Trans. Nonferrous Met. Soc. China 25(2015) 1307-1314
Sequential removal of selenium and tellurium from copper anode slime with high nickel content
Dian-kun LU, Yong-feng CHANG, Hong-ying YANG, Feng XIE
School of Materials and Metallurgy, Northeastern University, Shenyang 110819, China
Received 28 May 2014; accepted 20 October 2014
Abstract: A process using soda roasting-alkaline leaching-acid leaching to remove selenium, tellurium and copper sequentially from the copper anode slime with high content of Ni was tested. The mechanism of this process was outlined based on thermodynamic analysis and the change in the XRD patterns of different intermediate products. During soda roasting, copper which occurs as Cu4SeTe in the slime was oxidized to CuO and Cu3TeO6, while selenium and tellurium were oxidized to Ag2SeO4 and Cu3TeO6, respectively. Ag2SeO4 in the calcine is easily leached in the subsequent alkaline leaching, but CuTeO3 resulted from the decomposition of Cu3TeO6 remains inactive in this process through which selenium is leached out in preference to tellurium. The CuTeO3 and CuO in the alkaline leaching residue can be leached in the following sulfuric acid leaching process. More than 97% of selenium was leached with little tellurium leached under the optimal condition. Then, more than 96% of copper and almost all the tellurium were leached out in the following acid leaching process.
Key words: copper anode slime; tellurium; selenium; soda roasting; alkaline leaching; acid leaching
1 Introduction
Selenium and tellurium play important roles in the emerging thin film solar energy technology which is expected to have a larger share in the world’s future energy portfolio [1,2]. They are primarily by-products of electrolytic copper refining [3,4]. Approximately 90% of selenium and tellurium are obtained from anode slimes formed during the electrolytic refining of copper [1,5]. Copper anode slime is also an important raw material for recovering Au, Ag and platinum group metals. A variety of technologies for treating copper anode slimes have been developed. However, since copper, selenium and tellurium are chemically more active than Au, Ag and platinum group metals, they always bring troubles for the recovery of those precious metals. So they are usually removed from anode slime before the recovery of precious metals. Different methods for removing seleuium, tellurium and copper from copper anode slime have been reported, either through pyrometallurgical or hydrometallurgical processes. Typical pyrometallurgical processes are mostly based on sulfate-roasting, oxidation-converting, alkaline-roasting or direct-roasting in air [6-10]. Hydrometallurgical processes are usually based on acid-leaching, autoclave-leaching, or chlorinating [l1-15]. These technologies are usually designed with the purpose to recover precious metals, especially gold and silver with little attention on selenium and tellurium recovery. According to a previous survey of 56 worldwide electrolytic copper refiners, 52 plants reported that selenium was found in their anode slimes, but only about 50% of the selenium was recovered on average [1]. The average recovery of tellurium is generally 70%-80% [16,17]. This is probably because selenium and tellurium tend to disperse into different process products and thus are difficult to recover.
According to the properties of selenium and tellurium, soda roasting-acid leaching process is a cost-effective approach for recovering selenium and tellurium from anode slimes. The disadvantage of direct roasting in air to the environment can be avoided when soda-roasting is used. This process has been reported and discussed in many research works [7,15,18,19]. Na2SeO4 and Na2TeO4 were believed to form during alkaline- roasting. Na2SeO4 is readily dissolved in alkaline solution, but Na2TeO4 is virtually insoluble. Thus, selenium can be separated from tellurium in the followed water leaching step. After water leaching, Na2TeO4 can be leached as H2TeO4 in acid solution. This process has been simply mentioned in some researches, but no supporting experimental data were available. In this work, the recoveries of selenium and telurium through soda-roasting from the copper anode slime with relatively high content of nickel were examined. The potential reaction mechanism of the recovering processes was also discussed.
2 Experimental
2.1 Materials
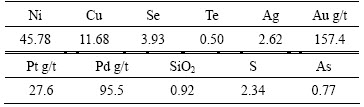
The wet copper anode slime sample (produced in a local copper-nickel refinery) was firstly dried at 80 °C and then ground to below 0.125 mm. Semi-quantitative analyses were carried out using X-ray diffraction fluorescence analysis (ZXS100e,Rigaku Corporation). Metals in the slime were determined by atomic absorption spectroscopy (AAS, Table 1). The result of the X-ray diffraction analysis (PW/3040/60,Netherlands PANalytical B.V company) is shown in Fig. 1. Nickel presents primarily as NiO and a part of copper as copper alkali sulfate and arsenate. Selenium and tellurium mainly occur as silver and copper selenides and tellurides, respectively. Reagents used in this research are all of analytical grade.
