
不同制冷剂/离子液体工质对的吸收式制冷循环性能分析
王晓坡,杜家浩,陈建林,何茂刚
(西安交通大学 能源与动力工程学院,热流科学与工程教育部重点实验室,陕西 西安,710049)
摘 要:
吸收式制冷工质对,对比分析19种制冷剂/离子液体工质对在单效吸收式制冷循环中的性能。首先,采用非随机双液活度系数模型对19种工质对的相平衡实验数据进行关联,验证模型的可靠性;其次,基于质量和能量守恒,建立单效吸收式制冷循环的热力学模型;最后,分析吸收器出口温度和发生温度等参数对溶液质量流量、性能系数及火用效率的影响。研究结果表明:溶液质量流量是影响系统性能的重要因素,不同的工质对会造成系统性能明显的差异,且碳链较短的氟化烷烃更易溶解于离子液体,有利于提升系统性能;随着吸收器出口温度降低和发生器出口温度增加,系统的性能系数均得到提升;在研究的工质对中,NH3/[omim]BF4,NH3/[hmim]BF4,H2O/[bmim]Tf2N,R32/[emim]Tf2N和R161/[hmim]Tf2N这5种工质对具有较高性能系数,其中NH3/[omim]BF4工质对性能最优,其性能系数最高可达到0.62;此外,蒸发温度每升高2 ℃可导致系统火用效率的最大值提高1.39倍,且火用效率随蒸发温度增加先急剧上升后逐渐降低,冷凝温度增加导致系统火用效率逐渐降低。
关键词:
中图分类号:TB651 文献标志码:A
文章编号:1672-7207(2021)06-1817-09
Performance analysis of absorption refrigeration cycle based on different refrigerant/ionic liquids working pairs
WANG Xiaopo, DU Jiahao, CHEN Jianlin, HE Maogang
(Key Laboratory of Thermo-Fluid Science and Engineering, Ministry of Education, School of Energy and Power Engineering, Xi'an Jiaotong University, Xi'an 710049, China)
Abstract: In order to find new alternative pairs, 19 kinds of refrigerant/ionic liquid working pairs were used in the single-effect absorption refrigeration cycle and the performance of the cycle was analyzed. Firstly, non-random two-liquid activity coefficient model was utilized to correlate the experimental phase equilibrium data of the studied pairs to validate the reliability of the model. Secondly, thermodynamic model of absorption refrigeration cycle was established based on the conservation of mass and energy. Finally, the effects of the outlet temperature of absorber and generation temperature on solution mass flow, coefficient of performance and exergy efficiency of the system were discussed. The results show that the mass flow of solution is an important factor affecting the system performance. The performance of the system has obvious difference due to the different working pairs. Fluorinated alkanes with shorter carbon chains are more easily dissolved in ionic liquid, which is conducive to improve the performance of the system. In addition, the coefficient of performance of the system increases with the decrease of the absorber outlet temperature and the increase of the generator outlet temperature. For the studied substances, five working pairs including NH3/[omim]BF4, NH3/[hmim]BF4, H2O/[bmim]Tf2N, R32/[emim]Tf2N and R161/[hmim]Tf2N have higher coefficient of performance. NH3/[omim]BF4 is the best, and the maximum coefficient of performance is 0.62. Moreover, the maximum value of the exergy efficiency increases by 1.39 times when the evaporation temperature increases by 2 ℃. The exergy efficiency increases sharply firstly and then decreases gradually with the increase of evaporation temperature, and the exergy efficiency decreases with the increase of the condensation temperature.
