
Acid mine drainage and heavy metal contamination in groundwater of
metal sulfide mine at arid territory (BS mine, Western Australia)
LEI Liang-qi(雷良奇)1, 2, SONG Ci-an(宋慈安)1,
XIE Xiang-li (谢襄漓)1, LI Yan-hong (李艳红)1, WANG Fei(王 飞)1
1. School of Earth Science, Gulin University of Technology, Guilin 541004, China;
2. Key Laboratory of Geological Engineering Centre of Guangxi Province,
Gulin University of Technology, Guilin 541004, China
Received 20 July 2009; accepted 19 April 2010
Abstract:
The issues of acid mine drainage (AMD) and heavy metals contamination in the metal sulfide mine in the arid district were explored, through studying the acidification and the heavy metals distribution and evolution of groundwater in the black swan (BS) nickel sulfide mine (Western Australia). The groundwater samples were collected from the drilling holes situated in the vicinity of tailings storage facility (TSF) and in the background of the mine (away from TSF), respectively, and the pH and electric conductivity (Ec) were measured in site and the metal contents were analysed by ICP-MS and ICP-AES, quarterly in one hydrological year. The results disclose that the TSF groundwater is remarkably acidified (pHmean≈5, pHmin=3), and the average contents of heavy metals (Co, Cu, Zn, Cd) and Al, Mn are of 1-2 orders of magnitude higher in TSF groundwater than in background groundwater. It may be due to the percolation of tailings waste water from mill process, which leads the tailings to oxidize and the deep groundwater to acidify and contaminate with heavy metals. Besides, the heavy metals concentration in groundwater may be controlled by pH mainly.
Key words:
nickel ore tailings; acid mine drainage; heavy metals; groundwater contamination;
1 Introduction
The acid mine drainage (AMD) released from the metal sulfide tailings (including waste rock) can bring about the environmental contamination in mine and nearby mine. Because the acidic water can promote metals leaching and removing in tailing medium[1], the heavy metal contents in AMD are enhanced generally, causing the AMD contamination to ecological environment in mine intensified.
Surface water percolating through sulfide tailing piles is one of the key factors to produce AMD[2-3], therefore, AMD happens more easily and causes the metal sulfide mine to contaminate more significantly in humid territory than in arid one. There have been lots of papers focusing on AMD in humid area (e.g. Refs.[4-9]), however, the AMD issue appearing in arid district has been ignored, and fewer publications concerned it.
Black swan (BS) nickel sulfide mine is situated in north-east Kalgoorlie, Western Australia, which is a Gobi desert territory (monthly mean precipitation of 15-35 mm). LEI et al[10-11] previously indicated that the tailings in BS mine were oxidized and AMD formed, and that the groundwater in the vicinity of tailings storage facility (TSF) was contaminated by the AMD, displaying a model of rare earth elements (REE) distribution with higher REE concentration and Ce depletion [10-11]. In this work, the characteristics of distribution and evolution on AMD and heavy metals in TSF groundwater in BS mine were further probed. The highlights may contribute to have a deeper knowledge of the mechanism concerning about releasing and removing of AMD and heavy metals from metal sulfide tailings under arid circumstance.
2 Background
BS sulfide nickel orebodies are hosted in Archaean komatiite and dacite lavas[12]. The nickel ores typically comprise banded pyrrhotite and pentlandite, associated with trace amounts of chalcopyrite, pyrite, violarite,gersdorffite and chromite. Major alteration minerals include magnesite, siderite, quartz and sericite, etc. Mg/Fe carbonates (magnesite-siderite series) are main acid-neutralizing minerals.
Based on the orebody occurrence in the mine, the ores can be classified into massive sulfide ore and impregnated sulfide ore. Both have been mined and disposed in flotation circuits in mill. The flotation wastes, e.g. massive sulfide tailings (MST, output: 58 000 t/a) and impregnated sulfide tailings (IST, 325 000 t/a), are processed in the form of slurry and discharged into two adjacent tailing ponds separately (Fig.1). The tailing assessment results disclosed that MST is classified totally as acid forming, while IST partially as acid forming (~16% by volume fraction of IST tailings)[10].