Table 1 Composition of copper anode slime (mass fraction, %)
2.2 Procedure
The soda-roasting tests were carried out with a small scale of sample (5 g) in a corundum crucible. A horizontal tubular electric furnace equipped with programmable temperature controller was used to roast fully mixed copper anode slime and soda. The roasted product then went through alkaline-leaching to remove selenium. The alkaline-leaching residue was dried in a drying cabinet with forced convection and then leached in the sulfuric acid solution. Both leach liquor and the residue were sent for elemental analysis. All alkaline leaching and acid leaching tests were conducted in a magnetic stirring apparatus equipped with a thermostatic water bath. Double distilled water was used throughout the whole tests.
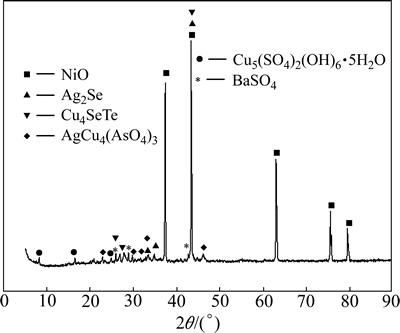
Fig. 1 XRD pattern of copper anode slime
3 Results and discussion
3.1 Preliminary tests
In the preliminary test, 5 g anode slime was mixed with 0.5 g Na2CO3 and then baked at 600 °C for 2.5 h in each batch. The calcination was then leached with 25 mL 100 g/L NaOH solution at 75 °C for 2.5 h. The alkaline leaching residue was water-washed and then leached with 100 mL of 72 g/L H2SO4 solution at 80 °C for 2 h. The test result is summarized in Table 2. It shows that the majority of selenium was leached from the calcination with little other elements leached during alkaline leaching. This produced a relative clean selenium pregnant solution which was convenient for the subsequent recovery of selenium and the recycling of the alkaline solution. The majority of tellurium and copper were leached from alkaline-leaching residue while little other elements were leached during acid-leaching. The result showed that selenium, tellurium and copper may be selectively recovered from these two kinds of solution and thus it is worthy of optimizing the process for better separating these metals in the slime.
Table 2 Preliminary test results of soda roasting-alkaline leaching-acid leaching process

3.2 Process optimization
3.2.1 Soda roasting
To examine the effect of roasting time on selenium removal, 5 g anode slime was mixed with 0.4 g Na2CO3 and baked at 600 °C for different time. The obtained calcine was then leached with 25 mL 100 g/L NaOH solution at 75 °C for 2.5 h. The results are shown in Fig. 2. It shows that the leaching recovery of selenium decreases with an increase of roasting time (see Fig. 2). This is probably because more SeO2 is volatilized for a prolonged roasting time. When the roasting time is 0.5 h, a selenium recovery of 97.5% is obtained.
To examine the effect of Na2CO3 dosage on selenium removal, 5 g anode slime was mixed with different amounts of Na2CO3 and baked at 600 °C for 0.5 h in each batch. The results of the effect of Na2CO3 dosage on selenium recovery are shown in Fig. 3. It shows that selenium recovery increases with an increase of Na2CO3 addition. When Na2CO3 dosage reaches 0.5 g, the recovery of selenium reaches 97.6%. So, 0.5 g Na2CO3 is the enough dosage for 5 g anode slime.
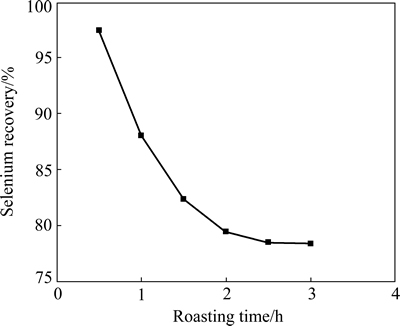
Fig. 2 Effect of roasting time on selenium recovery
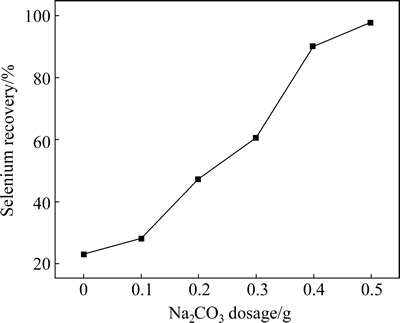
Fig. 3 Effect of Na2CO3 dosage on selenium recovery
To examine the roasting temperature on selenium removal, 5 g anode slime was mixed with 0.5 g Na2CO3 and baked at different temperatures for 0.5 h in each batch. It shows that selenium recovery increases with an increase of temperature below 500 °C, but drops slowly with further increasing temperature (Fig. 4). This is probably due to the volatilization of SeO2 at high temperature. So, 500 °C is the optimal temperature for soda-roasting, and the recovery of selenium reaches 98% at this temperature.
