
Sol-gel synthesis and photoluminescence of LaPO4?Eu3+ nanorods
GAO Rui(高 锐)1, QIAN Dong(钱 东)1, LI Wei(李 卫)1, 2
1. School of Chemistry and Chemical Engineering, Central South University, Changsha 410083, China;
2. State Key Laboratory of Powder Metallurgy, Central South University, Changsha 410083, China
Received 18 September 2009; accepted 14 January 2010
Abstract:
Monoclinic LaPO4 nanostructures with uniform rod shape have been successfully synthesized by a simple sol-gel method. The procedure involves formation of homogeneous, transparent, metal-citrate-EDTA gel precursors, followed by calcination to promote thermal decomposition of the gel precursors to yield the LaPO4 nanoparticles. Their morphologies and structures were characterized by XRD, TEM, TG-DSC and HRTEM. The results indicate that single monoclinic phase LaPO4 nanorods are readily obtained at 800 ℃ within 3 h. Furthermore, photoluminescence (PL) characterization of the Eu3+-doped LaPO4 nanocrystals was carried out. The effects of calcination temperatures and Eu3+ doping content on the PL properties were elaborated in detail. Room-temperature photoluminescence (PL) characterization reveals that the optical brightness as well as the intensity ratio of 5D0-7F1 to 5D0-7F2 is highly dependent on the calcination temperature, and the Eu0.05La0.95PO4 nanophosphor shows the relatively promising PL performance with the most intense emission.
Key words:
LaPO4; Eu doping; sol-gel; nanorods; photoluminescence;
1 Introduction
The useful applications of rare earth element compounds, especially lanthanide phosphate doped inorganic materials, have been touched upon broadly. Over the past a few years, they have been applied in many fields, such as optical display panels, cathode ray tubes, optoelectronic, sensitive device, nanoscale electronic and plasma display panels[1-4] due to their special chemical and physical properties.
Various solution-phase routes, including combustion[5], sol-gel[6-7], precipitation[8], water-oil microemulsion[9], polyol-mediated process[10], ultrasoni?cation[11], hydrothermal[12-14], and mechanochemical method[15], have been tried to lower the reaction temperature and obtain high-quality LaPO4-based nanoparticles. However, the simple and mass fabrication of LaPO4 nanocrystals with narrow grain size distribution and uniform morphology still remains a challenge. It appears that the best solution both to control powder morphology and to produce low cost thin films is the use of soft chemistry routes, in particular the sol-gel process since the intimate mixing of components ensure homogeneity of the final product. The sol-gel methods also have some obvious advantages compared with other ways, such as low processing temperature, easy coating of large surfaces, possible formation of porous films and homogeneous multicomponent oxide films as well as simple and cheap experimental setups. This sol-gel process is widely used for oxides materials which are the mostly used materials in phosphor technology by far.
In this regard, many research efforts have been taken on the upgrade of luminescence[16] of lanthanide compounds and control of the morphologies[17-18]. Nanoscale LaPO4 with small grain shape has been synthesized via sol-gel reaction by RAJESH et al[7], and the yield of product is 85%. This work mainly worked on the elevation of the emitting wavelength and intensity by synthesis of LaPO4 nannorods which could be used in biomedical image as quantum dots[19]. We adopted a simple sol-gel way to prepare LaPO4 with good morphologies and fine crystal structures; and its emission and intensity of luminescence were also studied.
2 Experimental
2.1 Synthesis of LaPO4
All the chemical reagents were analytically pure and used without further purification. In a typical procedure, 0.01 mol of ethylenediamine tetraacetic acid (EDTA) was dissolved in 1 mol/L ammonia solution, then 0.45 mol/L La(NO3)3 aqueous solution was added dropwise to 1 mol/L CA solution under vigorous stirring. After that, this mixed solution and 0.5 mol/L of NH4H2PO4 aqueous solution were added dropwise to the already dissolved EDTA solution by fiercely stirring. The pH of the solution under these steps was kept at 10-11 by using the ammonia solution as necessary. Then, the mixed solution was heated at 90 ℃ in a water bath with strong stirring as far as a transparent mixed sol was consequently obtained. The obtained sol was then transferred into an oven at 120 ℃ for 1 h to produce a viscous and black gel. The acquired gel subsequently was calcined for 3 h in muffle at 800 ℃ to fabricate the final white LaPO4 nanoparticle.
