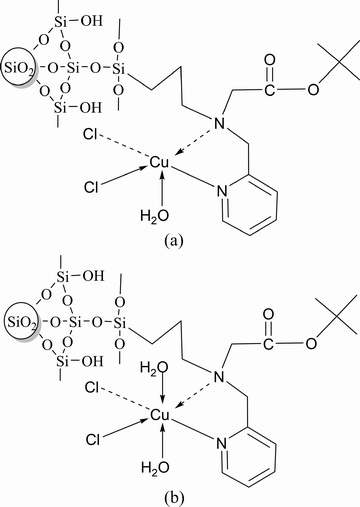
Fig. 14 Chelating forms between Cu(II) and Si-AMPY-1
4 Conclusions
1) A novel resin, silica-supported tert-butyl 2-picolyamino-N-acetate chelating resin (Si-AMPY-1) was prepared by reacting tert-butyl 2-picolyamino-N- acetate (AMPY-1) with γ-chloropropyltrimethoxysilane (CPTS) prior to immobilization on the silica surface.
2) The experimental data obtained for the adsorption of Cu(II) and Ni(II) on the Si-AMPY-1 resin were well obeyed the pseudo-second-order kinetics and the adsorption of Cu(II) was well fitted by the Langmuir isotherm model and the Si-AMPY-1 resin presented a preferential binding capacity of Cu(II). The adsorption of Ni(II) was well fitted by both Langmuir and Freundlich isotherm model with the correlation coefficients R2>0.989.
3) On the basis of the analysis of experimental data on the thermodynamic parameters, it was observed that the Si-AMPY-1 resin adsorbed Ni(II) mainly via physisorption, while adsorbed Cu(II) via chemisorption. Selectivity experiments showed that the Si-AMPY-1 resin displayed an excellent selectivity in the adsorption of Cu(II) over Ni(II) in the binary metal solutions.
4) Especially, in column experiment, the Si-AMPY-1 resin can efficiently separate Cu(II) from Ni(II) in a simulated nickel electrolyte before the breakthrough point. Moreover, the concentration of Cu(II) in the column effluent was reduced to less than 3 mg/L within the first 43 BVs, and the mass ratio of Cu/Ni exceeded 20:1 in the saturated resin, which completely satisfied the requirements of the nickel electrorefining process.
5) The XPS results indicated that Cu(II) could be chemically adsorbed onto the Si-AMPY-1 resin accompanied with coordinated water molecule and chloro anion in copper chloride aqueous solution. From the results obtained in this study, it can be concluded that the Si-AMPY-1 resin has great potential for the deep removal of Cu(II) from nickel electrolyte.
References
[1] CHEN Ai-liang, QIU Guan-zhou, ZHAO Zhong-wei, SUN Pei-mei, YU Run-lan. Removal of copper from nickel anode electrolyte through ion exchange [J]. Transactions of Nonferrous Metals Society of China, 2009, 19(1): 253-258.
[2] BAI Lan, HU Hui-ping, ZHANG Wen-jing, FU Jun-tian, LU Zhen-zhen, LIU Mu-hua, JIANG Hong, ZHANG Lu, CHEN Qi-yuan, TAN Ping. Amine/acid catalyzed synthesis of a new silica- aminomethyl pyridine material as a selective adsorbent of copper [J]. Journal of Materials Chemistry, 2012, 22(3): 17293-17301.
[3] LI Lin, CHEN Xing-yu, LIU Xu-heng, ZHAO Zhong-wei. Removal of Cu from the nickel electrolyte using amorphous MnS [J]. Hydrometallurgy, 2014, 146(3): 149-153.
[4] CHEN Xing-yu, CHEN Ai-liang, ZHAO Zhong-wei, LIU Xu-heng, SHI Yu-chen, WANG De-zhi. Removal of Cu from the nickel electrolyte using nickel thiocarbonate [J]. Hydrometallurgy, 2013, 133: 106-110.
[5] CHEN Ai-liang, ZHAO Zhong-wei, CHEN Xing-yu, LIU Xu-heng, CAO Cai-fang. Decoppering capability of nickel thiocarbonate in nickel electrolyte [J]. Hydrometallurgy, 2014, 144-145(4): 23-26.