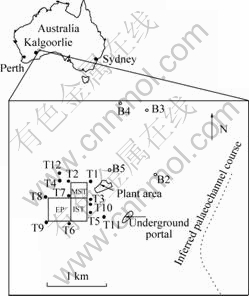
Fig.1 Map for sampling sites in BS mine: T1-T12—Monitoring bores; B2-B5—Hydrologic bores, MST—Massive sulfide tailing storage; IST—Impregnation sulfide tailing storage; EP—Evaporation pond with waste water
The mine district is covered by a veneer of clayey ferricrete, which has been recorded to have a depth of 4-20 m and underlain by saprolitic clay with minor ferruginous bands (depth of 62-79 m). Weathering front is even attained to Archaean mafic and ultramafic rocks in some place. Clayey and clay deposits generated from highly weathered Archaean rocks are of weak permeability. Surface runoff drains from south-west turning to south-east via a poorly defined paleochannel course, situated at eastern part of the mine (Fig.1). Groundwater is inferred flow to south-east.
3 Sampling and analysis results
It was performed to measure and sample the groundwater in TSF vicinity of BS mine quarterly in a whole hydrologic year (November 2003, February 2004, May 2004 and August 2004). The groundwater samples were collected from monitoring bores (T1-T12) in the proximity of TSF and hydrologic bores (B2-B5) in a distance from TSF (Fig.1). To get fresh groundwater, the sample collection was conducted through a Teflon-lined tube (approaching ~30 cm above bottom of the bore) after groundwater was pumped out from each bore in 15 min. Static water level (SWL) of groundwater had been measured before water was pumped out, and water pH and EC (electrical conductivity) were recorded on site as well. Each water sample was filtered through a Watman 0.45 ?m cellulose-nitrate filter membrane, and acidified with AR grade nitric acid, then poured in polyethylene bottles with lid to transport to laboratory for ICP-MS and ICP-AES analysis.
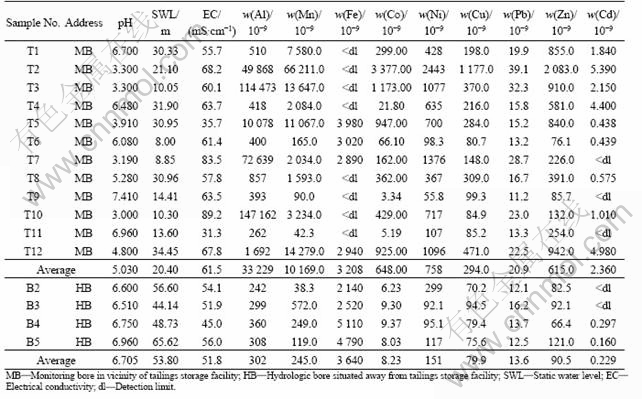
Table 1 displays analysis results of TSF groundwater in the first quarter of the hydrological year (November 2003).
4 Discussion
4.1 Characteristics of groundwater in BS mine
Table 1 shows that SWL values in TSF monitoring bores (T1-T12), with a varied depth range of 8.0-34.5 m and an average depth of 20.4 m, are significantly less than those in hydrological bores (B2-B5) which represent the regional watertable in the mine, with a varied range of 44.14-65.62 m and an average depth of 53.8 m, and the minimum SWL values (i.e. the highest watertable) are placed around the evaporation pond with waste water in TSF (T6 and T7). So, it is reasonably inferred that the rising of watertable (reducing SWL) in TSF vicinity is due to the water supplemented from TSF (waste water and tailing slurry).
The monitoring groundwater is acidic remarkably, with an average pH of 5.03 (measured by pH electrode meter on site) and a minimum pH of 3.00, while the regional groundwater in hydrological bores is neutral approximately, with an average pH of 6.7. Otherwise, compared with the regional groundwater, the mean EC value of monitoring groundwater is noticeably higher, confirming that acid water could promote the salts and heavy metals in tailing pile to dissolve and leach[13].
