
Antistatic coating material consisting of poly (butylacrylate-co-styrene) core-nickel shell particle
Min-Yeong JEONG1, Byung-Yoon AHN2, Sang-Koul LEE3, Won-Ki LEE4, Nam-Ju JO1
1. Department of Polymer Science and Engineering, Pusan National University, Busan, 609-735, Korea;
2. Kumho Petrochemical, Seongam-dong, Nam-gu 680-140, Ulsan, 680-140, Korea;
3. Samsung Corning Precision Glass, Tangjeong, Asan-City, Chungcheongnam-do, 336-840, Korea;
4. Division of Chemical Engineering, Pukyong National University, Busan, 606-739, Korea
Received 2 March 2009; accepted 30 May 2009
Abstract:
A transparent and antistatic coating material consisting of polymer core-metal shell particle was prepared. As a polymer core, poly(butylacrylate-co-styrene)s ([P(BA-co-sty)s]) with various compositions of butylacrylate and styrene were synthesized by emulsion polymerization. And the effect of comonomer composition on the thermal property of polymer core particle was investigated. By electroless plating method, the nickel particles were formed and deposited on the surface of P(BA-co-Sty) particles to form P(BA-co-Sty) core-nickel shell composite particles. SEM observation confirms that the nickel particles with size of 15 nm are distributed on the surface of the polymer core particles. The surface resistance of P(BA-co-Sty) core-nickel shell composite is 0.8×108 Ω/cm2, enough to act as antistatic coating material.
Key words:
antistatic coating; polymer core; metal shell; poly(butylacrylate-co-styrene); nickel; composite;
1 Introduction
Plastic is one of the most commercial and widespread materials in our life. Most plastics are good electrical insulator, tending to acquire a strong electrostatic charge which may cause trouble and malfunction in the operation of the electric devices, and are dangerous of being flamed[1-2]. The occurrence of these electrostatic charges is related to the surface resistance of material and the electrostatic problems can be solved by using internal or external antistatic agent which reduces the surface resistance[3-9]. Most of the common antistatic agents are anionic type, which have the ability to absorb moisture acting as conducting path on the surface of material in which the surface resistance is decreased. However, the antistatic ability of these materials heavily depend on the humidity in ambient air.
Therefore, the decrease of moisture can make the surface resistance significantly increase. So some antistatic coating materials were prepared to solve this problem, which consisted of polymer core-metal shell particles. The final aim of this work is the formation of transparent and antistatic coating material containing small quantities of metal, which does not depend on humidity. To form polymer core-metal shell, the polymer core must have functional groups to adsorb the metal shell[10-11]. In this work, poly(butylacrylate-co- styrene) [P(BA-co-sty)] particles as polymer core are synthesized by emulsion copolymerization. The object of this copolymerization is to introduce selective activation of functional group into the polymer particle, leading to the precise control over the active sites, and consequently, over the metal deposition process. The functional group applied is the carbonyl group in butylacrylate, which has strong interaction with the metal particles. But this strong interaction should induce the coagulation of polymer cores during the electroless chemical metal deposition process. So the interaction between each polymer core particles was controlled by introducing styrene comonomer. The reason why we applied electroless chemical metal deposition process to prepare polymer core-metal shell composite particles is that this process is more suitable and convenient than any other methods to achieve metal deposition in aqueous media[10].
2 Experimental
2.1 Materials
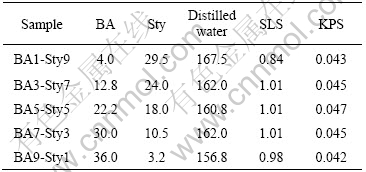
Butylacrylate (BA, Junsei Co. Ltd) and styrene (Sty, Junsei Co., Ltd) were purified by washing with a 5 mol/L NaOH aqueous solution, and washed out three times with purified water to make it neutral condition. Potassium persulfate (KPS, Shinyo Pure chemical) and sodium dodecyl sulfate (SLS, Junsei Co., Ltd.) were used as initiator and emulsifier, respectively. Nickel chloride hexahydrate and hydrazine monohydrate were used as perchased.
