J. Cent. South Univ. (2017) 24: 325-334
DOI: 10.1007/s11171-017-3434-3
Effect of liquid nitriding at 400–670 °C on microstructure and properties of C110 Steel
YAN Jing(闫静)1, 2, WANG Jun(王均)1, GU Tan(谷坛)2, PAN Dong(潘东)1,
WANG Dan-qi(王单奇)3, LIN Yuan-hua(林元华)4, FAN Hong-yuan(范洪远)1
1. School of Manufacturing Science and Engineering, Sichuan University, Chengdu 610065, China;
2. Southwest Oil and Gasfield Company Research Institute of Natural Gas, Chengdu 610231, China;
3. Department of Materials Science and Engineering, Case Western Reserve University,
Cleveland, OH, 44106, USA;
4. State Key Laboratory of Oil and Gas Reservoir Geology and Exploitation, Southwest Petroleum University,
Chengdu 610500, China
 Central South University Press and Springer-Verlag Berlin Heidelberg 2017
Central South University Press and Springer-Verlag Berlin Heidelberg 2017
Abstract:
Liquid nitriding of C110 steel was conducted in a wide range of temperatures (400–670 °C) using a kind of chemical heat-treatments, and the hardness, mechanical and corrosion properties of the nitrided surface were evaluated. Experimental results revealed that the microstructure and phase constituents of the nitrided surface alloy are highly depended on the processing condition. When C110 steel was subjected to liquid nitriding at 430 °C, the nitrided layer was almost composed of a thin ε-Fe2–3N layer. When C110 steel was subjected to liquid nitriding at 640 °C, the phase composition of the nitrided layer was greatly changed. The nitrided layer depth increased significantly with increasing the treating temperature. The liquid nitriding effectively improved the surface hardness. After liquid nitriding, the absorption energy of the treated sample decreased and the tensile strength increased by Charpy V-notch (CVN) test. But the elongation of treated sample decreased. The reason is that the nitrided layer of sample is hardened and there is brittlement by diffusion of nitrogen atom. Despite of treatment temperature, the liquid nitriding can improve the corrosion. After being nitrided at 430 °C, the nitrided layer of the C110 steel was mainly composed by ε-Fe2–3N, which has excellent corrosion resistance and high microhardness, the nitrided sample has the best corrosion resistance. After nitriding temperature over 580 °C, especially at 680 °C, the sample’s surface was covered by the thick oxide layer, which has very low hardness and corrosion resistance. So, the corrosion resistance of samples is severely compromised.
Key words:
liquid nitriding; treated temperature; C110 steel; corrosion; hardness; properties;
1 Introduction
Casing or tubing and down-hole tools of C110 carbon steel are often thread-jointed together in sour gas wells. Occasionally, they are exposed to highly corrosive sour environments composed of H2S, CO2, elemental sulfur, and brine water [1, 2]. However, its application is restricted due to the relatively poor corrosion performance in harsh corrosion environment. This has necessitated the development of advanced surface engineering technologies to address the problem [3, 4].
Compared to conventional gas-phase nitriding and plasma nitriding, the liquid nitriding treatment, also referred to as salt bath nitrocarburizing or liquid nitrocarburizing, was regarded as an effective, low-cost method with many advantages, such as low treatment temperature, short treatment time, high degree of shape and dimensional stabilities, and reproducibility [5–8]. Moreover, the previous studies suggest that the performance of surface corrosion resistance provided by salt bath nitriding is better than that provided by hard chrome plating or other galvanic layers [9–14]. Liquid nitriding is a process that a combination of high fatigue resistance and good wear and corrosion resistances can be achieved [12]. FUNATANI [13] insisted that the liquid nitriding process technology could solve the environmental problems and could be applicable to the hardening of steels with high reaction efficiency. However, some reports doubted that the liquid nitriding technology has pollution because that the composition of the medium of liquid nitriding are toxic. This may come from the historic viewpoint. Actually, the dominant components of the conventional liquid nitriding medium are urea, M2CO3 (M denotes some elements of halogen), chloride and some nontoxic trace components [11]. In the process of nitriding, some NH3 gas will be emitted. If the gas and some waster slag is controlled properly, the
liquid nitriding can be a green technology [13].
The conventional liquid nitriding known as quench-polish-quench (QPQ) or Tenifer process is also justified by the importance of the diffusion speed of nitrogen atoms into the surface [15–21]. The required temperature for this process (540–580 °C) will contribute to the heat treatment of the core properties. Nitrogen, while diffusing into the surface, reacts with iron to form a relatively hard compound layer [14–16]. The compound layer is of a thickness varying between 0 and 20 μm and is made of two phases: ε-Fe2–3N and γ′-Fe4N. It is widely applied in the mechanical engineering industry to improve the friction, wear and fatigue properties of steel and cast-iron parts [17].
With the development of liquid nitriding, some researches have indicated that high temperature liquid nitriding produce a deeper nitrided layer than that of conventional technology [22, 23]. The large thick nitrided layer can bear more serious frictional behavior [22]. But to authors’ knowledge, the related report was very limited.