2.2 Synthesis of Eu3+-doped LaPO4
The synthesis of the Eu3+-doped LaPO4 nanoparticles was the same with above described methods except for the addition of a certain amount of Eu(NO3)3 which was gained by Eu2O3 dissolved in 1 mol/L nitric acid after dropping of the La(NO3)3 aqueous solution. And the molar fractions of Eu3+ in the obtained products were 1%, 5% and 10%, respectively.
2.3 Sample characterization
Phase structures of the as-prepared samples were identified with X-ray powder diffraction (XRD) taken on a Rigaku-D-Max rA 12 kW diffractometer with Cu Kα radiation (λ=1.540 56 ?) at an operation voltage and current of 40 kV and 300 mA, respectively. The morphologies and sizes of the final products were characterized with a Hitachi H-800 transmission electron microscope (TEM) as well as high-resolution transmission electron microscope (HRTEM, JEOL-3010). The thermal decomposition behavior of the citrate gel precursors was examined by a thermogravimetric analyzer (TGA, Perkin-Elmer, TAC7/DX) using air as the working gas. For DSC measurements, the samples were heated from room temperature to 950 ℃ at a heating rate of 15 K/min. α-Al2O3 was used as the reference material, and the samples were run in open platinum pans. The PL measurements were carried out by using a Hitachi F-4500 fluorescence spectrophotometer. The excitation and emission spectra were corrected for the beam intensity variation in the Xe light source used. For comparison, all excitation and emission spectra were measured at room temperature with the same instrumental parameters.
3 Results and discussion
3.1 Thermal analysis
To clarify the chemical reaction during the thermal decomposition of the precursors, the thermal decomposition procedure of the obtained La-P-Citrate-EDTA gel precursors was studied by thermogravimetric/differential scanning calorimetry (TG-DSC) and the results are shown in Fig.1. From the TG curve, in the temperature range from ambient temperature to 600 ℃, three mass loss regions occur at 28-200 ℃(about 4.20%), 200-350 ℃ (about 27.20%), and 350-600 ℃ (about 41.05%). No significant mass loss can be observed from 600 to 800 ℃. Correspondingly, five discrete regions of thermal decomposition can be found in the DSC curve. The first endothermic peak centered at 99 ℃ corresponds to the elimination of water. Due to the elimination of molecule water, the second endothermic peak centered at 236 ℃ appears. The following two exothermic peaks centered at around 342 ℃ and 438 ℃ can also be ascribed to the combustion of the nitrate and the organic phases (EDTA). Subsequently, the third exothermic peak centered at 651 ℃ can be attributed to the elimination of the remaining organic materials (carbon and any other organic compounds) accompanied by the crystallization of the LaPO4 inorganic phase[6, 20].

Fig.1 TG-DSC curves of as-synthesized metal-citrate-EDTA precursors
3.2 XRD analysis
The XRD patterns of the as-obtained samples are shown in Fig.2. It can be observed that the samples synthesized by sol-gel method at different temperatures crystallize in the monazite LaPO4[8] (JCPDS 35-0731). From the main planes of ![]() , (200), (120),
, (200), (120),![]() and
and ![]() , we can conclude that the intensity of the diffraction peaks gradually increases with the increase of temperature, revealing that the particle size increases and crystallinity increases with temperature. Moreover, no diffraction peaks for other phases are found in the XRD patterns.