[6] READ D T, CHENG Y W, GEISS R. Morphology, microstructure, and mechanical properties of a copper electrodeposit [J]. Microelectronic Engineering, 2004, 75(1): 63-70.
[7] PREEZ J G H D, SHILLINGTON D P, BRECHT B J A M V. Polynitrogen reagents in metal separation. Part 1. Ditertiary and diquaternary ammonium extractants for cobalt(II) and copper(II) in HCl medium [J]. Solvent Extraction and Ion Exchange, 1984, 2(6): 839-858.
[8] HUANG Ting-chia, LIN Yih-kung, CHEN Chi-yih. Selective separation of nickel and copper from a complexing solution by a cation-exchange membrane [J]. Journal of Membrane Science, 1988, 37(2): 131-144.
[9] LI Lan-juan, LIU Fu-qiang, JING Xiao-sheng, LING Pan-pan, LI Ai-min. Displacement mechanism of binary competitive adsorption for aqueous divalent metal ions onto a novel IDA-chelating resin: Isotherm and kinetic modeling [J]. Water Research, 2011, 45(3): 1177-1188.
[10] MONIER M, AYAD D M, WEI Y, SARHAN A A. Adsorption of Cu(II), Co(II), and Ni(II) ions by modified magnetic chitosan chelating resin [J]. Journal of Hazardous Materials, 2010, 177(1): 962-970.
[11] HASSANIEN M M, ABOUELSHERBINI K S. Synthesis and characterization of morin-functionalised silica gel for the enrichment of some precious metal ions [J]. Talanta, 2006, 68(5): 1550-1559.
[12] CHEN Xue-rong, LI Qing, YU Shi-jin, LIN Bin, WU Kang-bing. Activated silica gel based carbon paste electrodes exhibit signal enhancement for quercetin [J]. Electrochimica Acta, 2012, 81(11): 106-111.
[13] JAL P K, PATEL S, MISHRA B K. Chemical modification of silica surface by immobilization of functional groups for extractive concentration of metal ions [J]. Talanta, 2004, 62(5): 1005-1028.
[14] QU Rong-jun, NIU Yu-zhong, SUN Chang-mei, JI Chun-nuan, WANG Chun-hua, CHENG Guo-xiang. Syntheses, characterization, and adsorption properties for metal ions of silica-gel functionalized by ester- and amino-terminated dendrimer-like polyamidoamine polymer [J]. Microporous and Mesoporous Materials, 2006, 97(1): 58-65.
[15] MRMC S, AIROLDI C. Urea derivatives anchored on silica gel [J]. Journal of Colloid and Interface Science, 1996, 183(2): 416-423.
[16] SIROLA K, LAATIKAINEN M, LAHTINEN M, PAATERO E. Removal of copper and nickel from concentrated ZnSO4 solutions with silica-supported chelating adsorbents [J]. Separation and Purification Technology, 2008, 64(1): 88-100.
[17] LAATIKAINEN K, LAHTINEN M, LAATIKAINEN M, PAATERO E. Copper removal by chelating adsorption in solution purification of hydrometallurgical zinc production [J]. Hydrometallurgy, 2010, 104(1): 14-19.
[18] ROSENBERG E, FISCHER R J. Materials and methods for the separation of copper ions and ferric iron in liquid solution [P]. U.S. Patent: 6576590, 2004.
[19] SALES J A A, FARIA F P, PRADO A G S, AIROLDI C. Attachment of 2-aminomethylpyridine molecule onto grafted silica gel surface and its ability in chelating cations [J]. Polyhedron, 2004, 23(5): 719-725.
[20] WEN Jun-jie. The fundamental research on removing copper from cobalt electrolyte and nickel electrolyte by ion-exchange with novel silica-polyamine organic-inorganic composite resin [D]. Changsha: Central South University, 2010. (in Chinese)
[21] BAI Lan. Synthesis of a noval chelating resin and its properties for copper removal from cobalt electrolyte [D]. Changsha: Central South University, 2012. (in Chinese)
[22] YANG Jin-peng, HU Hui-ping, CHENG Ze-ying, QIU Xue-jing, WANG Cai-xia. Structural insights into the coordination and selective extraction of copper (II) by tertiary amine ligands derived from 2-aminomethylpyridine [J]. Polyhedron, 2017, 128: 76-84.