Moreover, the development of a low temperature process is a recent advancement in liquid nitriding technology [24–27]. Low-temperature treatment of aluminum extrusion dies and other forming dies at 480 °C is acquiring a wide use and ensures enhanced wear resistance without deterioration of hardness even in repeated nitriding. In addition, low-temperature nitriding reduces the distortion of shafts and crankshafts for automotive applications [13]. Unfortunately, with the high natural melting point of the most composition of liquid nitriding, the development of low temperature liquid nitriding proved to be a hard work [24–27]. Therefore, it is of great interest and practical importance to understand and evaluate the effects of the different temperature liquid nitriding technologies on microstructure and properties of chemically treated products.
However, little knowledge exists in the effects of a wide range of temperatures on microstructure, mechanical properties and corrosion behaviors when the liquid nitriding is done on C110 steel. This is especially the case in low and high temperature liquid nitriding. Therefore, the aim of the study is to investigate the influence of nitriding temperature on surface microstructure, mechanism properties and corrosion resistance of C110 steel, mainly using corrosion test, X-ray diffraction (XRD) and scanning electron microscopy (SEM).
2 Experimental
Samples were prepared from grade C110 steel with the composition shown in Table 1. The samples of C110 steel were dipped in molten composite salts at (400– 490) °C for 4 h, (520–670 °C) for 2 h for nitriding and then cooled in air to room temperature. The treatment temperatures were divided into three typical treatment temperatures of the different technologies. The low temperature nitriding temperature was 400–490 °C. The nitriding salts with a special formula contain a certain oxidization agent developed by authors [26, 27]. Nitriding at 520–580 °C was the conventional nitriding temperature. 610–670 °C was the high temperature nitriding temperature. After conventional and high temperature nitriding, the process is followed by a post-oxidation process at 400 °C for 40 min. The polishing process was omitted in this research by simulating the real production process, comparing to the standard QPQ technology. The formula of nitriding salt and oxiding salt was kept in secret by the manufacturing factory. The different temperature nitriding technologies have different CNO- concentrations, ranging from 20% to 50%.
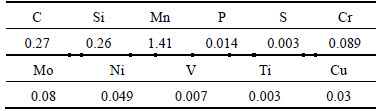
Table 1 Chemical composition of C110 steel (mass fraction, %)
The impact tests were carried out using a Tinius Olsen impact machine. Charpy V-Notch (CVN) samples were prepared in conformance with specifications in ASTM E-23. Three replicated tests were performed on samples of each nitriding temperature. The impact energy, E0 is 406.24 J, impact velocity is 5.47 m/s and test temperature is 24.0 °C. Cylindrical specimens for mechanical testing were machined from rod stock to have a reduced cross-section gauge region, which was 120 mm in length and 8 mm in diameter, with shoulder regions of 13 mm in radius. All of the specimens were longitudinally polished using 320 grit SiC to remove machining marks. Uniaxial tension tests were carried out in a MTS-100 (Eden Prairie, MN, USA) testing system. Erosion-corrosion tests were carried out by means of an erosion-corrosion device (detail described in Ref. [28]). Before the erosion-corrosion tests, the untreated and nitrided samples were surface polished and cleaned by alcohol. The surface area and the initial mass were measured. Samples were then immersed into 3.5% NaCl+100g/L Al2O3 solution at the speed of 500 r/min in room temperature at about 20 °C for 24 h. The abrasive particles used in this work were angular Al2O3 particles of 100–150 μm in diameter with a high hardness (HV 2500–3000), resulting in very severe erosion conditions [10]. The impact angle was kept at 90° to get the maximum slurry rate. The mass of the samples were measured by a precision electron balance model BSA124S (Beijing Sarturius Co., Ltd., China) with the balance to an accuracy of 0.1 mg. Dynamic polarization experiments were performed using a commercial electrochemical system (Model CS310, Wuhan CorrTest Instrument Co. Ltd., China). The scan rate was 5 mV/s and the experiments were conducted in 3.5% NaCl solution at room temperature (20 °C). The reference electrode was saturated calomel electrode (SCE), the counter electrode was platinum plate (Pt), and the samples connected with the working electrode. The electrodes were prepared by epoxy cold resin mounting of specimens, leaving areas for exposure to the electrolyte of about 1 cm2.
The structural changes in the modified layer were investigated using cross-sections for optical microscopy and the Hitachi S4800 scanning electron microscopy with the Philips EDS tester. X-ray diffractometer type Dmax-1400 with Cu Kα radiation and a nickel filter was used to determine the phases present in the modified layer.
3 Results and discussion
3.1 Metallography
It was observed that the microstructures produced during liquid nitriding of C110 steel changed according to treatment temperatures, as shown in Fig. 1. Optical microscopy (OM) observations showed bright nitrided layers near the surface of sample after nitriding at low temperature (430 °C), which had a different microstructure from the substrate. With a higher temperature (less than 580 °C), the nitrided layer had a larger thickness and few precipitates. After nitriding at a high temperature (more than 610 °C), the microstructure of the nitrided layer changed greatly. The nitrided layers are distinguished from the substrate due to the degree of etching of
microstructure being different. The cross-sectional microstructure of high temperature liquid nitrided specimens shows that the nitrided layer can be divided into four zones. Near the surface of the nitrided specimen, there is a loose oxide layer. Near the oxide layer is a bright layer of ε-Fe2-3N. Also, there is a gray columnar microstructure layer, γ′-Fe4N. Near the substrate is the composite layer of γ′-Fe4N and ferrite. The loose oxide sublayer, i.e., a porosity sublayer in the compound layer on the high temperature nitrided sample is mainly caused by a consequence of molecular nitrogen formation and corrosive attack by the salt bath [25].