, we can conclude that the intensity of the diffraction peaks gradually increases with the increase of temperature, revealing that the particle size increases and crystallinity increases with temperature. Moreover, no diffraction peaks for other phases are found in the XRD patterns.
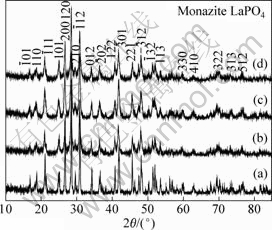
Fig.2 XRD patterns of as-prepared LaPO4 samples synthesized at different temperatures: (a) 1 000 ℃; (b) 900 ℃; (c) 800 ℃; (d) 700 ℃
Fig.3 shows the XRD patterns of the as-synthesized EuxLa1-xPO4 (x=0-0.1) with varying Eu3+ doping concentration compared with the LaPO4 sample. It is also found that all the diffraction peaks can be readily indexed into pure-phase LaPO4. Apparently, after doping with Eu3+, the obtained samples remain a single phase, which indicates that Eu3+ can be effectively doped into the LaPO4 lattice. And from the diffraction peaks of the main planes, we can also clearly see that the 5% Eu3+ doped sample has the best crystallinity.
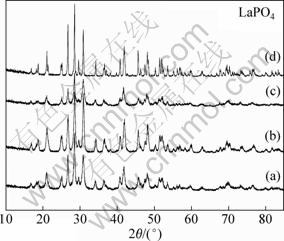
Fig.3 XRD patterns of LaPO4-based nanoparticles after substituting La3+ by various contents of Eu3+: (a) Eu0.01La0.99PO4; (b) Eu0.05La0.95PO4; (c) Eu0.10La0.90PO4; (d) LaPO4
3.3 TEM analysis
Fig.4 shows the TEM and HRTEM images of uniform LaPO4 and Eu0.05La0.95PO4 nanoparticles. It can be found from Fig.4(a) that the average length of Eu0.05La0.95PO4 nanorods is around 60 nm. Fig.4(b) demonstrates the pure LaPO4 nanorods sample is about 50 nm in length. From the HRTEM image of the sample shown in Fig.4(c), it can be seen that the as-synthesized Eu0.05La0.95PO4 primary nanoparticles are well crystallized and ordered in crystallography.
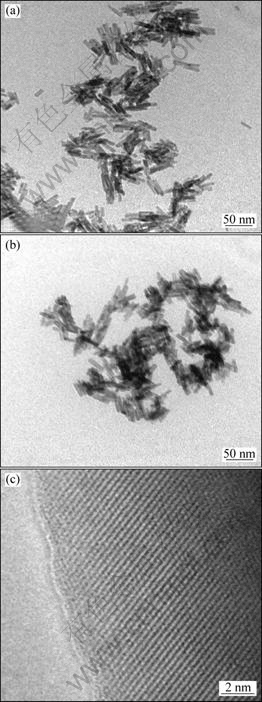
Fig.4 TEM images of as-prepared Eu0.05La0.95PO4 (a), LaPO4 (b) and HRTEM image of Eu0.05La0.95PO4 (c)
3.4 Photoluminescence characterization
The excitation and emission spectra of the nanoscale Eu0.05La0.95PO4 by sol-gel method are presented in Fig.5. Excitation spectrum of the sample is demonstrated in the left part of Fig.5. The broad band centered at 260 nm is attributed to the charge-transfer band (CTB) between Eu3+ and the surrounding oxygen anions[21-23]. There exist five emitting bands which can be seen in the right part of Fig.5. The emission spectrum of the Eu0.05La0.95PO4 mainly locates in the orange-red spectral area (from 550 to 720 nm). The emission located at 540-560 nm can be attributed to the 5D0 -7F0 transition, while emissions at 585-600 nm, 610-625 nm, 650-660 nm and 680-710 nm correspond to the 5D0 -7F1 transition, 5D0 -7F2 transition, 5D0 -7F3 transition and 5D0 -7F4 transition, respectively[21-23]. The splitting and intensity pattern of the emission lines demonstrate that the europium has been successfully doped in the sample[24]. The orange-red emission lines at around 595 nm originating from the magnetic dipole transition 5D0 -7F1 are the dominant bands for the as-synthesized Eu0.05La0.95PO4 nanocrystals.