4.2 Metal concentration in TSF groundwater
1) Metal contents
Mean contents of heavy metals (Co, Zn, Ni, Cu), Al, Mn, except Fe and Pb in TSF groundwater are of 1-2 orders of magnitude higher than those in regional groundwater, and Ni average content is enhanced as well (Table 1).
2) Correlation between metal elements
In groundwater, there are close relevances between
Table 1 Water quality data measured in November 2003
Ni and Cu, and between Mn and Zn, while these elements have very weak relevances to Al and Pb, similar to the element relevances in BS tailings (Fig.2). In addition, the heavy metals (Ni, Cu, Mn, Zn) concentrated in groundwater are also the major profitable elements in tailings. So, it may imply that the heavy metals in the groundwater are probably originated from the tailings.
The element relativity can reflect the geochemical environment and the element behaviour. OLIAS et al[5] indicated that in acidic water, Mn is present in the +2 oxidation state, behaving in a similar way to Zn. In the BS mine, there is a similar relativity of Mn-Zn in both groundwater and tailings, meaning that the groundwater is acidified and its acidification is relative to the tailings oxidation.
However, there are remarkable differences in relevance between Fe and other elements in both groundwater and tailings. The reason should be probed in further study.
3) Metals relevance to pH
In the groundwater, most metals (e.g. Al, Mn, Co, Ni, Cu, Pb, Zn, Cd, except Fe) display a negative correlation with pH (Fig.3), showing that the heavy metals concentration enhances with the acidity increasing in groundwater.
There have been lots of studies certifying that the metal elements in acidic waters display a negative correlation with pH, and the variations in pH control the mobility of dissolved metals[5, 7, 11, 14-15]. When pH
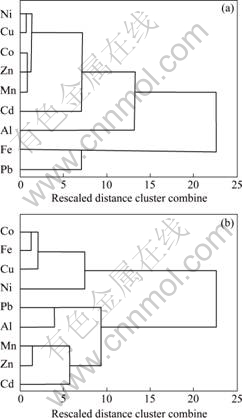
Fig.2 Dendrogram using average linkage (between groups) showing correlation between metal elements in BS tailings (20 samples) (a) and BS groundwater (b)
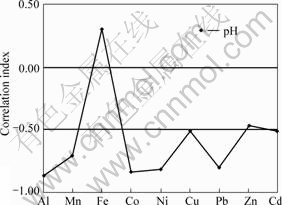
Fig.3 Correlation between metal elements and pH in groundwater
increases gently and attains a value >4.3, Al3+ and SO42- dissolved in AMD combine and form the Al hydroxysulfate/hydroxide precipitate[17-18]. Then, these precipitate play an important role in removal of heavy metals through adsorption and co- precipitation[19].
4.3 Heavy metal distribution in TSF groundwater
The contours of Co, Ni, Cu, Zn, Mn concentrations and pH value in TSF groundwater are constructed by using data in Table 1 (Fig.4). Fig.4 demonstrates that: 1) the central areas of these heavy metal concentrations appear at monitoring bore T2 in northeastern part of TSF, and place in the low pH (pH<4) area; 2) the contour axes of these heavy metal concentrations are situated at the

Fig.4 Contours of metal concentrations (w=10-9) and pH in groundwater in vicinity of TSF: (a) Co; (b) Ni; (c) Mn (Mn concentration is divided by 1 000); (d) Cu; (e) Zn; (f) pH
linkage line between monitoring bore T2 and T3, striking with northwest-southeast direction and running parallel to the contour axis of pH value; and 3) Both heavy metal concentration gradient and pH gradient are steeper and confined in TSF district. This indicates that there appears to be a closer relation between the heavy metals releasing and the acidic water leaching (pH) in BS mine, and that the contaminated plume centres of AMD and heavy metals are located at the TSF district, and the plume moving trend is similar to the southeast flow direction of the regional groundwater.
