
Preparation and characterization of PEG-PEI/Fe3O4 nano-magnetic fluid by co-precipitation method
PENG Jian(彭 健)1, ZOU Fen(邹 芬)1, LIU Lu(刘 路)1, TANG Liang(唐 亮)1,
YU Li(余 丽)1, CHEN Wei(陈 伟)1, LIU Hui(刘 辉)1,
TANG Jing-bo(唐静波)1, WU Li-xiang(邬力祥)2
1. National Key Laboratory of Nanobiological Technology, Xiangya Hospital, Central South University,
Changsha 410008, China;
2. Department of Biology, School of Basic Medical Science, Central South University,
Changsha 410078, China
Received 18 February 2008; accepted 3 March 2008
Abstract:
PEG-PEI/Fe3O4 nano-magnetic fluids with different mass fractions of reactant were prepared by co-precipitation method. Besides particle size analyzer
Key words:
Fe3O4; PEG; PEI; co-precipitation; nano-materials; magnetic fluids;
1 Introduction
In the recent years, gene therapy gets a rapid development, and it’s mainly focused on obtaining target genes and achieving high gene transfection efficiency. The type of vector used is a key to success in gene delivery[1]. Two approaches commonly used in gene transfection are viral vectors and non-viral vectors. The former has some disadvantages, such as inducing the immune response of organism, potential viral replication, expensive production cost, not being applied repeatedly, and without targeting. Though the non-viral vectors are easier to be produced without immunogenicity and much safer, the efficiency of them is not satisfied, particularly with the presence of serum protein[2]. An ideal gene delivery carrier would be a system that can safely transport the genetic materials without exhibiting any toxicity or immune responses, and can be produced on a large scale easily. According to MERDAN et al[3] and other scientists’ researches, cationic polymer as the nanometric gene vector in gene therapy is one of the best non-viral vectors.
PEG-PEI/Fe3O4 nano-magnetic fluids show the advantages of good magnetic drug targeting, prolonged circulation and low toxicity. There have been lots of reports on PEG/Fe3O4 magnetic fluids as drug carriers[4]. PEI/Fe3O4 or PEI-PEG as gene vector has also been reported[5-6]. In this study, we creatively combine the advantages of PEG and PEI to prepare PEG-PEI/Fe3O4 nano-magnetic fluids as a new gene carrier. This can dramatically prevent non-specific uptake by the reticular- endothelial system[7] and reduce the cytotoxicity of PEG-PEI/Fe3O4 magnetic fluids[8]. By applying magnetic field, we can control the concentration of PEG-PEI/Fe3O4 in targeted tissues or cells. This would further decrease the harm to normal cells and minimize dosage of drugs and obtain the standard of targeted drug carriers[9].
2 Experimental
2.1 Preparation of PEG-PEI/Fe3O4 nano-magnetic fluids and DNA adsorption on nanoparticles
Main reagents, FeCl3, FeCl2, NH3?H2O, polyethyleneimine (PEI, MW25000 Da), monomethoxy poly(ethylene glycol) (PEG, MW 2000) were used in this experiment.
Main instruments included Malvern Zetasizer 3000E (Malvern, UK), Asylum Research MFP-3D AFM Systems, Freeze dryer (LABCONCO), Ultraviolet- visible spectrophotometer (Japan), Supercentrifuge (BECKMAN ZK401), High speed refrigerated centrifuge (Heraeus Company) and Thermostatic circulator water bath oven.
The experimental sequence flowchart is shown in Fig.1.
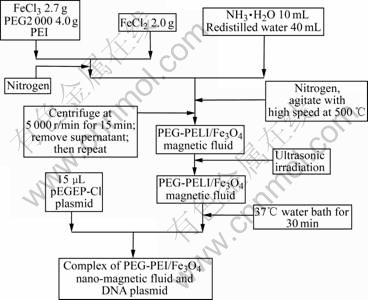
Fig.1 Experimental flowchart for preparation of PEG-PEI/Fe3O4 nano-magnetic fluids and DNA adsorption on nanoparticles
2.2 Characterization of PEG-PEI/Fe3O4 nano- magnetic fluid
Malvern Zetasizer 3000E(Malvern, UK) was used to detect the Zeta potential and particle size of the complex; and Asylum Research MFP-3D AFM System was used to observe the distribution and appearance of the nano-particles.