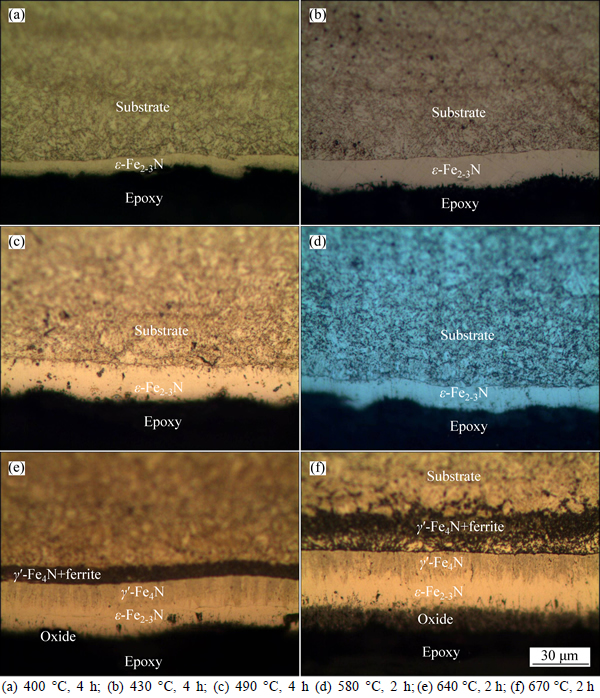
Fig. 1 Cross-sectional microstructures of nitrided samples:
The relations of nitrided layer thickness to the treating temperature are shown in Fig. 2. From the figure, it is very clear that the thickness of nitrided layers of C110 steel obtained at a certain temperature increases significantly with increasing temperature. This may be ascribed to the faster diffusion of nitrogen atoms at higher temperature.
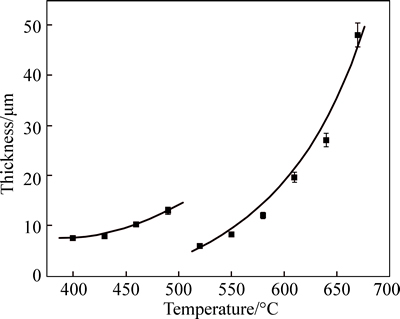
Fig. 2 Thickness of nitrided layer versus processing temperature
Figure 3 shows the plot of layer thickness [ln(d2)] against inversed temperature (1/T) for liquid nitriding C110. In this plot, the slope of the straight line gives the activation energy for the diffusion process. The activation energy was independent of the nitriding temperature, and was determined to be 213.5 kJ/mol(2.23 eV) at the high temperature and 94.5 kJ/mol (0.99 eV) at low temperature. When being nitrided at high temperatures, N diffuses in a multiphase matrix consisting of ε-Fe2-3N, γ′-Fe4N and ferrite. Therefore, fast diffusion of N is expected because of the short circuit diffusion along phase boundaries.
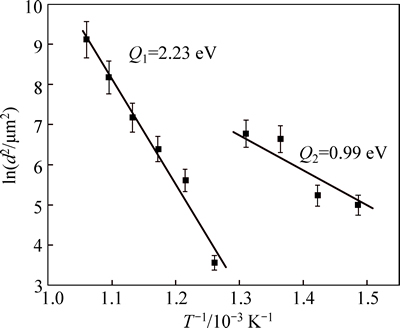
Fig. 3 Relationship of ln(d2) with inverse temperature (1/T)
3.2 Phase analysis of nitriding layer
Figure 4 shows the X-ray diffraction patterns of the nitrided and untreated samples. The phase composition of nitrided layers on C110 steel depends on the nitriding temperature and the nitrogen potential. As depicted in the figure, the phases present in the untreated C110 are dominated by ferrite (α). After nitriding treatment at a low temperature, the sample microstructure is characterized by peaks of ε-Fe2–3N and γ′-Fe4N, which is in line with the observations by LI et al [11]. The lower the treated temperature, the larger the amount of ε-Fe2–3N was observed. By increasing treatment temperature, the diffraction intensity of ε-Fe2–3N became stronger and that of γ′-Fe4N was weakened. From the diffraction patterns, differences in γ'-Fe4N and the iron oxide (FexOy) diffraction peak intensities are observable. It is obvious that higher peaks correspond to higher nitriding temperatures, and the FexOy diffraction peak intensity increased as the nitriding temperature increased. At a high temperature, the diffusivities of nitrogen and oxygen in the nitriding agent increase. So, there are more nitrides and oxides such as γ'-Fe4N and FexOy produced and the depth of nitrided layer is greater.