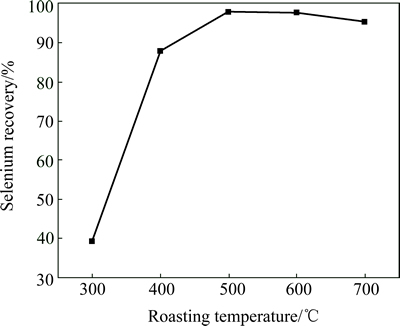
Fig. 4 Effect of roasting temperature on Se recovery
3.2.2 Alkaline leaching
According to the results of roasting tests, the optimal roasting condition is as follows: 0.5 g Na2CO3 is needed for 5 g anode slime, temperature is at 500 °C, roast time is 0.5 h. Under these conditions, a batch of 25 g anode slime was firstly roasted. The calcine was then divided into 5 equal parts for alkaline leaching test. The calcine was leached with 25 mL 100 g/L NaOH solution at 75 °C for different time. It shows that the selenium extraction is nearly unchanged when the alkaline leaching time increases from 0.5 h to 2.5 h. (Fig. 5). Leaching time of 1 h is enough for alkaline leaching and about 97.4% of selenium can be leached.
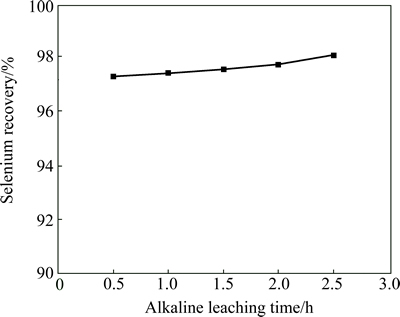
Fig. 5 Effect of alkaline leaching time on selenium recovery
The calcine samples were then leached with different concentrations of NaOH at 75 °C for 1 h. It was found that when NaOH concentration increased from 0 to 20 g/L, the selenium recovery increased from 78.1% to 95.4%. Then, the selenium recovery increased very slowly with an increase of NaOH concentration when it is above 20 g/L. When NaOH is 80 g/L, the selenium recovery only increases to 96.80% (Fig. 6). Thus, 20 g/L NaOH is the appropriate NaOH concentration for alkaline leaching.
The calcine sample was also leached with 25 mL 20 g/L NaOH solution at different temperatures for 1 h. When the alkaline leaching temperature increases from 20 °C to 80 °C, the selenium recovery increases gradually from 92.1% to 97.4% (Fig. 7). Thus, 80 °C is selected as the optimal temperature for alkaline leaching. Under this condition, selenium leaching recovery reaches up to 97.4%.
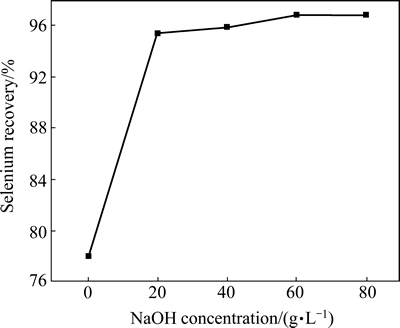
Fig. 6 Effect of NaOH concentration on selenium recovery
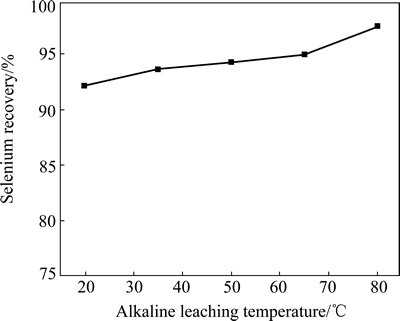
Fig. 7 Effect of alkaline leaching temperature on selenium recovery
3.2.3 Acid leaching
In this group of tests, all samples used for acid leaching are the residues of soda roasting-alkaline leaching tests. The main content of the sample is shown in Table 3.