After the complex was changed into fine powder by cryodesiccation, XRD (Phlips X’Pert PRO, XL-30) and FT-IR were used to analyze the functional group of PEG-PEI/Fe3O4 magnetic nano-particles. And the paramagnetism of the sample under certain condition was measured by VSM (Quantum Design, JDM-13).
The data were described as x±S (x is the mean value; S is the standard deviation). The measurement data were analyzed with the variance analysis and the group comparison was treated with the T-test or least significant difference(LSD).
3 Results and discussion
3.1 Particle size, Zeta potential and pEGFP-C1 DNA loading efficiency of PEG-PEI/Fe3O4 nano- magnetic fluid
The particle size of PEG-PEI/Fe3O4 nano-magnetic fluid was measured by Malvern Zetasizer 3000E (Malvern, UK). Almost all the particles are about 80 nm, as shown in Fig.2(a). This means the PEG-PEI/Fe3O4 nano-magnetic fluid has typical nano-material characteristics and can be efficiently taken up by cells.
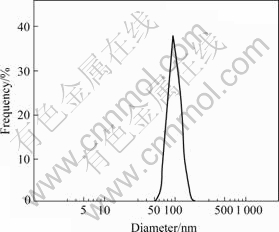
Fig.2 Particle size distribution measured by Malvern Zetasizer
The result observed with the atomic force microscope (Asylum Research MFP-3D AFM Systems) shows that, the particles in PEG-PEI/Fe3O4 nano-magnetic fluid are spheroid, the surface of them is smooth, and they are dispersed evenly without adhesion, as shown in Fig.3.
Through analyzing the result of superficial potential test of nanoparticle, on one hand, the stability of nano-suspension can be judged; on the other hand, because the charge of cell membrane is negative, the transfection system must be positive to make the transfection proceed smoothly[10]. The adsorption rate of plasmid DNA on nano-particles is one of the most direct indicators demonstrating whether nanoparticle could carry and transfect gene with high efficiency. The high DNA loading efficiency is helpful to enhancing the gene transfection efficiency of nanoparticle.

Fig.3 AFM photograph of nanoparticles
Table 1 proves the effects of PEI with different mass fractions of reactant on particle size, surface Zeta potential and plasmid pEGFP-C1 DNA loading efficiency of nanoparticle. The size of nanoparticles decreased as the amount of PEI in the nanoparticles was decreased. However, when the mass fraction of PEI was beyond 30%, the particle size dramatically increased into 100-200 nm. When the mass fraction of PEI was between 5% and 20%, the Zeta-potential of the resulting nanoparticles increased when the PEI concentration was increased. The surface potential was about 20 mV; and the pEGFP-C1 DNA loading efficiency was 40%-70%, which was not good for gene transfection. When the mass fraction of PEI was more than 25%, the surface potential would be more than 30 mV, and the pEGFP-C1 DNA loading efficiency could reach 90% or more, which could promote the efficiency of gene transfection.
Table 1 Effects of PEI on nanoparticle size, Zeta-potential and pEGFP-C1 DNA loading efficiency

As shown in Table 1, the optimum reaction condition was obtained: mass fraction of PEI=25%, then the nano-particle size was (79.99±7.3) nm, surface potential was (34.13±2.03) mV, and the adsorption rate of DNA plasmid pEGFP-C1 was (94.13±1.8)%.
3.2 X-ray diffraction pattern of PEG-PEI/Fe3O4 nano-magnetic fluids
Fig.4 shows the X-ray diffraction pattern of PEG-PEI/Fe3O4 nano-magnetic fluid. The parameters of PEG-PEI/Fe3O4 nano-magnetic fluid, such as the position and value of peak were close to the standard data of Fe3O4 alone in powder diffraction PDF card (JCPDS No.82-1533). The appearance of sample diffraction peaks at 2θ=30.16?, 35.70?, 43.33?, 53.60?, 57.10?, 62.9? corresponded to (220), (311), (400), (422), (511) and (440) crystal plane of Fe3O4 respectively, which indicated that the resulting particles were Fe3O4, with structures of cubic crystal.

Fig.4 X-ray diffraction pattern of PEG-PEI/Fe3O4 nano- magnetic fluid
Up to now, magnetic fluids have been used in medicine as magnetic targeting carriers for anti-cancer drug delivery systems. For application in biological organisms, these particles have good biocompatibility and low toxicity. The use of magnetic fluid as drug carriers aims to target drugs to a specific site through the application of a magnetic field to achieve prolonged release of high localized concentrations of the drug via the retention of magnetic particles in the region of interest. This can reduce the harm to healthy organs by limiting the circulation of the drug throughout the body. The magnetic particles, loaded with drug or gene, are attracted and held in the tumour region by a strong external magnetic field, which avoids non-specific uptake by the reticular-endothelial system[11].