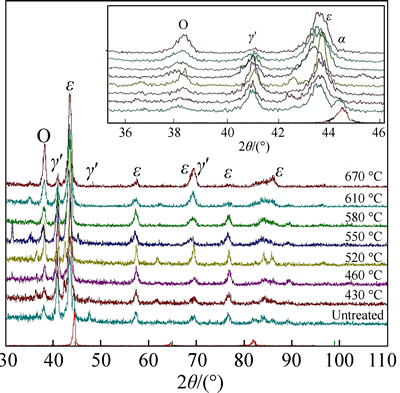
Fig. 4 XRD patterns of untreated and nitrided C110 steel at different temperatures
3.3 Microhardness
Figure 5 shows the micro-hardness of nitrided layers as a function of treated temperature. Very steep micro-hardness increase was found on the sample surface. After nitriding, the steel contains some additional elements which have a high affinity towards nitrogen (such as Cr and V). Some fine nitrides and precipitatesformed inside the diffusion layer, which led to a significant increase in hardness. For a low temperature nitriding, previous research indicated there are so many stacking faults and dislocation groups caused by nitrogen atom diffused in nitrided stainless steel [18]. For the 670 °C nitrided sample, the decline in hardness is probably due to the formation of the loose sublayer or/and the oxide sublayer.
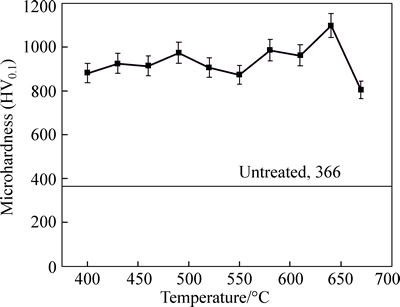
Fig. 5 Surface microhardness versus processing temperature
Figure 6 shows the micro-hardness of nitrided layers as a function of the depth of nitrided layer of 670 °C nitrided sample. From the microstructure shown in Fig. 1 and Fig. 5, it can be found that the hardness of oxide layer is very low and the hardness of ε-Fe2–3N is very high.
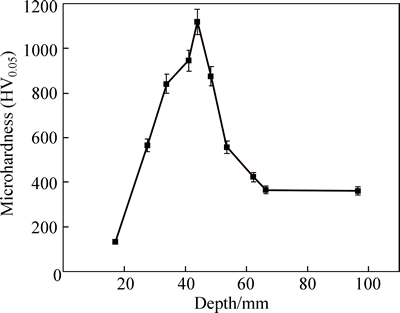
Fig. 6 Microhardness versus depth of nitrided layer of 670 °C nitrided sample
3.4 Mechanical properties
The load–displace and energy–displace oscilloscope traces at different nitriding temperature were recorded and then smoothened by a minicomputer-assisted instrumented system (shown in Fig. 7). The total Charpy impact energy of nitrided samples at different nitriding temperatures is shown in Fig. 8. The energy of the untreated sample also referred in figure. Form the figure,the nitrided samples have a similar situation: the displacement of impact and break process became short and the total absorption energy became small. According to Ref. [29], the impact fracture can be categorized into type I. This type trace refers to ductile fracture, which is Pgy
max>Pf as reflected in the P–d trace. In this type, the deflections correspond to fracture and the impact energy is great; the crack has been well stably propagated before specimen fracture. However, the absorption energy of the nitrided sample became smaller. This phenomenon indicated that liquid nitriding made the steel brittle. This agrees with Kahn’s report [30]. Kahn found that absorption energy of carburized 2205 duplex stainless steel at 380 °C decreased. And he ascribed that the decrease in Charpy impact energy after low temperature carburisation is not due to the surface layer but is most likely due to the incipient spinodal decomposition of the ferrite phase.
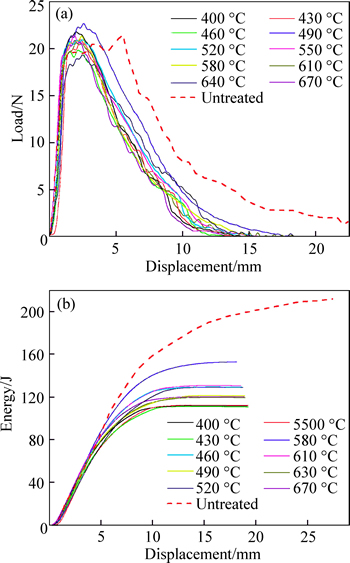
Fig. 7 Load–energy–time curves of CVN samples of nitrided C110 steel (a) P–d; (b) E–d
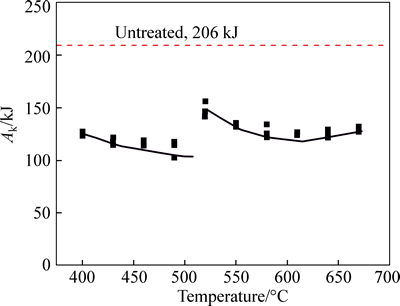
Fig. 8 Absorption energy of nitrided C110 steel CVN samples changing with treated temperature
Figure 9 shows some of the bulk tensile properties of the C110 steel nitrided at different temperatures. In the uniaxial tensile test, most of the nitrided specimen displayed an increase in both of the yield strength and the ultimate tensile strength, especially in the yield strength. Meanwhile, the elongation to failure of the nitrided specimen had a noticeable decrease.
