J. Cent. South Univ. Technol. (2007)02-0206-04
DOI: 10.1007/s11771-007-0041-0 ![]()
Effect of sintering atmosphere on microstructure and
properties of TiC based cermets
ZHOU Shu-zhu(周书助)1,2, WANG She-quan(王社权)2, WANG Lin-sen(王零森)2, DING Ze-liang(丁泽良)1
(1. Department of Metallurgy, Hunan University of Industry, Zhuzhou 412000, China;
2. State Key Laboratory of Powder Metallurgy, Central South University, Changsha 410083, China)
Abstract:
The effect of the sintering atmospheres (vacuum, N2, Ar) on the microstructures and properties of the TiC based cermets was studied using XRD, SEM/BSE and energy dispersive spectrometer. Compared with the alloy sintered in vacuum, the carbon content of the specimen sintered in N2 and Ar is lower by 0.5%; and the nitrogen content is higher by 0.3% when sintered in nitrogen. The central part of the ring structure may be carbide with either a high W or Ti content. The ring structures are (Ti, W, Ta, Mo, Co, Ni)C solid solutions with different metallic elements and distributions. The composition of the binder phase is (Co, Ni) solid solution with different Ti, W, Ta, Mo, C contents. The structures are uniform for the cermets sintered in vacuum and the properties are the best. When sintered in Ar or N2, the O2 and N2 in the atmosphere take part in the sintering reaction to break the carbon balance in the cermets to form a shell structure and defects, which results in poor density, microhardness (HV) and transverse rupture strength (TRS).
Key words:
TiC based cermets; sintering atmosphere; microstructure; mechanical properties ;
1 Introduction
TiC base cermets are considered as excellent cutting tool materials for high speed finish machining and semi-finishing machining of steels due to their superior anti-oxidation, anti-crater-abrasion and high temperature properties. Because TiC is cheaper and easy to gain, TiC base cermets have been studied extensively, especially in those developed countries whose tungsten resources are poor[1-5]. TiC base cermets have more complex composition and microstructure than common cemented carbides. In general, most of the hard-phase grains in the microstructure possess a stable core/rim microstructure, the black cores are undissolved TiC, while the gray rims are cubic carbides (Ti, Mo, W)C solid solutions[6-12]. The microstructure and mechanical properties of the TiC base cermets may be improved by sintering in different atmospheres. But the researches in this aspect are rarely reported[13-16]. The effects of sintering atmospheres (vacuum, N2, Ar) on the microstructure and properties of the TiC based cermets were investigated in the present work.
2 Experimental
The specimens containing 40%TiC-10%Mo2C- 15%(Ti-W-Ta)C-15%WC-10%Ni-10%Co were prepared. The powder mixtures were ball milled in ethyl alcohol for 72-96 h and blended. The mass ratio of ball to material was 5?1. The bar specimens, 6.2 mm×8.0 mm×25 mm in size, were dry pressed and then sintered in vacuum (1 460 ℃, 1 h) and N2(1 460 ℃, 1 h, N2 pressure 8 kPa) in atmosphere furnace (COV533R, made in PVA, Germany) and gas pressure sintered in Ar(1 460 ℃, 1 h, Ar pressure was 6 kPa) in pressure sintering furnace (COD533R, made in PVA, Germany), respectively.
The bulk density was measured using the Archimedes principle. The surface was sand blasted for the TRS measurement and polished for Vickers indentation. The microstructures were observed using FE-SEM (Germany LEO type1525). The sintered bar specimens were smashed to powders (<175 μm) for chemical analysis and X-ray diffraction(in D8ADVANCE, made in Germany Bruker). The scanning speed was 0.5 (?)/s in the range of 20?-80?.
3 Results and discussion
3.1 Effect of sintering atmospheres on composition of cermets
The chemical compositions of the mixed powders before and after sintering in different atmospheres are listed in Table 1. Before sintering the oxygen content is relatively high and it decreases remarkably after sintering. Due to the trace amount of oxygen in N2 and Ar, the oxygen content of the specimen sintered in N2 and Ar is nearly twice of that sintered in vacuum.
Table 1 Compositions of specimen before and after sintering in different atmospheres (mass fraction, %)

The total carbon content decreases obviously after sintering. The carbon content for the specimen decreases by 1.59% after being sintered in vacuum, and by 2.35% in N2, and by 2.36% in Ar. The main reason for the decrease of carbon is the deoxidizing of oxygen from the mixed powder, a fraction of carbon takes part in the gas reactions during sintering. Comparing the decrease of the carbon and oxygen in the mixed powder, the chemically bond oxygen content in the powder mixtures is 1.5%- 2.0%, the remains is adsorbed oxygen which is desorbed during the heating process.