Table 3 Main composition of soda roasting–alkaline leaching residue (mass fraction, %)
To examine the effect of H2SO4 concentration on Te and Cu leaching, 4 g alkaline leaching residue was leached with 100 mL sulfuric acid solution with different concentrations at 80 °C for 2 h. It shows that the recovery of tellurium increases with an increase of sulfuric acid concentration. The recovery of copper almost remains constant. When the sulfuric acid concentration is 90 g/L, more than 97% of tellurium and 97.6% copper were leached (Fig. 8). The test results of the effect of leaching temperature on Te and Cu leaching show that 70 °C is enough for obtaining high recoveries of tellurium and copper ( 97.2% and 96.9%, respectively, Fig. 9). The effect of the acid leaching time indicates that 1 h is enough to ensure high recoveries of tellurium and copper (99.9% and 96.4%, respectively, Fig. 10).
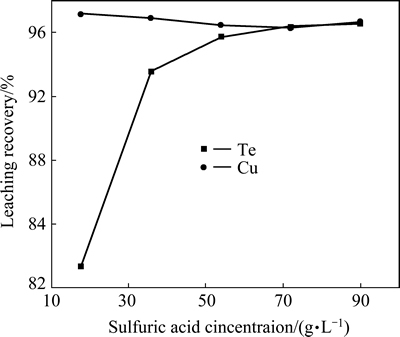
Fig. 8 Effect of H2SO4 concentration on recoveries of copper and tellurium
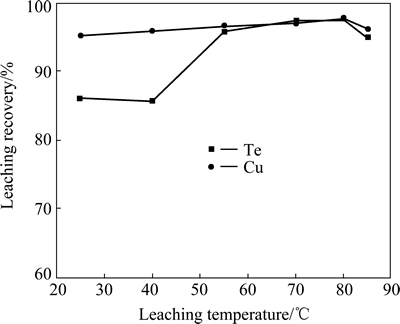
Fig. 9 Effect of acid leaching temperature on recoveries of copper and tellurium
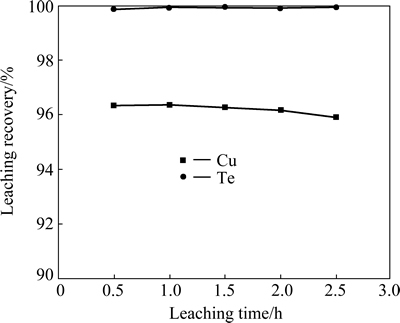
Fig. 10 Effect of acid leaching time on recoveries of copper and tellurium
3.3 Potential reaction mechanism
In early reports, the reactions taking place during soda roasting are simply described by Eqs. (1) and (2):
MeSe+Na2CO3+1.5O2=Na2SeO4+MeO+CO2↑ (1)
MeTe+Na2CO3+1.5O2=Na2TeO4+MeO+CO2↑ (2)
Na2SeO4 in the calcine can be dissolved in the followed water leaching process, but Na2TeO4 can not be leached in alkaline solution. Na2TeO4 can be leached in the followed sulfuric acid leaching process [7]. However, some research works reported that both Na2SeO3 and Na2SeO4 formed during soda roasting [19]. According to the composition of the anode slime sample used in this research, possible reactions for silver and copper selenides and tellurides can be described as follows.
Ag2Se+Na2CO3+O2=Na2SeO3+2Ag+CO2↑ (3)
Ag2Se+Na2CO3+1.5O2=Na2SeO4+2Ag+CO2↑ (4)
Ag2Te+Na2CO3+1.5O2=Na2TeO4+2Ag+CO2↑ (5)
Cu2Se+Na2CO3+2O2=Na2SeO3+2CuO+CO2↑ (6)
Cu2Se+Na2CO3+2.5O2=Na2SeO4+2CuO+CO2↑ (7)
Cu2Te+Na2CO3+2.5O2=Na2TeO4+2CuO+CO2↑ (8)
A simplified calculation on the change of Gibbs free energy for these reactions is shown in Fig. 11 [20]. It indicates that both Ag2Se and Cu2Se can be oxidized to either Na2SeO3 or Na2SeO4 below 700 °C. When the roasting temperature is below 450 °C, Na2SeO4 is produced in preference to Na2SeO3. On the other hand, Na2SeO3 is produced in preference to Na2SeO4 beyond 450 °C. Compared with selenides, tellurides are easier to be oxidized to high valence state.