Key words: absorption refrigeration; working pairs including ionic liquids; non-random two liquid model
吸收式制冷技术能够有效利用余热资源,一直是各界研究的热点。然而,传统的吸收式制冷工质对如H2O/LiBr或NH3/H2O等仍存在一些问题[1-3],如NH3有毒且需要在发生器出口安装精馏装置,增加了系统的运行成本;LiBr溶液易结晶,对金属有腐蚀性且对装置密封性要求比较高。因此,寻找新型吸收式制冷工质对一直是吸收式制冷领域一个重要的研究方向。
由于离子液体具有较高热稳定性和极低饱和蒸气压,以离子液体为吸收剂组成的新型吸收式制冷工质对引起了广泛关注。MARTIN等[4]比较了CO2与多种离子液体组成的工质对在吸收式制冷循环中的应用潜力,发现CO2/[bmpyrr]Tf2N的性能系数最高可达到0.55;KIM等[5-6]分析比较了不同HFCs与[bmim]PF6组成的工质对的性能,发现R134a/[bmim]PF6性能最优;WU等[7-8]分析了不同HFOs与[hmim]Tf2N组成的工质对在吸收式制冷循环中的性能,发现工质对R1234ze(E)/[hmim]Tf2N的性能系数可达0.498;SUN等[9-10]比较了R1234yf与[emim]BF4,[hmim]BF4,[omim]BF4和[hmim]Tf2N等组成的工质对的性能,发现R1234yf/[hmim]Tf2N的性能最好;张垚等[11]研究了R1234ze(E)与3种离子液体组成的工质对的性能,发现R1234ze(E)/[omim]PF6的性能系数最大,可达到0.21。
上述研究表明,研究人员研究了以离子液体为吸收剂的工质对,获得了一些有价值的结论。然而,目前的研究主要集中在对某一种或几种工质对的性能分析上,而离子液体的种类繁多,进一步对比分析多种离子液体工质对的性能,对于开发新型吸收式制冷工质对具有重要意义。基于此,本文对烷烃类、烯烃类以及NH3等制冷剂与不同离子液体组成的19种工质对在吸收式制冷循环中的性能进行分析,探究发生器出口温度、吸收器出口温度、蒸发温度和冷凝温度等参数对系统性能系数及火用效率的影响。
1 热力学模型的建立
1.1 工质的名称及相关信息
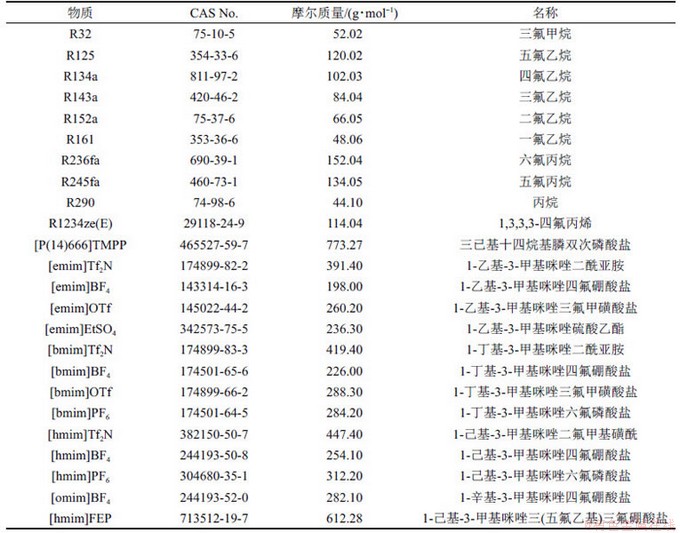
本文研究的制冷剂和离子液体的名称及相关信息如表1所示。
表1 制冷剂和离子液体的名称及相关信息
Table 1 Names and related information of refrigerants and ionic liquids
1.2 溶解度计算模型
由于离子液体的饱和蒸气压非常低,通常可以忽略,因此,当制冷剂与离子液体组成的混合物处于相平衡状态时,可认为气相中全部为制冷剂气体,即气相中制冷剂的摩尔分数y1=1。此时,相平衡方程可写为
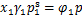
式中:x1为液相中制冷剂的摩尔分数;γ1为制冷剂的液相活度系数;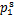
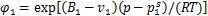
式中:R为气体常数;B1和v1分别为制冷剂的第二维里系数和饱和液相摩尔体积。
制冷剂的液相活度系数γ1由非随机双液模型(NRTL)[12]计算得到:
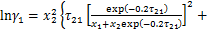
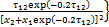
式中: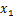
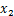
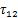
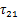
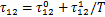
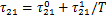
式(4)和(5)中,
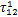
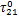
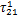
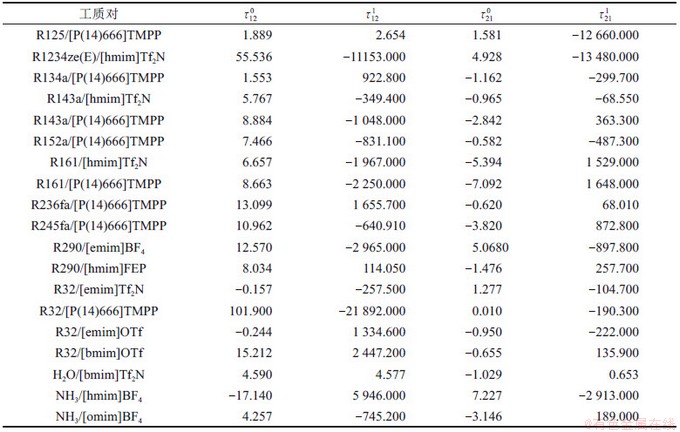
表2 制冷剂/离子液体工质对的NRTL模型系数回归结果
Table 2 Regressed results of coefficients in NRLT model for refrigerant/ionic liquid working pairs
为了验证NRTL模型对溶解度实验数据的复现性,以R152a/[P(14)666]TMPP为例,图1给出了由NRTL方程计算的制冷剂摩尔分数与实验值之间的偏差(δARD)分布。由图1可见:NRTL方程计算值与实验测量值具有良好一致性,偏差均在5%以内,故可以利用所得到的NRTL方程分析循环的性能。
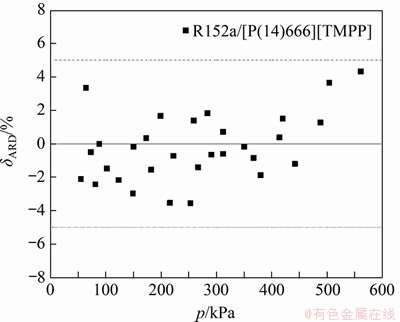
图1 NRTL模型预测值与实验值对比
Fig. 1 Comparison of predictive values from NRTL model and experimental data
1.3 循环模型
单效吸收式制冷循环的流程简图如图2所示。在分析时,假设系统处于稳态运行,忽略工质在管道和换热器中的热损失和压降,且出发生器和吸收器的蒸气和稀溶液均处于气液相平衡状态。
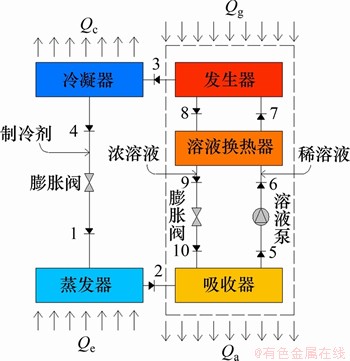
图2 单效吸收式制冷循环的流程图
Fig. 2 Flow chart of single-effect absorption refrigeration cycle
在进行循环的性能分析时,需要用到各状态点的焓。制冷剂/离子液体混合物的焓H可由下式计算:
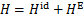
式中: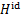
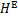
理想溶液的焓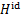
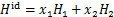
式中:H1和H2分别为制冷剂和离子液体的焓。离子液体的焓H2用下式计算得到:
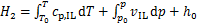