The microscopical fractographs of the nitrided specimens with various conditions of the different treated temperatures are shown in Figs. 10–13. In all fractography observation of the nitrided samples, it can be concluded that the fracture type of surface of sample are typically brittle fracture and the core of the sample are typical ductile fracture. Cracks were found in some microscopical fractographs of the nitrided layer (Figs. 10(a), 11(c), 12(a)). Form the observation of the impact fracture of 640 °C nitrided sample by SEM, the fourfold microstructure can be clearly found (Fig. 13(a)). This is in agreement with the optical microscopy observation (Fig. 1). Each layer has a different fracture microstructure. More cleavage planes exist in the ε-Fe2–3N layer than γ'-Fe4N layer.
After nitriding, the nitrided layer of C110 steel has a very high microhardness (Fig. 4) due to the formation of ε-Fe2–3N and other nitrides.These nitride precipitates result in hardening by a precipitation hardening mechanism. Also, these nitrides make the layer more
brittle. Ahuge amount of interstitial atoms diffuse into the surface of the sample, which causes high compressive residual stresses in the sample surface after nitriding. Hoeft’s research results showed that for treatment temperatures less than 450 °C, high compressive residual stresses in the range of 2–3 GPa were found and the fracture resistances of these layers were less than 7 MPa·m1/2 [26]. WANG et al [28] and HUANG et al [32] found some cracks in the nitrided layer of 17-4 PH stainless steel and 2205 duplex steel after low temperature nitriding, respectively. The main reason for the formation of crack in the nitrided layer is the significant residual stresses. So, the nitride layer has high hardness and less toughness. Thus, in the process of tensile and impact test, the type of the nitrided layer fracture is typical brittlement fracture. The pre-existing crack induced by liquid nitriding can make the materials fracture easily. But, beyond the nitrided layer, the fracture surface is typically ductile without the effect of the high level of residual stresses due to good comprehensive mechanical properties of C110 steel.
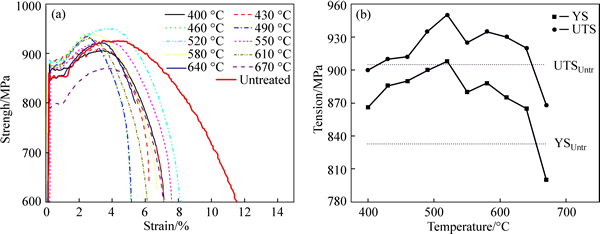
Fig. 9 Bulk tensile properties of C110 steel nitrided at different temperatures

Fig. 10 Microscopical fractographs of 430 °C nitrided sample:
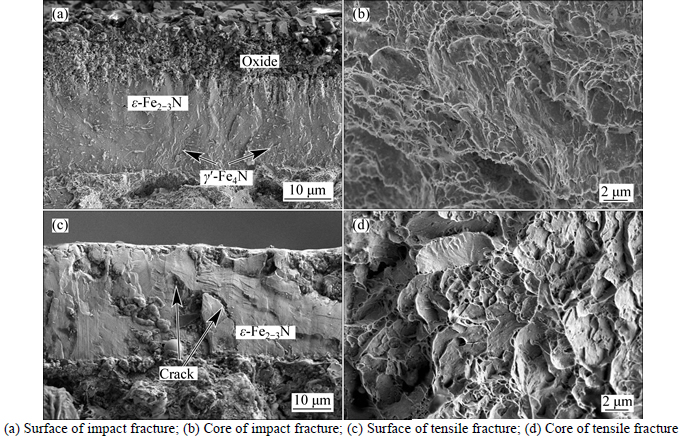
Fig. 11 Microscopical fractographs of 490 °C nitrided sample:
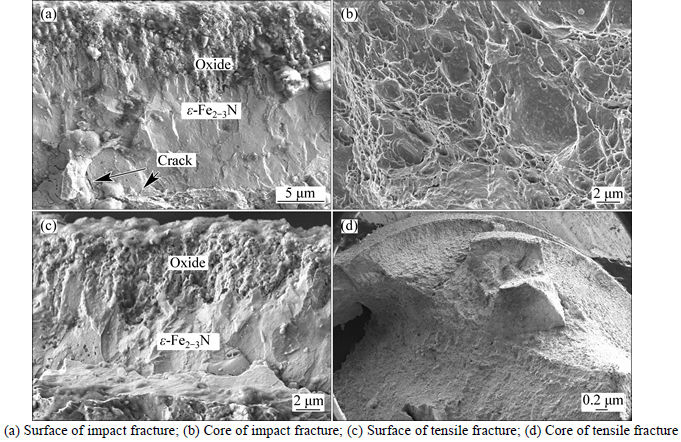
Fig. 12 Microscopical fractographs of 580 °C nitrided sample:
3.5 Corrosion behavior
The polarization corrosion of the specimens after different treatment conditions was studied in the polarization method. Jcorr, anodic/cathodic Tafel slopes, and corrosion rates measured for specimens in different conditions are presented in Table 2. And typical polarization curves of the different conditions obtained in 3.5% NaCl solution at room temperature are shown in Fig. 14.