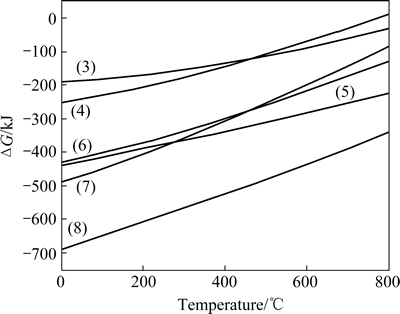
Fig. 11 Effect of temperature on Gibbs free energy change
The thermodynamic analysis above is in agreement with those experimental data which show that copper and tellurium in the anode slime have been leached by sulfuric acid before soda roasting. The selenides and tellurides may be decomposed to some extent before soda roasting. Due to the presence of a large quantity of nickel in the anode slime, the possible reactions taking place during soda roasting, alkaline leaching and acid leaching processes may be different from those reported in literatures. In order to identify the potential difference, 10 g anode slime was roasted and then went through alkaline and acid leaching, under the optimal conditions above. All the residues from roasting, alkaline leaching and acid leaching were sent to XRD analysis. The results are shown in Figs. 12, 13 and 14, respectively.
By comparing Fig. 12 with Fig. 1, it can be found that Ag2Se and Cu4SeTe are converted into selenate and tellurate during soda roasting, i.e., Ag2SeO4 and Cu3TeO6. AgCu4(AsO4)3 is turned into Ni4.35(AsO4)3 and BaSO4 is reduced to Bax(SO3)z. Cu5(SO4)2(OH)6·5H2O are turned into CuO and Na2SO4 after soda roasting. Because AgO is not stable and may be decomposed into Ag and O2 under the roasting conditions [21]. The released O2 from the decomposition of AgCu4(AsO4)3 and BaSO4 may help the formation of Ag2SeO4 and Cu3TeO6.
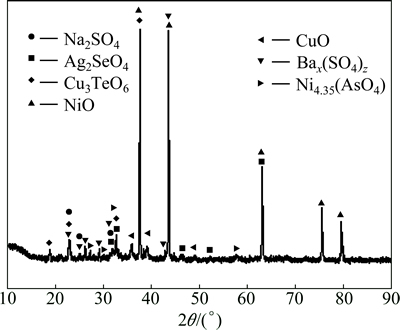
Fig. 12 XRD analysis of calcine alkaline
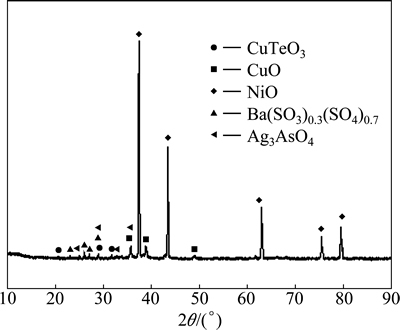
Fig. 13 XRD analysis of alkaline leaching residue
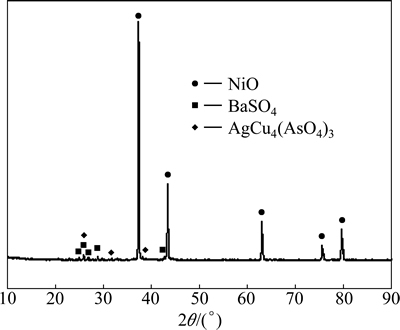
Fig. 14 XRD analysis of acid leaching residue
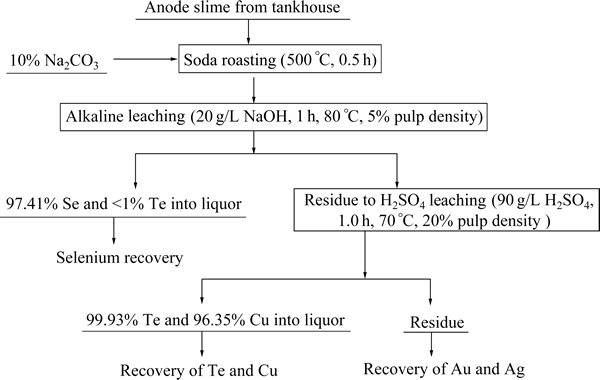
Fig. 15 Simplified flowsheet for sequential separating selenium and tellurium from copper anode slime
Because  is the most stable species in alkaline leaching conditions, Ag2SeO4 will be reduced into Na2SeO3 during alkaline leaching. The presence of CuTeO3 in the alkaline leaching residue (Fig. 13) indicates that Cu3TeO6 is also reduced into CuTeO3 during alkaline leaching. Because Na2SeO3 is easily dissolved during alkaline leaching while CuTeO3 could not be leached, the majority of selenium is selectively leached by alkaline leaching process while the majority of tellurium remains in the residue. This is verified by the XRD patterns of the calcination and the alkaline leaching residue, in which Ag2SeO4 in Fig. 12 disappears in Fig. 13. Apart from these changes, Bax(SO3)z is partly oxidized to Ba(SO3)0.3(SO4)0.7, and Cu3TeO6 and Ag2SeO4 may function as oxidants. At the same time, Ag3AsO4 is found in alkaline leaching residue which indicates that Ni4.35(AsO4)3 decomposes during the alkaline leaching. The needed AgO to form Ag3AsO4 may come from the decomposition of Ag2SeO4. In the following acid leaching process, virtually all CuTeO3 and CuO are dissolved in sulfuric acid solution. The presence of Cu2+ turns Ag3AsO4 into AgCu4(AsO4)3. Partial AgO in Ag3AsO4 is released into sulfuric acid solution which may decompose into Ag and O2. The released O2 again oxidized Ba(SO3)0.3(SO4)0.7 back into BaSO4 (Fig. 14). Nickel occurs as NiO through the whole process (Figs. 1, 12, 13 and 14). According to above XRD analysis, the reactions taking place during soda roasting, alkaline leaching and sulfuric acid leaching can be prospected as following:
is the most stable species in alkaline leaching conditions, Ag2SeO4 will be reduced into Na2SeO3 during alkaline leaching. The presence of CuTeO3 in the alkaline leaching residue (Fig. 13) indicates that Cu3TeO6 is also reduced into CuTeO3 during alkaline leaching. Because Na2SeO3 is easily dissolved during alkaline leaching while CuTeO3 could not be leached, the majority of selenium is selectively leached by alkaline leaching process while the majority of tellurium remains in the residue. This is verified by the XRD patterns of the calcination and the alkaline leaching residue, in which Ag2SeO4 in Fig. 12 disappears in Fig. 13. Apart from these changes, Bax(SO3)z is partly oxidized to Ba(SO3)0.3(SO4)0.7, and Cu3TeO6 and Ag2SeO4 may function as oxidants. At the same time, Ag3AsO4 is found in alkaline leaching residue which indicates that Ni4.35(AsO4)3 decomposes during the alkaline leaching. The needed AgO to form Ag3AsO4 may come from the decomposition of Ag2SeO4. In the following acid leaching process, virtually all CuTeO3 and CuO are dissolved in sulfuric acid solution. The presence of Cu2+ turns Ag3AsO4 into AgCu4(AsO4)3. Partial AgO in Ag3AsO4 is released into sulfuric acid solution which may decompose into Ag and O2. The released O2 again oxidized Ba(SO3)0.3(SO4)0.7 back into BaSO4 (Fig. 14). Nickel occurs as NiO through the whole process (Figs. 1, 12, 13 and 14). According to above XRD analysis, the reactions taking place during soda roasting, alkaline leaching and sulfuric acid leaching can be prospected as following:
Roasting:
Ag2Se+2O2=Ag2SeO4 (9)
2Cu4SeTe+11O2+4Ag=2Ag2SeO4+2Cu3TeO6+2CuO (10)
AgCu4(AsO4)3+4.35NiO=Ni4.35(AsO4)3+4CuO+Ag+0.175O2 (11)
Cu5(SO4)2(OH)6·5H2O+2Na2CO3=2Na2SO4+5CuO+5H2O+2CO2 (12)
xBaSO4=Bax(SO3)z+(x-0.5z)O2+(x-z)SO2 (13)
Alkaline leaching:
2Ag2SeO4+4NaOH=2Ag2O+2Na2SeO3+2H2O+0.5O2 (14)
2Cu3TeO6=2CuTeO3+4CuO+O2 (15)
Ni4.35(AsO4)3+4.5Ag2O=3Ag3AsO4+4.35NiO+0.075O2 (16)
Bax(SO3)z+0.35zO2=xBa(SO3)0.3z/x(SO4)0.7z/x (17)
Sulfuric acid leaching:
2CuTeO3+2H2SO4=2CuSO4+2H2TeO3 (18)
CuO+H2SO4=CuSO4+H2O (19)
3Ag3(AsO4)3+4CuSO4=AgCu4(AsO4)3+4Ag2SO4 (20)
Ba(SO3)0.3(SO4)0.7+0.15H2O+0.15Ag2SO4=BaSO4+0.3Ag+0.15H2SO4(21)
On the basis of the experimental work in this research, a simplified flowsheet diagram for sequentially separating selenium and tellurium from copper anode slime with high content of nickel is outlined as Fig. 15. In this process, selenium, tellurium and copper can be first sequentially separated from copper anode slime and then the residue can be sent to recover precious metals through either pyrometallurgy or hydrometallurgy processes.