PEG-PEI/Fe3O4 magnetic fluid mainly consists of nano sized Fe3O4 particles. The surface charge of Fe3O4 is neutralized by PEG. Effects of PEG on the resulting magnetic fluid also includes increasing dissolubility, which allows the resulting suspension to place for a long time without more agglomeration.
3.3 IR spectra of PEG-PEI/Fe3O4 nano-magnetic fluid
By comparing the absorption peaks of Fe3O4, PEG, PEI and PEG-PEI/Fe3O4 nano-magnetic fluid, the bond is identified among PEG, PEI and Fe3O4. Figs.5(a-d) show the FT-IR spectra in the region of 400-4 000 cm-1 of the PEG-PEI/Fe3O4 magnetic fluid by using infrared spectrometer (NICOL ET 200SXV FT—IR) and the KBr tabletting technique.
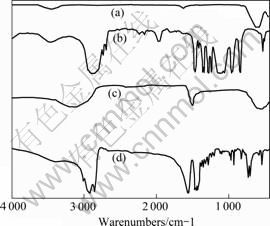
Fig.5 IR spectra of Fe3O4, PEG, PEI and PEG-PEI/Fe3O4:(a) Fe3O4; (b) PEG; (c) PEI; (d) PEG-PEI/Fe3O4
Fig.5(a) shows the characteristic absorption peak of Fe3O4 at 576 cm-1. Fig.5(b) shows the characteristic absorption peaks of PEG at 1111 cm-1 (C—O—C), 3 450 cm-1 (OH), 844 cm-1 (CH2CH2O), 2 875 cm-1, 1 465 cm-1, 1 349 cm-1 and 1 249 cm-1. Fig.5(c) shows the IR-spectra of PEI. The characteristic bands at 688 cm-1 (—NH wagging vibration), 1 637.5 cm-1 (NH2- scissor- ing vibration and C—H stretching vibration), 2 357.5 cm-1 and 2 110 cm-1 (NH+ asymmetrical stretching vibration) can be seen.
Fig.5(d) shows the IR-spectra of PEG-PEI/Fe3O4 magnetic fluid. It had the characteristic absorption peaks of PEG and PEI at 1 225.5 cm-1 (C—O—C), 3 470 cm-1 (OH), 870 cm-1 (CH2CH2O) and 1 629 cm-1, 2 851.4 cm-1, 1 446.2 cm-1, 1 425.5 cm-1 and 1 252 cm-1, respectively. We could also observe an absorption peak at 1 384 cm-1, which was a PEI characteristic absorption peak. A weaker intensity of —OH stretching band appeared at 2 921.7 cm-1, which showed an obvious red shift, indicating that the —OH radicals might interact with cationic [—CH2—CH2—NH2+-]n radicals in absorption process. This meant that PEI was absorbed on the surface of Fe3O4. Compared the wide and multiplet absorption peak of hydroxyl group in PEG-PEI/Fe3O4 shown in Fig.5(d) with singlet absorption peak of hydroxyl group in PEG, the different properties were due to the hydrogen bonds formed by the hydroxyl atoms in hydroxyl groups[12]. Therefore, both physical adsorption and hydrogen bonds could be found in magnetic colloid particles. Surface modification of Fe3O4 with PEG and PEI would prevent the aggregation of nano-particles and ensure a good stability.
PEI has been investigated extensively due to its higher transfection efficiency in polycation non-viral gene delivery vector. There is one nitrogen atom protonated in two carbon atoms of PEI molecule. Due to the different pKa values of the primary amino groups, secondary amino groups and tertiary amino groups consist of these nitrogen atoms, PEI has the ability to capture the protons at any different pH conditions, namely “proton sponge” mechanism[13].
Bonding PEI with the hydrophilic PEG molecule could increase the solubility of PEG-PEI/Fe3O4, neutralize surface potential, decrease interaction with proteins in blood, prolong circulation in vivo and decrease toxicity[14-16].