式中:h0依据国际制冷学会的标准选取;当T0=273.15 K时(p0=psat(T0)),h0=200 J/g;cp,IL和vIL分别为离子液体的比定压热容和比体积。离子液体定压比热容根据GARDAS等[22]提出的基团贡献法计算:
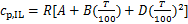
式中:A,B和D为离子液体中各基团贡献值加和求得的特征参数,具体数值可参考文献[22]。离子液体的比体积vIL由JACQUEMIN等[23]提出的基团贡献法得到:
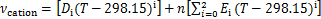
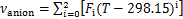
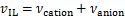
式中:vcation和vanion分别为阳离子和阴离子的比体积;Di,Ei和Fi分别为离子液体中各基团贡献值加和求得的特征参数,具体数值可以参考文献[23]。
二元溶液的过量焓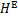
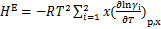
根据式(6)~(13)可以计算得到各点的焓。在此基础上,根据质量守恒和能量守恒,可以建立单效吸收式制冷循环中各部件的质能方程,得到各部件的热负荷Q。
对于发生器,
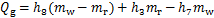
对于吸收器,
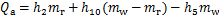
对于冷凝器,

对于蒸发器,
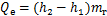
对于溶液换热器,
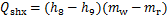
式中:Qg,Qa,Qc,Qe和Qshx分别为发生器、吸收器、冷凝器、蒸发器和溶液换热器的热负荷。对于溶液换热器,本文取其能效系数为0.8[24]。h为比焓,各下标分别对应于图2中的各状态点;mr为制冷剂蒸汽的质量流量;mw为进入发生器的稀溶液质量流量,

xw为制冷剂在稀溶液中的质量分数;xs为制冷剂在浓溶液中的质量分数。
单效吸收式制冷循环的性能系数用η表示,其计算公式为
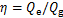
当忽略溶液泵功时,循环火用效率ECOP可以用下式计算:
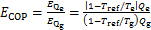
式中:
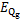
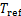
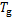
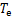
2 吸收器出口温度(ta)对溶液质量流量的影响
分析单效吸收式制冷循环的性能时,其计算工况如下:蒸发温度和冷凝温度分别为25 ℃和50 ℃,蒸发器出口工质过热5 ℃,冷凝器出口工质过冷5 ℃,循环制冷量为100 W。