4 Conclusions
1) The optimal roasting-alkaline leaching conditions are as follows: Na2CO3 dosage is 10% of anode slime, roasting time is 0.5 h and roasting temperature is 500 °C; the alkaline leaching time is 1.0 h, NaOH concentration is 20 g/L and the alkaline leaching temperature is 80 °C. Under this condition, 97.4% of selenium can be recovered.
2) The optimal acid leaching conditions are as follows: H2SO4 concentration is 90 g/L, acid leaching temperature is 70 °C, leaching time is 1 h. The leaching efficiencies of copper and tellurium under this condition are 96.4% and 99.9%, respectively.
3) It is believed that during soda roasting, Ag2Se, Cu2Se and Cu2Te are converted into CuO, Ag2SeO4 and Cu3TeO6. Ag2SeO4 will be reduced to Na2SeO3 and easily dissolved in alkaline leaching process. At the same time, Cu3TeO6 is reduced to CuTeO3, which cannot be leached in alkaline solution. But CuTeO3 can be leached in acid solution. This difference leads to the feasibility to selectively separate Se and Te sequentially through the suggested process in this work.
References
[1] KAVLAK G, GRAEDEL T E. Global anthropogenic selenium cycles for 1940–2010 [J]. Resources, Conservation and Recycling, 2013, 73: 17-22.
[2] KAVLAK G, GRAEDEL T E. Global anthropogenic tellurium cycles for 1940–2010 [J]. Resources, Conservation and Recycling, 2013, 76: 21-26.
[3] HOFFMANN J E, KING M G. Kirk-othmer encyclopedia of
chemical technology [M]. New York: Wiley-Interscience, 2010: 1-36.
[4] GEORGE M W. Minerals yearbook-selenium and tellurium [M]. Washington: US Geological Survey, 2011: 651-657.
[5] Roskill Information Services. The economics of selenium [R]. London: Roskill Information Services Ltd., 1990.
[6] YASIN K, GULDEM K, SERVET T. An investigation of coppe rand selenium recovery from copper anode slimes [J]. International Journal of Mineral Processing, 2013, 124: 75-82.
[7] HOFFMANN J E. Recovering selenium and tellurium from copper refinery slimes [J]. Journal of the Minerals Metals Materials Society, 1989, 7: 33-38.
[8] SYED S. Recovery of gold from secondary sources—A review [J]. Hydrometallurgy, 2012, 115: 30-51.
[9] MORRISON B H. The evolution of copper refinery slime processing and precious metal treatment at CCR division-noranda minerals [M]// HARRIS B. Precious Metals. Boulder, Colorado: International Precious Metals Institution, 1989: 403-413.
[10] WANG Yong-lu. Progress of the metallurgical engineering technology of precious metals in China [J]. Precious Metals, 2011, 32: 59-71. (in Chinese)
[11] TOUGARINOFF B, van GOETSENNHOVEN F, DEWULF A. Recovery by nitric acid cycle of gold and platinum metals from the anode slimes arising from the electrolysis of dore metal [M]// HOLMES. Advances in Extractive Metallurgy. London: Institution of Mining and Metallurgy, 1968: 741-758.
[12] BIGUM M, BROGAARD L, THOMAS H C. Metal recovery from high-grade WEEE: A life cycle assessment [J]. Journal of Hazardous Materials, 2012, 207: 8-14.
[13] ZHENG Ya-jie, CHENKun-kun. Leaching kinetics of selenium from selenium-tellurium-rich materials in sodium sulfite solutions [J]. Transactions of Nonferrous Metals Society of China, 2014, 24(2): 536-543.