Due to the decrease of the total carbon and oxygen, the nitrogen content increases comparatively. Since N2 takes part in the sintering reactions, the corresponding nitrogen content in the specimen sintered in N2 increases by about 0.3%.
3.2 Effect of sintering atmosphere on microstructure of cermets
Since the back scattered electrons are very sensitive to the atomic number, the regions having higher atomic number in specimen will gather more back scattered electrons and the image will be brighter. The change of the compositions in microstructure can be investigated using back scattered electrons analysis. The SEM/BSE micrographs of the specimen sintered in three atmospheres are shown in Fig.1. The interior structures are almost the same for the specimens sintered in vacuum and Ar. The ring structure is formed by the dissolving and precipitation process in liquid phase sintering, the center of the ring structure is TiC or WC. It can be seen from the EDS spectra (Table 2) of spot A in Fig.1(b) that a layer of about 10 μm thickness, having higher carbon, oxygen, Ti and W content, lower Co and Ni content is formed and the ring structure disappears on the surface for the specimen sintered in Ar, indicating that the cobalt and nickel transfer from surface to inside. While for the specimen sintered in N2 which is not an inert gas for the sintering of TiC base cermets, N2 penetrates from surface to inside and takes part in reactions, which increases the total content of C and N, breaks the carbon balance in the cermets. A two phase structure is formed, in which the binder phase is mainly Ni-Co multiple solid solution and the hard phase is mainy (Ti, W, Ta, Mo, Co, Ni)(C, N) multiple solid solution. There is a strait band about 100 μm away from the surface, within which the compositions change and the grain size is smaller. It can be concluded from the contrast of SEM/BSE micrographs of the band and the EDS spectra (Table 2) of spot C in Fig.1(d) that the band mainly consists of TiCxN1-x hard phase.

Fig.1 SEM/BSE micrographs of specimen sintered in different atmospheres
(a) In vacuum; (b) In Ar; (c) and (d) In N2
Due to the higher gross of carbon of specimen, the saturation degree of the carbide of W, Mo, Ti, Ta etc transition metals may be high. The hard phase is equiaxial and the ring structure is not obvious or disappeared.
3.3 Effect of sintering atmospheres on phase composition of cermets
The main components in the powder mixture are TiC, Mo2C, (Ti-W-Ta)C, WC, Ni and Co. The results of XRD analysis for the powder mixture and the corresponding cermets sintering in different atmospheres are shown in Fig.2. There are mainly TiC phase and Ni phase in alloy. The binder phase is a Ni solid solution consisting of Co, Mo, W, Ti and Ta etc. Since the lattice constant of Mo, W, Ti and Ta is larger than that of Ni (0.355 nm), the lattice constant of the binder phase is larger than that of Ni. The hard phase is mainly TiC solid solution consisting of W, Ti, Ta, Mo, Co etc. The center of the ring structure may be carbides mainly consisting f heavy metal atoms W, Mo etc or other metal atoms dissolving TiC. Its phase composition is (Ti, W, Ta, Mo, Co, Ni)C solid solution with different Ti, W, Ta, Mo, Co, Ni etc contents and distribution. The binder phase is mainly (Co, Ni)(Ti, W, Ta, Mo, C) solid solution consisting of different solubilities of Ti, W, Ta, Mo, C etc. Since the sintering reactions are very complex, and the crystal structures and compositions of the phases possibly formed are similar or rare, their diffraction peaks might be overlapped or did not appear. It may be very difficult to determine accurately the phase compositions of the TiC base cermets only using XRD.

Fig.2 XRD spectra of cermet powders sintered in three atmospheres
3.4 Effect of sintering atmospheres on property of cermets
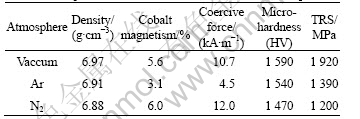
The cobalt magnetism, coercive force, density, microhardness and TRS of the cermets sintered in three different atmospheres are listed in Table 3. The maximum density, microhardness and TRS are obtained for the cermets sintered in vacuum. The density of cermets sintered in Ar atmosphere is not increased but decreased. Because of the lower density, higher porosity, the lower total carbon, and the coarsening of micrograin, the microhardness decreases. The shell structure on the surface of cermets makes the TRS decrease. N2 takes part in metallurgy reaction when sintering in N2, which increases the total content of carbon and nitrogen and breaks the carbon balance in cermets, so the disorder structure and surface defects appear. The mechanical properties of cermets sintered in N2 decrease more than that sintered in Ar. Investigating the total content of carbon and nitrogen of cermets in Table 2 and the cobalt magnetism and coercive force of corresponding cermets in Table 3, the changing tendency are the same. It can be seen from above investigations that the change of properties of the TiC base cermets is correspondent to the change of composition and microstructures.