图3所示为循环中进入发生器的稀溶液质量流量随着吸收器出口温度的变化趋势。从图3可见:溶液质量流量随吸收器出口温度增加而增加。吸收器出口温度增加,制冷剂在稀溶液中的质量分数降低,从而导致溶液质量流量随之增大。其中,工质对R143a/[P(14)666]TMPP,R143a/[hmim]Tf2N和R290/[hmim]FEP的溶液质量流量较大,当吸收器出口温度由35 ℃增加到50 ℃时,上述3种工质对的溶液质量流量增加幅度分别为98.68%,91.53%和91.22%。
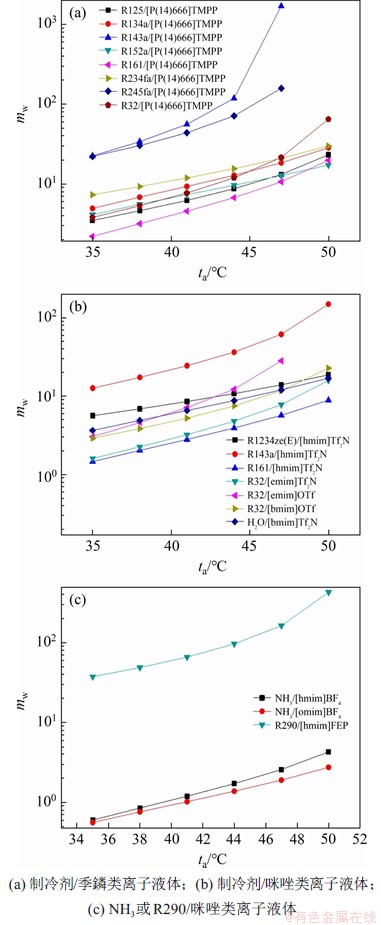
图3 溶液质量流量随着吸收器出口温度的变化趋势
Fig. 3 Trends of solution mass flow with absorber outlet temperature
3 吸收器出口温度(ta)对系统性能的影响
结合工业实际的需要,设定发生器出口温度为80 ℃。图4所示为系统的性能系数随吸收器出口温度的变化趋势。由图4可见:吸收器出口温度增加,引起制冷剂在浓溶液中的溶解度减小,浓溶液和稀溶液中的制冷剂质量分数的差相应减小,系统出现循环倍率单调增大,系统性能系数呈现单调减小的趋势。
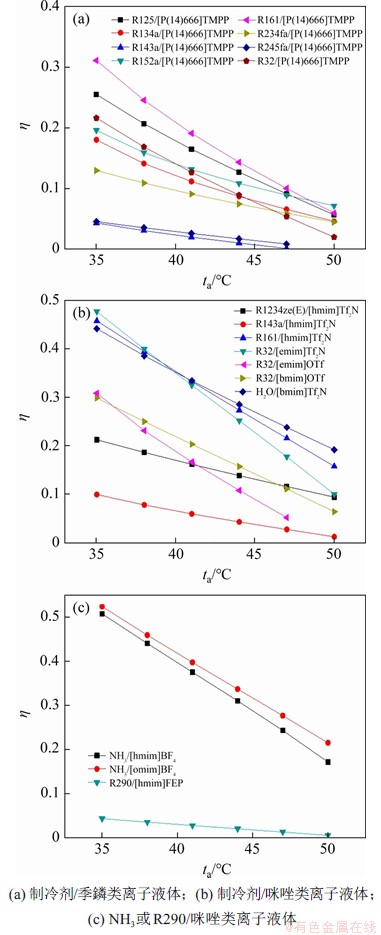
图4 系统性能系数随着吸收器出口温度的变化趋势
Fig. 4 Trends of coefficient of performance of system with absorber outlet temperature
此外,从图4还可见:在相同工况下,NH3/[omim]BF4和NH3/[hmim]BF4具有较高的性能系数,而含有[P(14)666]TMPP的工质对的性能系数普遍偏低。此外,结合溶解度实验数据以及图4(a)和(b)可知,对相同的离子液体,碳链较短的氟化烷烃的溶解度更大,这有利于提升系统性能。同时,降低吸收器出口温度可以显著提升系统性能系数。
4 发生温度(tg)对系统性能的影响
图5所示为系统性能系数随发生器出口温度的变化趋势。由图5可见:当发生器出口温度较低时(tg<80 ℃),制冷剂在稀溶液中的质量分数不变,制冷剂在浓溶液中的质量分数减小,溶液的循环倍率减小,导致发生器需要提供的加热量减小,此时,性能系数随发生器出口温度增加而急剧增加。当发生器出口温度达到一定程度后(tg>80 ℃),溶液质量流量随发生器出口温度的变化率逐渐减小,但此时发生器出口焓却逐渐增大,两者共同作用导致热负荷几乎不变,系统性能系数逐渐保持平稳。因此,在所研究的工质对中,NH3/[omim]BF4具有最高的性能系数,可达到0.62,R290/[hmim]FEP具有最低的性能系数。
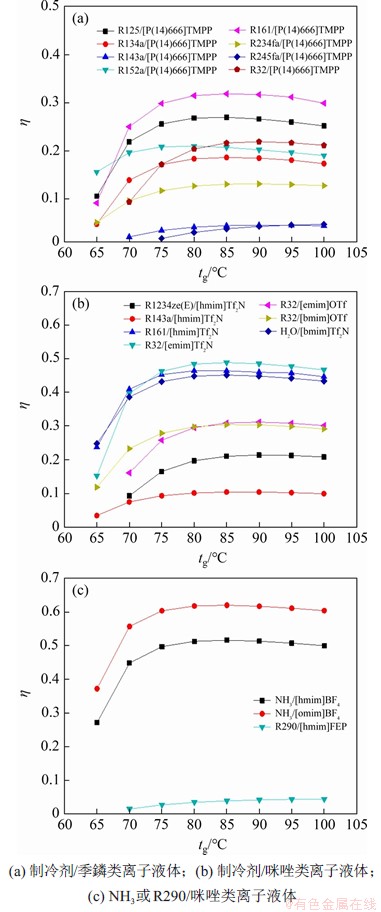
图5 系统性能系数随着发生器出口温度的变化趋势
Fig. 5 Trends of coefficient of performance of system with generator outlet temperature
5 系统火用效率的影响因素
本文以R161/[P(14)666]TMPP工质对为例,分别研究蒸发温度和冷凝温度对循环火用效率的影响,结果如图6所示。不同蒸发温度时对应的机组运行所需最低热源温度(t8min)、峰值温度(tpeak)以及系统火用效率的最大值(ECOPmax)如表3所示。
表3 同蒸发温度对应的t8min,tpeak和ECOPmax数值
Table 3 Values of t8min, tpeak and ECOPmax at different evaporator temperatures