Experimental results revealed that there is a great difference in the corrosion rate in different heat treatment conditions. Nitrided sampled have better corrosion resistance than untreated samples. When nitrided at 430 °C, the corrosion rate of the specimen was 2.69× 10–5 mm/a. This was less than the rate of the untreated sample (4.45×10–3 mm/a) obviously. The best corrosion performance of nitrided samples is the 430 °C nitrided sample.
The erosion corrosion rates of the untreated and different temperature nitrided samples were shown in Fig. 15 in 3.5% NaCl+100 g/L Al2O3 aqueous solution. It can be seen from the result that low temperature nitriding (400–460 °C) and conventional ferrite nitriding (520–580 °C) can greatly improve the erosion-corrosion
resistance and that the 430 °C treated sample has the best erosion-corrosion behavior. The mass loss is less than the 10% of untreated sample. Moreover, the high temperature (610–670 °C) nitrided sample has no good erosion-corrosion resistance. All of the nitrided samples have better erosion resistance than the untreated sample. The best corrosion science of nitrided samples is the 430 °C nitrided sample. This is consistent with the polarization test result (Fig. 14). A high hardness and corrosion resistance nitrided layer was formed at the surface of the C110 steel to protect the damage of the erosion corrosion. After treatment temperature at 430 °C, the nitrided layer of the C110 steel was mainly composed by ε-Fe2–3N, which has excellent corrosion resistance and high microhardness. Our results indicated that the 430 °C treated sample has the best corrosion behavior. In fact, the 430 °C treated sample has similar corrosion resistance with the 580 °C treated sample because the ε-Fe2–3N are mainly composition in the nitrided layer of both of the treated samples. After nitriding temperature over 580 °C, especially at 680 °C, the very thick oxide layer cover the sample’s surface and some crack formed because the huge compress stress [28]. Previous results demonstrated that oxide layer has very low hardness, the corrosion resistance is severely compromised [10].

Fig. 13 Microscopical fractographs of 640 °C nitrided sample:
Table 2 Corrosion parameters for various specimens obtained in 3.5% NaCl solution at room temperature by polarization method
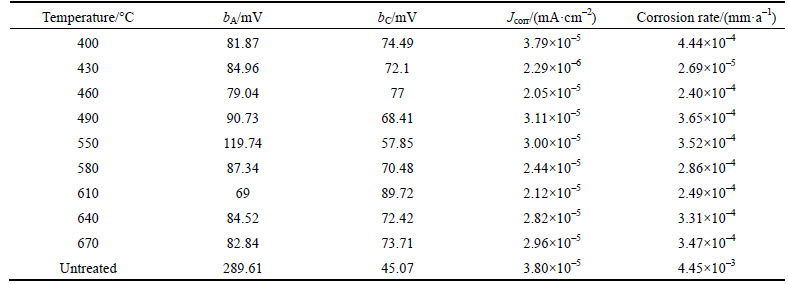
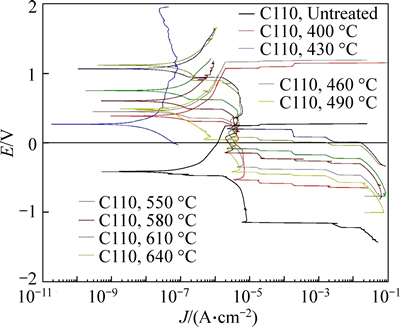
Fig. 14 Comparative polarization curves for different conditions in 3.5% NaCl solution at room temperature
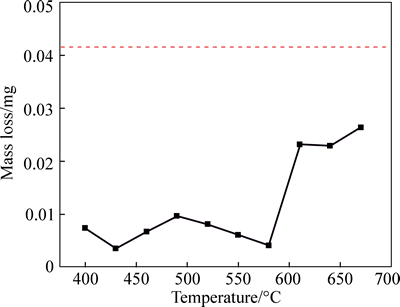
Fig. 15 Relationship of mass loss of C110 steel liquid nitrocarburizing after erosion-corrosion with nitrocarburizing temperature
4 Conclusions
1) A modified layer was formed on the surface of substrate with the thickness changing intensively with the temperature. For nitriding temperature at 430 °C, the nitrided layer of the C110 steel was mainly composed by ε-Fe2–3N .
2) After liquid nitriding, the absorption energy of CVN samples decreased and the tensile strength increased. But the elongation of nitrided sample decreased. The reason is that the nitrided layer of the sample is hardened and embrittled by diffusion of nitrogen atom.
3) The liquid nitriding can effectively improve the surface hardness, corrosion resistance and erosion resistance. After treatment at 430 °C, the nitrided layer of the C110 steel was mainly composed by ε-Fe2–3N, which has excellent corrosion resistance and high microhardness. The 430 °C treated sample has the best corrosion behavior. After nitriding at temperature over 580 °C, especially at 680 °C, the very thick oxide layer covers the sample’s surface. Previous results demonstrated that oxide layer has very low hardness, and the corrosion resistance is severely decreased.