[23] ARMAREGO W L F, CHAI C L L. Purification of laboratory chemicals [M]. Boston: Elsevier, 2007.
[24] LEE B C, KIM D H, LEE J H, SUNG H J, CHOE Y S, CHI D Y, LEE K H, CHOI Y, KIM B T. 99mTc(CO)3-15-[N-(acetyloxy)- 2-picolylamino] pentadecanoic acid: A potential radiotracer for evaluation of fatty acid metabolism [J]. Bioconjugate Chemistry, 2007, 18(4): 1332-1337.
[25] SUHARSO, BUHANI, SUMADI. Immobilization of S. duplicatum supported silica gel matrix and its application on adsorption– desorption of Cu (II), Cd (II) and Pb (II) ions [J]. Desalination, 2010, 263(1): 64-69.
[26] PRADO A G S, AIROLDI C. The pesticide 3-(3,4-dichlorophenyl)- 1,1-dimethylurea (diuron) immobilized on silica gel surface [J]. Journal of Colloid and Interface Science, 2001, 236(1): 161-165.
[27] GOSWAMI A, SINGH A K. Silica gel functionalized with resacetophenone: Synthesis of a new chelating matrix and its application as metal ion collector for their flame atomic absorption spectrometric determination [J]. Analytica Chimica Acta, 2002, 454(2): 229-240.
[28] GUBBUK I H, GUP R, KARA H, ERSOZ M. Adsorption of Cu(II) onto silica gel-immobilized Schiff base derivative [J]. Desalination, 2009, 249(3): 1243-1248.
[29] ZHANG Ying, QU Rong-jun, SUN Chang-mei, JI Chun-nuan, CHEN Hou, YIN Ping. Improved synthesis of silica-gel-based dendrimer-like highly branched polymer as the Au(III) adsorbents [J]. Chemical Engineering Journal, 2015, 270: 110-121.
[30] WANG Shao-bin, LI Hui-ting. Dye adsorption on unburned carbon: Kinetics and equilibrium [J]. Journal of Hazardous Materials, 2005, 126(1): 71-77.
[31] XIONG Chun-hua, LI Yan-li, WANG Guo-tao, FANG Lei, ZHOU Su-guo, YAO Cai-ping, CHEN Qing, ZHENG Xu-ming, QI Dong-ming, FU Ya-qin, ZHU Yao-feng. Selective removal of Hg(II) with polyacrylonitrile-2-amino-1,3,4-thiadiazole chelating resin: Batch and column study [J]. Chemical Engineering Journal, 2015, 259: 257-265.
[32] FOO K Y, HAMEED B H. Insights into the modeling of adsorption isotherm systems [J]. Chemical Engineering Journal, 2010, 156(1): 2-10.
[33] MUNGASAVALLI D P, VIRARAGHAVAN T, JIN Y C. Biosorption of chromium from aqueous solutions by pretreated Aspergillus niger: Batch and column studies [J]. Colloids and Surfaces A: Physicochemical and Engineering Aspects, 2007, 301(1): 214-223.
[34] ATIA A A, DONIA A M, ALAMRANI W A. Effect of amine type modifier on the uptake behaviour of silica towards mercury(II) in aqueous solution [J]. Desalination, 2009, 246(1): 257-274.
[35] TAO Xue-wen, LIU Fu-qiang, BAI Zhi-ping, WEI Dong-yang, ZHANG Xiao-peng, WANG Jun-fei, GAO Jie, SUN Xiao-wen, LI Bao-hua, LI Cheng-hui, LI Ai-min. Insight into selective removal of copper from high-concentration nickel solutions with XPS and DFT: New technique to prepare 5N-nickel with chelating resin [J]. Journal of Environmental Sciences, 2016, 48(10): 34-44.
[36] ZHAO Zhong-wei, CHEN Ai-liang, SUN Pei-mei, CHEN Xing-yu, HUO Guang-sheng, LI Hong-gui. Removing copper from nickel electrolyte solution [J]. The Chinese Journal of Nonferrous Metals, 2009, 19(4): 749-753. (in Chinese)
[37] SUN Chang-mei, QU Rong-jun, JI Chun-nuan, WANG Qun, WANG Chun-hua, SUN Yan-zhi, CHENG Guo-xiang. A chelating resin containing S, N and O atoms: Synthesis and adsorption properties for Hg(II) [J]. European Polymer Journal, 2006, 42(1): 188-194.