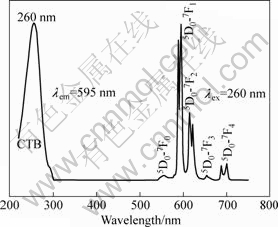
Fig.5 Excitation and emission spectra of Eu0.05La0.95PO4 nanophosphors obtained at 800 ℃
Physical techniques such as laser ablation have been used to elaborate thin films of Y2O3?Eu and YVO4?Eu phosphors, but they are often difficult to control for complex oxide matrices or doping compositions. Sol-gel routes at relatively high temperature (800-1 000 ℃) have also been largely developed, leading to polycrystalline thin oxide films. The sol-gel process enables a fine control of chemical and physical properties of the matrix in which the nanophosphors are dispersed, and is thus an easy way to obtain transparent and luminescent thin films by spin or dip-coating[25]. Because of these properties, the photoluminescence of nanophosphors synthesized by sol-gel method could be upgraded lagerly though the result is not shown here.
Emission spectra for comparison of different Eu3+ doping contents are shown in Fig.6. We can easily conclude that the optimized content of Eu3+ is found to be around 5%. The PL intensity is highly depended on activator content. Generally speaking, the brightness tends to increase with increasing activator content. However, the luminescence begins to decrease because pairing or aggregation of activator atoms at high content leads to efficient resonant energy transfer between Eu3+ ions and a fraction of energy migration to distant killers or quenchers followed by the appearance of quenching behavior[20, 26]. The concentration quenching behavior of the samples can be evidently observed in Fig.6.
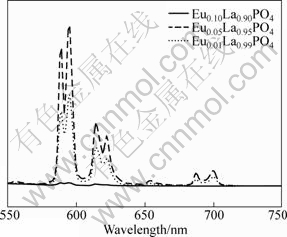
Fig.6 Evolution of emission spectra of EuxLa1.0-xPO4 nanophosphors with varying Eu3+ doping content
Fig.7 shows the emission spectra of the samples prepared by sol-gel method at different temperatures. It is clear that higher calcination temperature is favorable for brightness of the photoluminescence. The fluorescent materials would quench because of the presence of impure NO3- and OH- ions[27], and high temperature can effectively reduce these quenching centers. There is also another reason that the crystal structure of the sample obtained at higher temperature is reasonably ordered. However, the emission intensity becomes saturated when the temperature is raised above the critical point (1 000 ℃) in these samples.
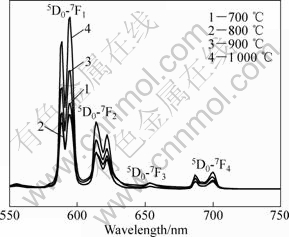
Fig.7 Emission spectra of Eu0.05La0.95PO4 nanophosphors synthesized at different temperatures
4 Conclusions
1) LaPO4:Eu phosphors are successfully synthesized through sol-gel method at moderate temperature (800 ℃).
2) The prepared LaPO4 crystallizes in a single-phase, monoclinic structure with a rod shape, approximately 50 nm in length. A high fraction of europium ions could be effectively doped into the crystal lattice of the monoclinic LaPO4 through the same method.
3) The PL characterization demonstrates that the Eu0.05La0.95PO4 nanophosphor shows the most intense emission, and the higher temperature tends to improve the emission intensity. The sol-gel process can upgrade the photoluminescence properties lagerly. Therefore, the LaPO4?Eu phosphors can be easily applied in various types of lamp and display due to its morphologies and good PL performance. In this regard, our target product is a very promising phosphor. Moreover, the synthesis methods are simple with low-cost, and also be readily extended to other rare earth phosphates.