References
[1] REN C Q, ZENG D Z, LIN J H, SHI T H, CHEN W G. Sour corrosion of C110 steel and its influence by galvanic couple and stress [J]. Industrial and Engineering Chemistry Research, 2012, 51: 4894–4904 .
[2] BOSCH C, WANZENBERG E, GATEAUD A, MARCHEBOIS H, DELATTRE L, CURY R, LINNE C. DCB testing of C110 material in standard and mild sour test conditions [C]// Corrosion/2010. San Antonio, Texas, 2010, 10315.
[3] HASSANI S H, ROBERTS K P, SHIRAZI S A, SHADLEY J R, RYBICKI E F, JOIA C. Flow loop study of NaCl concentration effect on erosion, corrosion, and erosion corrosion of carbon steel in CO2-saturated systems [C]// Corrosion/2011. Houston, Texas, 2011, 11237.
[4] MORJS M F, CORRIGAN P, BIRBILIS N, COLE I S. A green MnMgZn phosphate coating for steel pipelines transporting CO2 rich fluids [J]. Surface and Coatings Technology, 2012, 210: 183–189.
[5] WEN D C. Erosion and wear behavior of nitrocarburized DC53 tool steel [J]. Wear, 2010, 268: 629–636.
[6] PSYLLAKI P, KEFALONIKAS G, PANTAZOPOULOS G, ANOTONIOU S, SIDERIS J. A Microstructure and tribological behaviour of liquid nitrocarburized tool steels [J]. Surface and Coatings Technology, 2002, 162: 67–78.
[7] FARES M L, TOUHAMI M Z, BELAID M, BRUYAS H. Surface characteristics analysis of nitrocarburized (Tenifer) and carbonitrided industrial steel AISI 02 types [J]. Surface and Interface Analysis, 2009, 41: 179–186.
[8] QING Y H, GE S R, XUE Q J. Study on the structure and wear resistance of two-step salt bath nitrocarburized steel [J]. Wear, 1998, 218: 232–236.
[9] MARUSIC K, OTMACIC H, LANDEK D, CAJNER F. Modification of carbon steel surface by the Tenifer process of nitrocarburizing and post-oxidation [J]. Surface and Coatings Technology, 2006, 201: 3415–3421.
process of nitrocarburizing and post-oxidation [J]. Surface and Coatings Technology, 2006, 201: 3415–3421.
[10] PAN Dong, LIN Yuan-hua, LUO De-fu, ZENG De-zhi, HUANG Rui-bo, WANG Jun, Effect of salt bath nitriding process on erosion corrosion resistance of C110 steel [J]. Transactions of Materials and Heat Treatment, 2013, 34(11): 216–220. (in Chinese)
[11] LI H Y, LOU D F, YEUNG C F, LAU K H. Microstructural studies of QPQ complex salt bath heat-treated steels [J]. Journal of Materials Processing Technology, 1997, 69: 45–49.
[12] ZHANG J W, LU L T, SHIOZAWA K, ZHOU W N, ZHANG W H. Effect of nitrocarburizing and post-oxidation on fatigue behavior of 35CrMo alloy steel in very high cycle fatigue regime [J]. International Journal of Fatigue, 2011, 33: 880–886.
[13] FUNATANI K. Low-temperature liquid nitrocarburizing of steels [J]. Metal Science and Heat Treatment, 2004, 46: 277–281.
[14] JACQUET P, COUDERT J B, LOURDIN P. How different steel grades react to a liquid nitrocarburizing and post-oxidation process: Influence of alloying elements [J]. Surface and Coatings Technology, 2011, 205: 4064–4067.
[15] BALA SRINIVASAN P, KRISHNAKUMAR C V, KRISHNARAJ N. Sliding wear behavior of salt bath nitrocarburized medium carbon steel [J]. Journal of Materials Engineering and Performance, 2002, 11: 509–515.
[16] CHIU L H, WU C H, CHANG H. Wear behavior of nitrocarburized JIS SKD61 tool steel [J]. Wear, 2002, 253: 778–786.
[17] CAI W, MENG F N, GAO X Y, JING H. Effect of QPQ nitrocarburizing time on wear and corrosion behavior of 45 carbon steel [J]. Applied Surface Science, 2012, 261: 411–414.
[18] PANTAZOPOULOS G, PSYLLAKI P, KANAKIS D, ANTONIOU S, PAPADIMITRIOU K, SIDERIS J. Tribological properties of a liquid nitrocarburized special purpose cold work tool steel [J]. Surface and Coatings Technology, 2006, 200: 5889–5895.
[19] CHIU L H, WU C H, CHANG H. Wear behavior of nitrocarburized JIS SKD61 tool steel [J]. Wear, 2001, 253: 778–786.
[20] KRISHNARAJ N, IYER K J L, SUNDARESAN S. Scuffing resistance of salt bath nitrocarburized medium carbon steel [J]. Wear, 1997, 210: 237–244.
[21] QIANG Y H, GE S R, XUE Q J. Sliding wear behavior of nitrocarburized bearing steel [J]. Materials Science and Engineering A, 2000, 278: 261–266.