[38] ABE T, WATANABE A. X-ray photoelectron spectroscopy of nitrogen functional groups in soil humic acids [J]. Soil Science, 2004, 169(1): 35-43.
[39] TANG Cui-hua, ZHU Jian-xi, ZHOU Qing, WEI Jing-ming, ZHU Run-liang, HE Hong-ping. Surface heterogeneity of SiO2 polymorphs: An XPS investigation of α-quartz and α-cristobalite [J]. The Journal of Physical Chemistry C, 2004, 118(45): 26249-26257.
[40] LOPEZ G P, CASTNER D G, RATNER B D. XPS O1s binding energies for polymers containing hydroxyl, ether, ketone and ester groups [J]. Surface & Interface Analysis, 1991, 17: 267-272.
[41] AVILA-TORRES Y, HUERTA L, BARBA-BEHRENS N. XPS-characterization of heterometallic coordination compounds with optically active ligands [J]. Journal of Chemistry, 2013(3): 603-617.
模拟镍电解液深度除铜用硅胶负载N-叔丁基羰基亚甲基新型螯合树脂的合成及吸附性能
王彩霞1,2,胡慧萍1,2,邱雪景1,2,程泽英1,2,孟璐佳1,祝 莉1
1. 中南大学 化学化工学院,长沙 410083;
2. 中南大学 锰资源高效清洁利用湖南省重点实验室,长沙 410083
摘 要:制备一种硅胶负载N-叔丁基羰基亚甲基新型螯合树脂(Si-AMPY-1),分别采用元素分析、FT-IR谱、SEM和13C CP/MAS NMR手段对树脂进行结构表征。采用静态吸附法与动态吸附法分别研究Si-AMPY-1螯合树脂对Cu(II)和Ni(II)的吸附性能。静态吸附结果表明,Si-AMPY-1螯合树脂对Cu(II)的吸附主要为化学吸附,Si-AMPY-1螯合树脂对Ni(II)的吸附则主要为物理吸附。动态吸附结果表明,Si-AMPY-1螯合树脂具有模拟镍电解液良好的深度除铜效果,在前43 BV流出液中除铜后液的铜浓度小于3 mg/L,解吸液的铜/镍质量比为21:1,满足镍电解液深度除铜的工业要求。因此,Si-AMPY-1螯合树脂有望用于镍电解液深度除铜。
关键词:螯合树脂;选择性吸附;深度除铜;模拟镍电解液;合成
(Edited by Xiang-qun LI)
Foundation item: Project (2014CB643401) supported by the National Basic Research Program of China; Projects (51134007, 51474256) supported by the National Natural Science Foundation of China; Project (2016TP1007) supported by the Hunan Provincial Science and Technology Plan Project in China
Corresponding author: Hui-ping HU; Tel/Fax: +86-731-88879616; E-mail: phuhuiping@126.com
DOI: 10.1016/S1003-6326(18)64902-7
Abstract: A novel silica-supported tert-butyl 2-picolyamino-N-acetate chelating resin (Si-AMPY-1) was successfully synthesized and characterized by elemental analysis, FT-IR, SEM and 13C CP/MAS NMR. The adsorption behaviors of the Si-AMPY-1 resin for Cu(II) and Ni(II) were studied with batch and column methods. The batch experiments indicated that the Si-AMPY-1 resin adsorbed Ni(II) mainly via physisorption, while adsorbed Cu(II) via chemisorption. The column dynamic breakthrough curves revealed that the Si-AMPY-1 resin can efficiently separate Cu(II) from the simulated nickel electrolyte before the breakthrough point. Moreover, the concentration of Cu(II) in the column effluent was decreased to be less than 3 mg/L within the first 43 BV (bed volumes), and the mass ratio of Cu/Ni was 21:1 in the saturated resin, which completely satisfied the industrial requirements of the nickel electrorefining process. Therefore, it was concluded that the Si-AMPY-1 resin can be a promising candidate for the deep removal of Cu(II) from the nickel electrolyte.