4.4 Water quality evolution
Fig.5 discloses the quarterly variation of pH, EC, SWL, and heavy metal (Ni, Co, Zn, Cu and Mn) contents in TSF groundwater in the monitoring year. In this year, the groundwater pH values are constant with pH median values fluctuating around 5.0; and there is not a significant difference between EC, SWL, and heavy metals contents, except May 2003. In that month, EC and SWL median values are enhanced, while the heavy metal concentrations decline noticeably (Fig.5).
In May 2004, increase of SWL median value reflects relative decline of groundwater level at some place in TSF district, concerning about the shortage of water leaching from TSF. It was investigated and confirmed that the time when BS processed mill stoped for routine machine maintenance was just in May every year. At that moment, the amount of wastewater and tailing slurry from the mill was greatly reduced. Because water is the main carrier of acid and metals in removing, the shortage of water derived from TSF could cause the amount of acidified water and heavy metals into groundwater to decrease temporarily.
5 Conclusions
1) The groundwater in the vicinity of tailings storage facility in BS nickel mine is contaminated by AMD and heavy metals (including Ni, Co, Zn, Cu mainly) noticeably. The contamination is caused by the percolation of acid water and heavy metals from TSF, demonstrating that: the associations and relevances of heavy metal in groundwater are similar to those of beneficial elements in ore tailings; the contamination plumes of both heavy metals and acid water are confined in TSF district, and are of steeper gradient; and the reduction of heavy metal contents and groundwater level may correlate to the shortage of water from TSF.
2) The heavy metals concentration in groundwater can be controlled by pH mainly.
3) The wastewater and tailing slurry in tailing storage facility can provide water for oxidation metal sulfides and forming AMD, therefore even if a metal
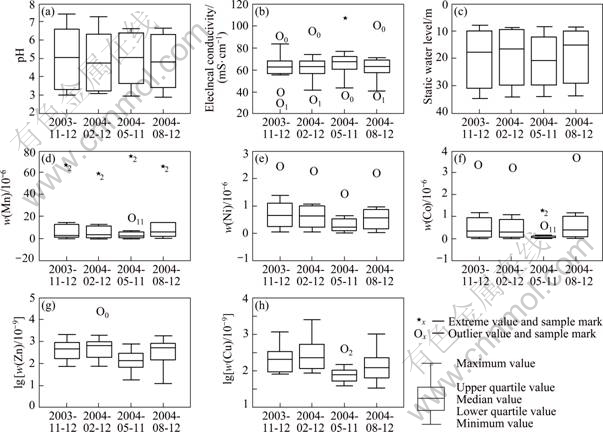
Fig.5 Box-and-whisker plots of quarterly evolution of groundwater quality in one hydrological year
sulfide mine is located in arid territory, there could be a potentiality of producing AMD and releasing heavy metals, contaminating the deep groundwater.
Acknowledgements
The authors acknowledge very much the invaluable assistances from colleagues, Dr. Troy Cook, Dr. Dave Oldmeadow, Dr. Ryan Noble and Dr. Margeret Smiths in Curtin University of Technology (Western Australia). Especially, many thanks to Professor Ron Watkins who provided the necessary condition for sample tests, and Ms Miranda Taylor who supplied fundamental documents concerning to hydrogeology of the study district and helped in field sampling.
References
[1] SULLIVAN P J, YELTON J L. An evaluation of trace element release associated with acid mine drainage [J]. Environment Geology Water Science, 1988, 12: 181-186.
[2] KELLY M. Mining and the freshwater environment [M]. England: Elsevier Science Publishers Ltd. 1988: 33-42.
[3] PARKER G, ROBERTSON A. Acid drainage [C]//Australia Minerals & Energy Environment Foundation, 1999, 11: 11-37.
[4] DINELLI E, LUCCHINI F, FABBRI M, CORTECCI G. Metal distribution and environmental problems related to sulfide oxidation in the Libiola copper mine area (Ligurian Apennines, Italy) [J]. Journal of Geochemical Exploration, 2001, 74: 141-152.
[5] OLIAS M, NIETO J M, SARMIENTO A M, CERON J C, CANOVAS C R. Seasonal water quality variations in a river affected by acid mine drainage: the Odiel River (South West Spain) [J]. Science of the Total Environment, 2004, 333: 267-281.