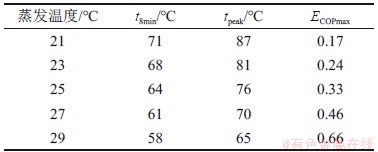
从表3可见:蒸发温度平均每升高2 ℃可导致系统火用效率最大时对应的峰值温度(tpeak)降低6 ℃左右,系统火用效率的最大值(ECOPmax)可以提高1.39倍。从图6(a)可见:随蒸发温度增加,火用效率先急剧上升,火用效率达到最大值时的“峰值温度”(图中箭头所指方向)后逐渐降低。
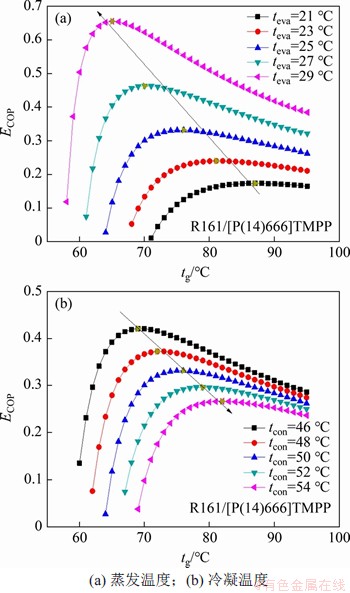
图6 蒸发温度和冷凝温度对系统火用效率ECOP的影响
Fig. 6 Influence of evaporation temperature and condensation temperature on system exergy efficiency ECOP
从图6(b)可见:增加冷凝温度导致系统火用效率ECOP沿着图中箭头所指方向逐渐降低,一方面,增加冷凝温度会增大溶液质量流量,导致发生器和溶液换热器等由于摩擦和压降等增加不可逆损失;另一方面,冷凝器本身由于存在温差也会增加传热火用损失。增加冷凝温度会提高发生器对于热源温度的要求,如当冷凝温度由46 ℃升高到54 ℃时,发生器所需的最低热源温度将会由60 ℃升高到70 ℃左右。
6 结论
1) NH3较大的蒸发潜热导致NH3/[omim]BF4具有最高的性能系数,最高可达0.62。而含有离子液体[P(14)666]TMPP的工质对的循环的性能系数普遍偏低。
2) 增加吸收器出口温度导致系统性能系数减小,而提高发生器出口温度,性能系数先增加后逐渐平稳。
3) 火用效率随蒸发温度增加先急剧上升,火用效率达到最大值时的“峰值温度”逐渐降低,而增加冷凝温度导致系统火用效率逐渐降低。
4) 增加蒸发温度或者减少冷凝温度都会降低发生器对于最低热源温度的要求。
参考文献:
[1] FONG K F, LEE C K. Performance advancement of solar air-conditioning through integrated system design for building[J]. Energy, 2014, 73: 987-996.
[2] WU Wei, WANG Baolong, SHI Wenxing, et al. Absorption heating technologies: a review and perspective[J]. Applied Energy, 2014, 130: 51-71.
[3] WANG Kai, ABDELAZIZ O, KISARI P, et al. State-of-the-art review on crystallization control technologies for water/LiBr absorption heat pumps[J]. International Journal of Refrigeration, 2011, 34(6): 1325-1337.
[4] MARTIN A, BERMEJO M D. Thermodynamic analysis of absorption refrigeration cycles using ionic liquid+supercritical CO2 pairs[J]. The Journal of Supercritical Fluids, 2010, 55(2): 852-859.