In vivo distribution of the resulting magnetic fluid could be affected by different amount of PEG. As reported, with the increase of modification degree with PEG, the derease of accumulation in the lung of composite injected by vein was observed[17]. The degree of modification with PEG could also affect gene transfection efficiency. With proper degree of modification with PEG (when the number of PEG segmer connected to each PEI molecule is less than 2), the gene transfection efficiency could be enhanced. While higher degree of modification with PEG (when the number of PEG segmer connected to each PEI molecule is more than 10) would reduce the transfection efficiency in vitro[18]. PEG could also change the nano structure and shape of PEG-PEI/Fe3O4 magnetic fluid[19] and weaken non-specific interaction between the composite and cellular membrane to reduce the internalization by cells.
3.4 Magnetism of PEG-PEI/Fe3O4 nano-magnetic fluid
As a magnetic targeting material, the paramagnetism property of PEG-PEI/Fe3O4 nano-magnetic fluid is considered as a key performance parameter. In this work, the magnetic properties of resulting magnetic fluid with different Fe3O4/PEI mass ratios were measured by using the VSM (Quantum Design, JDM-13). Normally, when Ms≥40 A?m2?kg-1, the materials could be classified as qualified magnetic materials, which could be controlled by external magnetic field.
Fig.6(a) displays the magnetization curve of pure Fe3O4 nanoparticles, and the magnetization of Fe3O4 changed according to different external field strength. Fig.6(a) showed a typical curve for ferrimagnetic material. The initial increase of external magnetic field intensity led to rapid increase in magnetization intensity of Fe3O4. After the field intensity increased to a certain level, the increase in magnetization intensity of Fe3O4 gradually slowed down until the saturated magnetization intensity(Ms) was reached (approximately when H= 6 000×79.528 A/m, Ms=60.22 A?m2?kg-1). When the external magnetic field intensity decreased, the magnetization intensity of Fe3O4 would decrease accordingly. When the external magnetic field intensity changed to 0, the magnetization intensity of Fe3O4 would change to 0 either.
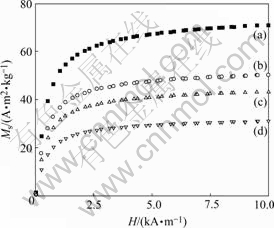
Fig.6 Magnetization curves for pure Fe3O4 and PEG-PEI/Fe3O4 nano-magnetic fluid with different mass fraction of PEI: (a) Pure Fe3O4; (b) 25%; (c) 30%; (d) 35%
Nanotechnology is the science and technology that study movement rule and interaction of system composed of matter on the nanometer length scale (1-100 nm)[20], and performing intelligent functions and portending particular use. Ferrimagnetism was observed in PEG-PEI/Fe3O4 nano-magnetic fluid. Figs.6(b-d) also display typical curves of ferrimagnetic material, and indicated that the PEG-PEI/Fe3O4 nano-magnetic fluid retained ferrimagnetism. When the mass fraction of PEI was 25%, the saturated magnetization intensity can be obtained in H=6 000× 79.528 A/m, and Ms=60.22 A?m2?kg-1. By further increasing the amount of PEI, the saturated magnetization intensity would be decreased. PEG-PEI/ Fe3O4 nana-magnetic fluid is a kind of stable colloidal solution, in which ferric oxide nanoparticles are well-distributed to hydrofacies, even the gravitational force, centrifugal force or magnetic force can not separate those elements. It has the magnetism of solid magnetic material and fluidity of liquid material with small particle size and strong magnetic response.
4 Conclusions
1) With ferric chloride, ferrous chloride, ammonia water, PEG and PEI as raw materials, the co-precipitation method was adopted to prepare colloid particles with size of about 80 nm and strong magnetism response. The experiment indicated that, through experimental condition optimization, when the PEG mass fraction is 25%, and ripening at 55 ℃ for 2 h, magnetic colloid particles obtained have the most superior synthesis characteristics, such as small size, positive Zeta potential and strong magnetism response. The morphology of magnetic fluid modified by PEG-PEI is spherical and smooth.
2) High plasmid pEGFP-C1 DNA loading efficiency indicates that PEG-PEI/Fe3O4 nano magnetic fluid could interact strongly with phosphate framework of DNA with negative charge, promote DNA concentration and combination with itself, and can also be easily absorbed by cellular membrane with negative charge.
References
[1] KOHN D B. SADELAIN M. GLOFIOSO J C. Occurrence of leukaemia following gene therapy of X-linked SCID [J]. Nat Rev Cancer, 2003, 3(7): 477-488.