References
[1] LETANT S E, VAN BUUREN T W, TERMINELLO L J. Nanochannel arrays on silicon platforms by electrochemistry [J]. Nano Letters, 2004, 4(9): 1705-1707.
[2] WANG Xun, ZHUANG Jing, PENG Qing, LI Ya-dong. A general strategy for nanocrystal synthesis [J]. Nature, 2005, 437(7055): 121-124.
[3] GAO Pu-xian, DING Yong, MAI Wen-jie, HUGHES W L, LAO Chang-shi, WANG Zhong-lin. Conversion of zinc oxide nanobelts into superlattice-structured nanohelices [J]. Science, 2005, 309(5741): 1700-1704.
[4] BUDDHUDU S, KAM C H, NG S L, LAM Y L, OOI B S, ZHOU Y, WONG K S, RAMBABU U. Green color luminescence in Tb3+: (La, Ln)PO4 (Ln=Gd or Y) photonic materials [J]. Materials Science and Engineering B, 2000, 72(1): 27-30.
[5] COLOMER M T, GALLINI S, JURADO J R. Synthesis and characterisation of a green NiO/La(Sr)PO4-delta cermet anode for phosphate based solid oxide fuel cells [J]. Journal of the European Ceramic Society, 2007, 27(13/15): 4237-4240.
[6] NEDELEC J M, MANSUY C, MAHIOU R. Sol-gel derived YPO4 and LuPO4 phosphors, a spectroscopic study [J]. Journal of Molecular Structure, 2003, 651: 165-170.
[7] RAJESH K, SHAJESH P, SEIDEL O, MUKUNDAN P, WARRIER K G K. A facile sol-gel strategy for the synthesis of rod-shaped, nanocrystalline, high-surface- area lanthanum phosphate powders and nanocoatings [J]. Advanced Functional Materials, 2007, 17(10): 1682-1690.
[8] GALLINI S, JURADO J R, COLOMER M T. Synthesis and characterization of monazite-type Sr: LaPO4 prepared through coprecipitation [J]. Journal of the European Ceramic Society, 2005, 25(12): 2003-2007.
[9] GUO Yan, WOZNICKI P, BARKATT A. Sol-gel synthesis of microcrystalline rare earth orthophosphates [J]. Journal of Materials Research, 1996, 11(3): 639-649.
[10] FELDMANN C. Polyol-mediated synthesis of nanoscale functional materials [J]. Advanced Functional Materials, 2003, 13(2): 101-107.
[11] BROWN S S, IM H J, RONDINONE A J, DAI S. Facile, alternative synthesis of lanthanum phosphate nanocrystals by ultrasonication [J]. Journal of Colloid and Interface Science, 2005, 292(1): 127-132.
[12] FUJISHIRO Y, ITO H, SATO T, OKUWAKI A. Synthesis of monodispersed LaPO4 particles using the hydrothermal reaction of an La(edta)- chelate precursor and phosphate ions [J]. Journal of Alloys and Compounds, 1997, 252(1): 103-109.
[13] YOSHIMURA M, SUDA H, OKAMOTO K, IOKU K. Hydrothermal synthesis of needle-like apatite crystal [J]. Nihon Kagaaku Kaishi, 1991, 10: 1402-1407.
[14] FERHI M, HORCHANI-NAIFER K, FERID M. Hydrothermal synthesis and photoluminescence of the monophosphate LaPO4?Eu(5%) [J]. Journal of Luminescence, 2008, 128(11): 1777-1782.
[15] DIAZ-GUILLEN J A, FUENTES A F, GALLINI S, COLOMER M T. A rapid method to obtain nanometric particles of rhabdophane LaPO4?nH2O center dot nH2O by mechanical milling [J]. Journal of Alloys and Compounds, 2007, 427: 87-93.