[22] XU Wen-ting, LUO De-fu, DENG Hui, WU Yang, ZHANG Kai. Corrosion resistance of 45 steel after deep layer QPQ treatment [J]. Heat Treatment of Metals, 2012, 37: 91–94. (in Chinese)
[23] WANG J, LIN Y H, FAN H Y, ZEN D Z, QIAN P,SHEN B L. Effects of temperature on microstructure and wear of salt bath nitrided 17-4PH stainless steel [J]. Journal of Materials Engineering and Performance, 2012, 21: 1708–1713.
[24] HAMDY A S, MARX B, BUTT D. Corrosion behavior of nitride layer obtained on AISI 316L stainless steel via simple direct nitridation route at low temperature [J]. Materials Chemistry and Physics, 2011, 126: 507–514.
[25] TSUJIMURA H, GOTO T, ITO Y. Electrochemical formation and control of chromium nitride films in molten LiCl–KCl–Li3N systems [J]. Electrochimica Acta, 2002, 47: 2725–2731.
[26] WANG J, LIN Y H, YAN J, ZEN D Z, ZHANG Q, HUANG R B, FAN H Y. Influence of time on the microstructure of AISI 321 austenitic stainless steel in salt bath nitrocarburizing [J]. Surface and Coatings Technology, 2012, 206: 3399–3404.
[27] WANG J, XIONG J, ZHANG T. Nitride salt used in low salt bath nitriding process, comprises specified amount of urea, potassium carbonate, sodium carbonate, lithium carbonate, sodium chloride and potassium chloride [P]. China Patent, ZL2011 10184878.1.
[28] WANG J, LIN Y H, LI M X, FAN H Y, ZENG D Z, XIONG J. Effects of the treating time on microstructure and erosion corrosion behaviour of salt bath nitrided 17-4PH stainless steel [J]. Metallurgical and Materials Transactions B: Process Metallurgy and Materials Processing Science, 2013, 44: 1010–1016.
[29] SREENIVASAN P R, MOITRA A, RAY S K, MANNAN S L,CHANDRAMOHAN R. Dynamic fracture toughness properties of a 9Cr-1Mo weld from instrumented impact and drop-weight tests [J]. International Journal of Pressure Vessels and Piping, 1996, 69: 149–159.
[30] KAHN H, HEUER A H, MICHAL G M, EMST F, SHARGHI-MOSHTAGHIN R, GE Y, NATISHAN P M, RAYNE R J, MARTIN F J. Interstitial hardening of duplex 2205 stainless steel by low temperature carburization: Enhanced mechanical and electrochemical performance [J]. Surface Engineering, 2012, 28: 213–219.
[31] HOEFT D, LATELLA B A, SHORT K T. Residual stress and cracking in expanded austenite layers [J]. Journal of Physics: Condensed Matter, 2005, 17: 3547–3558.
[32] HUANG Y B, WANG J, ZHONG S, LI M X, XIONG J, FAN H Y. Surface modification of 2205 duplex stainless steel by low temperature salt bath nitrocarburizing at 430 °C [J]. Applied Surface Science, 2013, 271: 93–97.
(Edited by YANG Bing)
Cite this article as:
YAN Jing , WANG Jun, GU Tan, PAN Dong, WANG Dan-qi, LIN Yuan-hua, FAN Hong-yuan. Effect of Liquid Nitriding at 400-670oC on Microstructure and Properties of C110 Steel [J]. Journal of Central South University, 2017, 24(2): 325-334.
DOI:https://dx.doi.org/10.1007/s11171-017-3434-3Foundation item: Projects(51471112, 51611130204) supported by the National Natural Science Foundation of China
Received date: 2015-12-07; Accepted date: 2016-08-01
Corresponding author: FAN Hong-yuan, Professor, PhD; Tel: +86–13688058426; E-mail: fanhy@scu.edu.cn
Abstract: Liquid nitriding of C110 steel was conducted in a wide range of temperatures (400–670 °C) using a kind of chemical heat-treatments, and the hardness, mechanical and corrosion properties of the nitrided surface were evaluated. Experimental results revealed that the microstructure and phase constituents of the nitrided surface alloy are highly depended on the processing condition. When C110 steel was subjected to liquid nitriding at 430 °C, the nitrided layer was almost composed of a thin ε-Fe2–3N layer. When C110 steel was subjected to liquid nitriding at 640 °C, the phase composition of the nitrided layer was greatly changed. The nitrided layer depth increased significantly with increasing the treating temperature. The liquid nitriding effectively improved the surface hardness. After liquid nitriding, the absorption energy of the treated sample decreased and the tensile strength increased by Charpy V-notch (CVN) test. But the elongation of treated sample decreased. The reason is that the nitrided layer of sample is hardened and there is brittlement by diffusion of nitrogen atom. Despite of treatment temperature, the liquid nitriding can improve the corrosion. After being nitrided at 430 °C, the nitrided layer of the C110 steel was mainly composed by ε-Fe2–3N, which has excellent corrosion resistance and high microhardness, the nitrided sample has the best corrosion resistance. After nitriding temperature over 580 °C, especially at 680 °C, the sample’s surface was covered by the thick oxide layer, which has very low hardness and corrosion resistance. So, the corrosion resistance of samples is severely compromised.