2.2 Synthesis of polymer core particles
Emulsion copolymerization of P(BA-co-Sty) was carried out at 75 ℃ in a 250 mL four necked reactor purged with nitrogen continuously and equipped with a teflon stirrer, condenser, thermocouple, and feeding funnel. The agitation speed for all runs was 200 r/min continuously. The reaction time for all runs was about 12 h to complete reaction. The recipe of all runs is listed in Table 1. Under the same recipe and reaction condition including uniform agitation speed, the reproducibility of the reaction was good. Latex stability was also good, which was confirmed from the results of experiments that coagulum does not adhere on the reactor and since then no separation or coagulation was observed on the fixed state for one month.
Table 1 Copolymerization condition of polymer core particle and sample code (mass, g)
2.3 Preparation of P(BA-co-Sty) core-nickel shell composite particles
Electroless plating was conducted by stirring fine copolymer particles in aqueous solution with metal compounds. The pH value of the electroless plating solution, 0.3 mol/L nickel chloride aqueous solution, was adjusted at 11. And then, this electroless plating solution was added into the emulsion solution. The bath was heated to 70 ℃ and the reducing agent, hydrazine monohydrate, was introduced. The reaction was continued for 30 min at 70 ℃, and maintained the quality of pH=11.
2.4 Characterization
The size and morphology of copolymer particles and composite particles were investigated by a Hitachi S-4250 scanning electron microscope(SEM). Differential scanning calorimeter(DSC) and thermo gravimetric analysis(TGA) were performed on a TA Q100 and TA. The surface resistance was estimated by a surface resistivity meter (Changmin CMT-ST 1000).
3 Results and discussion
3.1 Morphology and glass transition temperature of polymer core particles
The distribution and shape of all the P(BA-co-Sty) copolymer core particles were observed by using SEM as shown in Fig.1. It can be seen that all of the polymer core particles are monodispersed and has spherical shape. And the particle size is generally similar and the diameter is about 150 nm. The glass transition temperature(Tg) of every polymer core series was investigated by DSC and the result is shown in Fig.2. In the DSC results, all of the polymer cores show mono Tg peak in all range. And it be expected that the mone Tg is due to the similar reactivity ratio of the butylacrylate and styrene. Therefore, it can be considered that these copolymers must become random or alternated copolymer. Also, higher Tg is observed at higher styrene contents, and each Tg value is almost in accordance with theoretically calculated Tg value. From this result, it is confirmed that the emulsion copolymerization of BA and Sty is successfully completed.

Fig.1 SEM images of poly(butylacrylate-co- styrene) copolymer particles prepared at different mass ratio of butylacrylate to styrene: (a) BA1-Sty9 copolymer particle; (b) BA3- Sty7 copolymer particle; (c) BA5-Sty5 copolymer particle; (d) BA7-Sty3 copolymer particle; (e) BA9-Sty1 copolymer particle
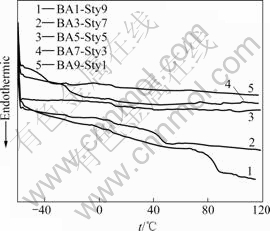
Fig.2 DSC curves of poly(BA-co-Sty) copolymers
3.2 Characterization of polymer core-nickel shell particles
P(BA-co-Sty) core-nickel shell composite particles were prepared by chemical metal deposition (electroless plating). Fig.3 shows typical SEM images of P(BA-co- Sty) core-nickel shell composite particles. It can be seen that some small nickel particles (10-20 nm in size) form on the surface of the P(BA-co-Sty) particles. When BA content is higher than that in case of BA3-Sty7, the formation of nickel shells can be observed. However, when the BA content is higher than that in case of BA5- Sty5, serious coagulation occurs. This coagulation is caused by the high interaction of BA with metal. So it can be considered that the best composition ratio is BA5-Sty5.
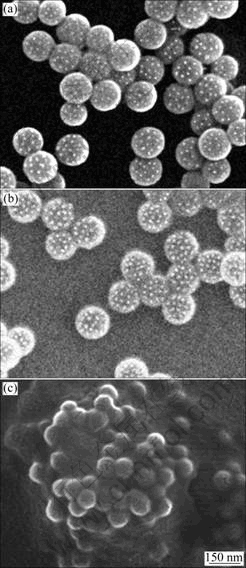
Fig.3 SEM images of Ni treated poly(butylacrylate-co-styrene) copolymer particle prepared at different mass ratios of butylacrylate to styrene: (a) Ni treated BA3-Sty7 copolymer particle; (b) Ni treated BA5-Sty5 copolymer particle; (c) Ni treated BA7-Sty3 copolymer particle
The quantity of adsorbed nickel was checked by TGA analysis. In the result of TGA, the core polymers become burned out after 350 ℃ and the adsorbed nickel still remains. The TGA measurement results of the composite particles are given in Fig.4. It could be confirmed from Fig.4 that the quantity of adsorbed nickel increases with increasing BA content in the polymer core particle.