[5] KIM Y J, KIM S, JOSHI Y K, et al. Thermodynamic analysis of an absorption refrigeration system with ionic-liquid/refrigerant mixture as a working fluid[J]. Energy, 2012, 44(1): 1005-1016.
[6] KIM S, PATEL N, KOHL P A. Performance simulation of ionic liquid and hydrofluorocarbon working fluids for an absorption refrigeration system[J]. Industrial & Engineering Chemistry Research, 2013, 52(19): 6329-6335.
[7] WU Wei, ZHANG Haiyang, YOU Tian, et al. Performance comparison of absorption heating cycles using various low-GWP and natural refrigerants[J]. International Journal of Refrigeration, 2017, 82: 56-70.
[8] WU Wei, ZHANG Haiyang, YOU Tian, et al. Thermodynamic investigation and comparison of absorption cycles using hydrofluoroolefins and ionic liquid[J]. Industrial & Engineering Chemistry Research, 2017, 56(35): 9906-9916.
[9] SUN Yanjun, DI Gaolei, WANG Jian, et al. Performance analysis of R1234yf/ionic liquid working fluids for single-effect and compression-assisted absorption refrigeration systems[J]. International Journal of Refrigeration, 2020, 109: 25-36.
[10] 孙艳军, 邸高雷, 夏娟, 等. 以离子液体为吸收剂的吸收式制冷循环热力学分析[J]. 化工学报, 2018, 69(S2): 38-44.
SUN Yanjun, DI Gaolei, XIA Juan, et al. Thermodynamic analysis of absorption refrigeration cycles using ionic liquids as absorbents[J]. CIESC Journal, 2018, 69(S2): 38-44.
[11] 张垚, 朱山杉, 王晓坡, 等. 采用R1234ze(E)/离子液体工质对的吸收式制冷循环性能分析[J]. 西安交通大学学报, 2019, 53(5): 9-15.
ZHANG Yao, ZHU Shanshan, WANG Xiaopo, et al. Performance analysis on the absorption refrigeration cycle using R1234ze(E)/ionic liquids as working pairs[J]. Journal of Xi'an Jiaotong University, 2019, 53(5): 9-15.
[12] 方一波, 管文洁, 华超, 等. 吸收式工质对R152a+DMETrEG的气液相平衡特性[J]. 化工学报, 2017, 68(11): 4025-4034.
FANG Yibo, GUAN Wenjie, HUA Chao, et al. Vapor liquid equilibrium of absorption working pair R152a+DMETrEG[J]. CIESC Journal, 2017, 68(11): 4025-4034.
[13] SHIFLETT M B, HARMER M A, JUNK C P, et al. Solubility and diffusivity of difluoromethane in room-temperature ionic liquids[J]. Journal of Chemical & Engineering Data, 2006, 51(2): 483-495.
[14] DONG Li, ZHENG Danxing, SUN Guangming, et al. Vapor-liquid equilibrium measurements of difluoromethane+[emim]OTf, difluoromethane+[bmim]OTf, difluoroethane+[emim]OTf, and difluoroethane+[bmim]OTf systems[J]. Journal of Chemical & Engineering Data, 2011, 56(9): 3663-3668.
[15] LIU Xiangyang, QI Xuetao, LU Nan, et al. Gaseous absorption of fluorinated ethanes by ionic liquids[J]. Fluid Phase Equilibria, 2015, 405: 1-6.
[16] LIU Xiangyang, HE Maogang, LU Nan, et al. Solubilities of R-161 and R-143a in 1-hexyl-3-methylimidazolium bis(trifluoromethylsulfonyl)imide[J]. Fluid Phase Equilibria, 2015, 388: 37-42.
[17] LIU Xiangyang, LU Nan, SU Chao, et al. Solubilities of R32, R245fa, R227ea and R236fa in a phosphonium-based ionic liquid[J]. Journal of Molecular Liquids, 2016, 218: 525-530.