[14] XIE Hong-yan, WANG Ji-kun, LU Hui. Status on Recovery of telluium from copper anode slime [J]. Hydrometallurgy of China, 2010, 29: 143-146. (in Chinese).
[15] HYVZNEN O, ROSENBERG E, LINDROOS L. Selenium and precious metals recovery from copper anode slimes at outokumpu pori refinery [M]// KUDRY D A, CORRIGAN N, LIANG W W. Precious Metals. Warrendale, Pennsylvania: TMS-AIME, 1984: 537-548.
[16] BJORN M L, STIG R L. Anode slimes treatment: The boliden experience [J]. Journal of the Minerals Metals Materials Society, 2003, 4: 41-44.
[17] WANG Shi-jie. Tellurium, its resourcefulness and recovery [J]. Journal of the Minerals Metals Materials Society, 2011, 63: 90-93.
[18] FERNHDEZ M A, SEGARRA M, ESPIELL F. Selective leaching of arsenic and antimony contained in the anode slimes from copper refining [J]. Hydrometallurgy, 1996, 41: 255-267.
[19] HAIT J, JANA R K, SANYAL S K. Processing of copper electrorefining anode slime: A review [J]. Mineral Processing and Extractive Metallurgy, 2009, 118: 240-252.
[20] ZHONG Xian-lin. Study on the removal of selenium, tellurium and copper from copper anodic slime with high nickel content [D]. Shenyang: Northeastern University, 2013: 27-29. (in Chinese).
[21] DUTTON W A, van DEN STEEN A J, THEMELIS N J. Recovery of selenium from copper anode slimes [J]. Metallurgical Transactions B, 1971, 2: 3091-3097.
高镍铜阳极泥中硒和碲的依次脱除
路殿坤,畅永锋,杨红英,谢 锋
东北大学 材料与冶金学院,沈阳 110819
摘 要:
对苏打焙烧-碱浸-酸浸从高镍铜阳极泥中依次脱除硒和碲的工艺进行试验研究。通过热力学分析结合各工序中间产物的XRD图谱变化推断整个过程的反应机理。在苏打焙烧过程中,铜阳极泥中以Cu4SeTe 形式存在的铜被氧化成CuO 和Cu3TeO6,而硒和碲则分别转化为Ag2SeO4 和Cu3TeO6。在焙砂碱浸过程中,Ag2SeO4 容易溶解浸出, 但Cu3TeO6 转化为CuTeO3仍然难以浸出,因此在焙烧-碱浸过程硒优先于碲被浸出。残留在碱浸渣中的CuTeO3和CuO很容易在接下来的酸浸过程中浸出。试验研究结果显示,在最佳的苏打焙烧-碱浸过程中,超过97%的硒被浸出,而碲几乎不浸出,从而实现了硒与碲的分离。在随后的酸浸过程中,超过96% 的铜和几乎所有的碲被浸出进入酸浸液中。
关键词:
(Edited by Yun-bin HE)
Foundation item: Project (2012BAE06B05) supported by the National Science and Technology Support Plan of China
Corresponding author: Dian-kun LU; Tel: +86-24-83687729; E-mail: ludk@smm.neu.edu.cn
DOI: 10.1016/S1003-6326(15)63729-3
摘 要:对苏打焙烧-碱浸-酸浸从高镍铜阳极泥中依次脱除硒和碲的工艺进行试验研究。通过热力学分析结合各工序中间产物的XRD图谱变化推断整个过程的反应机理。在苏打焙烧过程中,铜阳极泥中以Cu4SeTe 形式存在的铜被氧化成CuO 和Cu3TeO6,而硒和碲则分别转化为Ag2SeO4 和Cu3TeO6。在焙砂碱浸过程中,Ag2SeO4 容易溶解浸出, 但Cu3TeO6 转化为CuTeO3仍然难以浸出,因此在焙烧-碱浸过程硒优先于碲被浸出。残留在碱浸渣中的CuTeO3和CuO很容易在接下来的酸浸过程中浸出。试验研究结果显示,在最佳的苏打焙烧-碱浸过程中,超过97%的硒被浸出,而碲几乎不浸出,从而实现了硒与碲的分离。在随后的酸浸过程中,超过96% 的铜和几乎所有的碲被浸出进入酸浸液中。