[6] SAINZ A, GRANDE J A, DE LA TORRE M L. Characterisation of heavy metal discharge into the Ria of Huelva [J]. Environment International, 2004, 30: 557-566.
[7] XU Xiao-chun, CHEN Fang, WANG Jun, XIE Qiao-qin, LU San-ming, WU Wen-tao, CHEN Tian-hu. Acid mine drainage and heavy metal elements of solid waste in Tongling mines [J]. Acta Petrologica et Minerralogica, 2005, 24(6): 591-597. (in Chinese)
[8] LI Xiao-hu, TANG Zhong-li, CHU Feng-you. Chemical speciation and distribution of heavy metals in different environmental mediums around Ni-Cu mine area [J]. Journal of Jilin University (Earth Science Edition), 2008, 38(5): 847-853. (in Chinese)
[9] DU Li-yu, LIANG Cheng-hua, LIU Gui-qin. The distribution characteristics of heavy metals in Cu mine tailing and effect of heavy metals on pollution of soils in the areas of Hongtou Mountians [J]. Chinese Journal of Soil Science, 2008, 39(4): 938-941. (in Chinese)
[10] LEI Liang-qi, WATKINS R. Acid drainage re-assessment of mining tailings, black swan nickel mine, Kalgoorlie, Western Australia [J]. Applied Geochemistry, 2005, 20: 661-667.
[11] LEI Liang-qi, SONG Ci-an, XIE Xiang-li, LI Yan-hong. REE behavior and effect factors in AMD-type acidic groundwater at the sulfide tailings pond, BS nickel mine, W.A. [J]. Transactions of Nonferrous Metals Society of China, 2008, 18(4): 955-961.
[12] HILL R E T, BARNES S J, THORDARSON T, DOWLING S E. Volcanic evolution of the black swan succession and contained orebodies [J]. AGSO-Geoscience Australia, 2001(2): 158-160.
[13] HU Hong-wei, SHU Wen-sheng, LAN Chong-yu, WANG Bo-sun. Studies on acid form ing and heavy metal mobility of Lechang Pb/Zn mine tailings in lysimeters [J]. Environmental Science & Technology, 1999(3): 1-3. (in Chinese)
[14] CRAVOTTA C A, TRAHAN M K. Limestone drains to increase pH and remove dissolved metals from acidic drainage [J]. Applied Geochemistry, 1999, 14: 581-606.
[15] YU Chang-wu, XU Shi-guo, CHEN Guo-wei, ZHOU Li-dai. Impact on heavy metal Mo leached statically from Mo tailings by acid/alkali and temperature [J]. Environmental Science & Technology, 2008, 31(7): 1-3. (in Chinese)
[16] HUANG Kang-jun, XIE Shu-yun, BAO Zheng-yu, DONG Zhi-cheng, YU Chao. Environmental geochemistry of heavy metal and trace elements in tailings of Tonglushan Cu and Fe mine, Daye, Hubei province [J]. Geochimica, 2008, 37(3): 213-222. (in Chinese)
[17] NORDSTROM D K. The effect of sulphate on aluminium concentrations in natural waters: Some stability relations in the system Al2O3-SO3-H2O [J]. Geochim Cosmochim Acta, 1982, 46: 681-692.
[18] NORDSTROM D K, BILL J W. The geochemical behaviour of aluminium in acidified surface waters [J]. Science, 1986, 232: 54-58.
[19] COSTON J A, FULLER C C, DAVIS J A. Pb2+ and Zn2+ adsorption by a natural aluminium and iron-bearing surface coating on an aquifer sand [J]. Geochim Cosmochim Acta, 1995, 59: 3535-3547.
(Edited by LI Xiang-qun)
Foundation item: Projects(40972220, 40873030) supported by the National Natural Science Foundation of China; Project (0991024) supported by the Special Project for Applied Basic Research of Guangxi, China
Corresponding author: LEI Liang-qi; Tel: +86-773-5896341; E-mail: leilq@glite.edu.cn, llq927@hotmail.com
DOI: 10.1016/S1003-6326(09)60326-5