[2] FOX J L. Gene therapy safety issues come to fore [J]. Nat Bioteeh, 1999, 17(9): 1153-1157.
[3] MERDAN T, KOPECEK J, KISSEL T. Prospects for cationic polymers in gene and oligonucleotide therapy against cancer [J]. Adv Drug Deliv Rev, 2002, 54(5): 715-758.
[4] REN J, HONG H Y, REN T B. Preparation and characterization of magnetic PLA-PEG composite particles [J]. Mater Lett, 2005, 59 (21): 2655-2658.
[5] CHEN J Y, LIAO Y L, WANG T H. Transformation of Escherichia coli mediated by magnetic nanoparticles in pulsed magnetic field [J]. Enzyme Microb Tech, 2006, 39(3): 366-370 .
[6] KICHLER A, CHILLON M, LEBORGUE C. Intranasal gene delivery with a polyethylenimine-PEG conjugate [J]. J Controlled Release, 2002, 81(3): 379-388.
[7] CAI Jia, CHEN Jun, HUANG Ping. Research about gene delivery with a polyethylenimine-PEG [J]. China Medical Industry, 2006, 37(11): 737-741.
[8] XIE J, XU C. Controlled PEGylation of monodisperse Fe3O4 nanoparticles for reduced non-specific uptake by macrophage cells [J]. Adv Mater, 2007, 19(20): 3163-3174.
[9] SUN J, ZHOU S B. Synthesis and characterization of biocompatible Fe3O4 nanoparticles [J]. Biomed Mater Res, 2007, 80A(2): 333-341.
[10] ZHANG X, PAN S R. Transfection of vascular endothelial growth factor gene VEGF165 mediated with PEG-PEI copolymers and its effect on the growth of endothelial cells [J]. Prog Biochem Biophys, 2007, 34(10): 1065-1071.
[11] YUE Chang. Preparation and characterization of thennosensitive magnetic paraticles [J]. Materials Science and Engineering, 2000, A333: 155-159.
[12] PANG X F, LIU L W. Biological effects of the carbon nano-tubes [C]// Proceedings of 27th Annual International Conference of the IEEE Engineering in Medicine and Biology Society (EMBS). Shanghai: 2005, 365.
[13] KICHLER A, LEBORGNE C, COEYTAUX E. Polyethylenimine mediated gene delivery: A mechanistic study [J]. J Gene Med, 2001, 3(2): 135-144.
[14] MERCHANT S, SUM J H. Dying cells program their expedient disposal: Serum amyloid P component upregulation in vivo and in vitro induced by photodynamic therapy of cancer [J]. Photoch Photobio SCI, 2007, 6(12): 1284-1289.
[15] KICHLER A, CHILLON M, LEBORGUE C. Intranasal gene delivery with a polyethylenimine-PEG conjugate [J]. J Controlled Release, 2002, 81(3): 379-388.
[16] KIRCHEIS R, WIGHTMAN L, WAGNER E. Design and gene delivery activity of modified polyethylenimines [J]. Adv Drug Deliv Review, 2001, 53(3): 341-358.
[17] KIM E M, JEONG H J, PARK I K. Monitoring the effect of PEGylation on polyethylenimine in vivo using nuclear imaging technique [J]. Nucl Med Biol, 2004, 31(6): 781-784.
[18] HE K, XU C Y, Hydrothennal synthesis and characterization of single-crystalline Fe3O4 nanowires with high aspect ratio and uniformity [J]. Mater Lett, 2007, 61(14/15): 3159-3162.
[19] MISHRA S, WEBSTER P, DAVIS M E. PEGylation significantly affects cellular uptake and intracellular trafficking of nonviral gene delivery particles [J]. Eur J Cell Biol, 2004, 83(3): 97-111.
[20] REMANT K, LUCAS B. Protection of oligonucleotides against enzymatic degradation by pegylated and nonpegylated branched polyethylene eimine [J]. Biomacromolecules, 2007, 8(4): 1333- 1340.
Foundation item: Project(20060390884) supported by the Postdoctoral Science Foundation of China; Project(2006FJ4240) supported by the Postdoctoral Science Foundation of Hunan Province, China; Project(30672047) supported by the National Natural Science Foundation of China
Corresponding author: WU Li-xiang; Tel: +86-13874991058; E-mail: ywlx@mail.csu.edu.cn