[18] FALLANZA M, ORTIZ A, GORRI D, et al. Propylene and propane solubility in imidazolium, pyridinium, and tetralkylammonium based ionic liquids containing a silver salt[J]. Journal of Chemical & Engineering Data, 2013, 58(8): 2147-2153.
[19] LIU Xiangyang, LU Nan, BAI Lihang, et al. Solubilities of propane and cyclopropane in 1-hexyl-3-methylimidazolium tris(pentafluoroethyl) trifluorophosphate[J]. International Journal of Refrigeration, 2016, 67: 69-76.
[20] KATO R, GMEHLING J. Measurement and correlation of vapor-liquid equilibria of binary systems containing the ionic liquids [EMIM][(CF3SO2)2N], [BMIM][(CF3SO2)2N], [MMIM][(CH3)2PO4] and oxygenated organic compounds respectively water[J]. Fluid Phase Equilibria, 2005, 231(1): 38-43.
[21] LI Guihua, ZHOU Qing, ZHANG Xiangping, et al. Solubilities of ammonia in basic imidazolium ionic liquids[J]. Fluid Phase Equilibria, 2010, 297(1): 34-39.
[22] GARDAS R L, COUTINHO J A P. A group contribution method for heat capacity estimation of ionic liquids[J]. Industrial & Engineering Chemistry Research, 2008, 47(15): 5751-5757.
[23] JACQUEMIN J, HUSSON P, MAYER V, et al. High-pressure volumetric properties of imidazolium-based ionic liquids: effect of the anion[J]. Journal of Chemical & Engineering Data, 2007, 52(6): 2204-2211.
[24] AYOU D S, BRUNO J C, CORONAS A. Integration of a mechanical and thermal compressor booster in combined absorption power and refrigeration cycles[J]. Energy, 2017, 135: 327-341.
(编辑 秦明阳)
收稿日期: 2020 -11 -28; 修回日期: 2021 -01 -12
基金项目(Foundation item):国家自然科学基金资助项目(51936009) (Project(51936009) supported by the National Natural Science Foundation of China)
通信作者:王晓坡,教授,从事流体热物性和制冷循环优化研究;E-mail:wangxp@xjtu.edu.cn
DOI: 10.11817/j.issn.1672-7207.2021.06.011
引用格式:王晓坡, 杜家浩, 陈建林, 等. 不同制冷剂/离子液体工质对的吸收式制冷循环性能分析[J]. 中南大学学报(自然科学版), 2021, 52(6): 1817-1825.
Citation:WANG Xiaopo, DU Jiahao, CHEN Jianlin, et al. Performance analysis of absorption refrigeration cycle based on different refrigerant/ionic liquids working pairs[J]. Journal of Central South University(Science and Technology), 2021, 52(6): 1817-1825.
摘要:为了寻找新型的吸收式制冷工质对,对比分析19种制冷剂/离子液体工质对在单效吸收式制冷循环中的性能。首先,采用非随机双液活度系数模型对19种工质对的相平衡实验数据进行关联,验证模型的可靠性;其次,基于质量和能量守恒,建立单效吸收式制冷循环的热力学模型;最后,分析吸收器出口温度和发生温度等参数对溶液质量流量、性能系数及火用效率的影响。研究结果表明:溶液质量流量是影响系统性能的重要因素,不同的工质对会造成系统性能明显的差异,且碳链较短的氟化烷烃更易溶解于离子液体,有利于提升系统性能;随着吸收器出口温度降低和发生器出口温度增加,系统的性能系数均得到提升;在研究的工质对中,NH3/[omim]BF4,NH3/[hmim]BF4,H2O/[bmim]Tf2N,R32/[emim]Tf2N和R161/[hmim]Tf2N这5种工质对具有较高性能系数,其中NH3/[omim]BF4工质对性能最优,其性能系数最高可达到0.62;此外,蒸发温度每升高2 ℃可导致系统火用效率的最大值提高1.39倍,且火用效率随蒸发温度增加先急剧上升后逐渐降低,冷凝温度增加导致系统火用效率逐渐降低。