The surface resistance of polymer core-metal shell particle is 0.8×108 Ω/cm2, and this value is enough to act as an antistatic coating material.

Fig.4 TGA curves of Ni untreated and treated poly (butylacrylate-co-styrene) copolymer particle prepared at different mass ratios of butylacrylate to styrene
4 Conclusions
1) Monodispersed P(BA-co-Sty) copolymer particles with the diameter of about 150 nm were synthesized by emulsion polymerization as the polymer core. All of the polymer cores have mono Tg peak. This reveals that the emulsion polymerization of BA and Sty was successfully accomplished.
2) By electroless plating method, nickel particles form and deposit on the surface of P(BA-co-Sty) particles to form P(BA-co-Sty) core-nickel shell composite particles. With increasing BA content in the emulsion polymerization system, the adsorbed quantity of nickel increases. However, with increasing BA content, serious coagulation of composite particles resulted from the high interaction of BA with nickel occurs.
3) The best composition ratio of comonomer is the mass ratio of BA to Sty of 5?5. The surface resistance of P(BA-co-Sty) core-metal shell composite particle is 0.8×108 Ω/cm2, enough to act as antistatic coating material.
Acknowledgement
This work was supported by the Korea Research Foundation Grant funded by the Korean Government (MOEHRD, Basic Research Promotion Fund) (KRF-2007-313-D00212)
References
[1] BAJAJ P, GUPTA A P, OJHA N. Antistatic and hydrophilic synthetic fibers: A critique [J]. Journal of Macromolecular Science, Reviews in Macromolecular Chemistry and Physics, 2000, C40 (2/3): 105-138.
[2] HAUSMANN K. Permanent antistatic agent offers long term performance for films and containers [J]. Plastics, Additives & Compounding, 2007, 9(3): 40-2.
[3] ZHANG F X, SRINIVASAN M P. Crosslinked polyimide- polythiophene composite with reduced surface resistivities [J]. Thin Solid Films, 2005, 479(1/2): 95-102.
[4] KIM H K, KIM Y B, CHO J D, HONG J W. Synthesis and characterization of radiation-curable monomers for antistatic coatings [J]. Progress in Organic Coatings, 2003, 48(1): 34-42.
[5] WOUTERS M E L, WOLFS D P, VAN DER LINDE M C, HOVENS J H P, TINNEMANS A H A. Transparent UV curable antistatic hybrid coatings on polycarbonate prepared by the sol-gel method [J]. Progress in Organic Coatings, 2004, 51(4): 312-319.
[6] LEHMANN W. Plastics with antistatic properties [J]. Kunststoffe, 1992, 82(10): 991-992.
[7] LI Zheng, WANG Xi-cun. Research and development on inner antistatic agents of plastics [J]. Hecheng Shuzhi Ji Suliao, 1995, 12(2): 54-56.
[8] JONAS F, LERCH K. Electrically conductive plastics [J]. Kunststoffe Plast Europe, 1997, 87(10): 48-49.
[9] RICHARD F. Grossman. Antistatic agents [J]. Journal of Vinyl Technology, 1993, 15: 164-173.
[10] WARSHAWSKY A, UPSON D A. Zerovalent metal-polymer composites (I) [J]. Metallized Beads, 1989, 27(9): 2963-2994.
[11] LEE T S, JEON D W, KIM J K, HONG S L. Formation of metal complex in a poly(hydroxamic acid) resin bead [J]. Fibers and Polymers, 2001, 2(1): 13-17.
Foundation item: Project(KRF-2007-313-DO0212) supported by the Korea Research Foundation Grant
Corresponding author: Nam-Ju JO; Tel: +82-51-510-2462; E-mail: namjujo@pusan.ac.kr
(Edited by LONG Huai-zhong)