[16] GIAUME D, BUISSETTE V, LAHLIL K, GACOIN T, BOILOT J P, CASANOVA D, BEAUREPAIKE E, SAUVIAT M P, ALEXANDROU A. Emission properties and applications of nanostructured luminescent oxide nanoparticles [J]. Progress in Solid State Chemistry, 2005, 33(2/4): 99-106.
[17] FANG Yue-ping, XU An-wu, SONG Rui-qi, ZHANG Hua-xin, YOU Li-ping, YU J C, LIU Hua-qin. Systematic synthesis and characterization of single-crystal lanthanide orthophosphate nanowires [J]. Journal of the American Chemical Society, 2003, 125(51): 16025-16034.
[18] CAO Min-hua, HU Chang-wen, WU Qing-yin, GUO Cai-xin, QI Yan-juan, WANG En-bo. Controlled synthesis of LaPO4 and CePO4 nanorods/nanowires [J]. Nanotechnology, 2005, 16(2): 282-286.
[19] WANG Ying, TANG Zhi-yong, NICHOLAS A K. Bioapplication of nanosemiconductors [J]. Materials Today, 2005, 8(5): 20-31.
[20] LI Wei, LEE Joonho. Microwave-assisted sol-gel synthesis and photoluminescence characterization of LaPO4?Eu3+, Li+ nanophosphors [J]. Journal of Physical Chemistry C, 2008, 112(31): 11679-11684.
[21] PATRA C R, ALEXANDRA G, PATRA S, JACOB D S, GEDANKEN A, LANDAU A, GOFER Y. Microwave approach for the synthesis of rhabdophane-type lanthanide orthophosphate(Ln=La, Ce, Nd, Sm, Eu, Gd and Tb) nanorods under solvothermal conditions [J]. New Journal of Chemistry, 2005, 29(5): 733-739.
[22] YI S S, BAE J S, MOON B K, JEONG J H, PARK J C, KIM I W. Enhanced luminescence of pulsed-laser-deposited Y2O3?Eu3+ thin-film phosphors by Li doping [J]. Applied Physics Letters, 2002, 81(18): 3344-3346.
[23] SCHMECHEL R, KENNEDY M, SEGGERN H V, WINKLER H, KOLBE M, FISCHER R A, LI X M, BENKER A, WINTERER M, HAHN H. Luminescence properties of nanocrystalline Y2O3?Eu3+ in different host materials [J]. Journal of Applied Physics, 2001, 89(3): 1679-1686.
[24] YAN Ruo-xue, SUN Xiao-ming, WANG Xun, PENG Qing, LI Ya-dong. Crystal structures, anisotropic growth, and optical properties: Controlled synthesis of lanthanide orthophosphate one-dimensional nanomaterials [J]. Chemistry A European Journal, 2005, 11(7): 2183-2195.
[25] BUISSETTE V, GIAUME D, GACOIN T, BOILOT J P. Aqueous routes to lanthanide-doped oxide nanophosphors [J]. Journal of Materials Chemistry, 2006, 6(16): 529-539.
[26] HUANG Hai, XU Guo-qin, CHIN Wee-shong, GAN Leong-ming, CHEW Chwee-har. Synthesis and characterization of Eu?Y2O3 nanoparticles [J]. Nanotechnology, 2002, 13(3): 318-323.
[27] HONG G Y, JEON B S, YOO Y K, YOO J S. Photoluminescence characteristics of spherical Y2O3?Eu phosphors by aerosol pyrolysis [J]. Journal of the Electrochemical Society, 2001, 148(11): H161-H166.
Foundation item: Project(50972166) supported by the National Natural Science Foundation of China; Project supported by the Scientific Research Foundation for the Returned Overseas Chinese Scholars, State Education Ministry, China
Corresponding author: LI Wei; Tel: +86-731-88830768; E-mail: csu973@163.com
DOI: 10.1016/S1003-6326(09)60158-8