Table 2 EDS results of measuring points in Fig.1 (mass fraction, %)
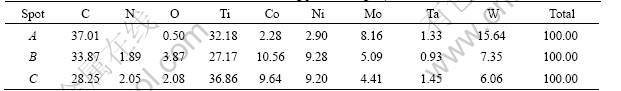
Table 3 Properties of cermets sintered in different atmospheres
4 Conclusions
1) The reduced amount of total carbon content in the TiC base cermets sintered in N2 is approximately equal to that sintered in Ar and it is 0.5% lower than that sintered in vacuum. The nitrogen content increases by about 0.3% after sintering in N2.
2) The gross of the carbon and nitrogen in TiC base cermets has a great effect on the form of the ring structure. The central part of the ring structure may be carbide with either a high W or Ti content. The ring structures are (Ti, W, Ta, Mo, Co, Ni)C solid solutions with different metallic elements and distributions. The compositions of the binder phase are (Co,Ni) solid solutions with different Ti, W, Ta, Mo, C contents.
3) The structure of TiC base cermets sintered in vacuum is relatively uniform and the properties are the best. For the specimens sintered in Ar or N2, the oxygen or nitrogen takes part in sintering reaction and breaks the carbon balance in cermets, resulting in a shell structure or surface defects in the surface layer, leading to a degradation of density, microhardness and TRS. N2 has a greater effect than Ar. The change of mechanical properties of TiC base cermets is correspondent to the change of its compositions and microstructures.
References[1] TATSUZAWA K, MATSUBARA H, KIHARA J, IWAMA K. Preparation of TiC-Ni cermets using composite powders[J]. Journal of the Japan Society of Powder and Powder Metallurgy, 1990, 37(7): 1009-1012.
[2] KINOSHITA S, UEKI M, SUZUKI H. Creep characteristics of TiC-Mo2C-Ni cermet sintered after nitrification in the course of raising temperature[J]. Journal of the Japan Society of Powder and Powder Metallurgy, 1993, 40(8): 820-822.
[3] TOKUMOTO K, SHINOAKI H, KITADA T, SAKAGUCHI S. Microstructures and mechanical properties of Ti-M-TiC(M:Cr,Mo,W) sintered hard alloy[J]. Journal of the Japan Society of Powder and Powder Metallurgy, 1994, 41(1): 27-32.
[4] SABATELLO S, FRAGE N, DARIEL M P. Graded TiC-based cermets[J]. Materials Science and Engineering A 2000, 288(1): 12-18.
[5] NOMURA N, YOSHIMI K, KONNO T, HANADA S. Fracture toughness improvement of TiC by Nb and Mo precipitates[J]. Journal of Materials Science Letters, 2000, 19(21): 1879-1881.
[6] MATSUBARA H, SHIN S G, SAKUMA T. Growth of carbide particles in TiC-Ni and TiC-Mo2C-Ni cermets during liquid phase sintering[J]. Materials Transactions JIM, 1991, 32(10): 951-956.
[7] CHUN D I, KIM D Y, EUN K Y. Microstructural evolution during the sintering of TiC-Mo-Ni cermets[J]. Journal of the American Ceramic Society, 1993, 76(8): 2049-2052.
[8] YAMAMOTO T, JAROENWORALUCK A, IKUHARA Y, SAKUMA T. Nanoprobe analysis of core-rim structure of carbides in TiC-20wt%Mo2C-20wt%Ni cermet[J]. Journal of Materials Research, 1999, 14(11): 4129-4131.
[9] ZARIPOV N G, KABIROV R R, BLOSHENKO V N. Structural peculiarities of cermets design based on titanium carbide[J]. Journal of Materials Science, 1996, 31(19): 5227-5230.
[10] CHOI K, CHOI J W, KIM D Y, HWANG N M. Effect of coalescence on the grain coarsening during liquid-phase sintering of TaC-TiC-Ni cermets[J]. Acta Materialia, 2000, 48(12): 3125-3129.
[11] LINDAHL P, ROLANDER U, ANDR?N H ?. Atom-probe analysis of the binder phase in TiC-TiN-Mo2C-(Ni,Co) cermets[J]. International Journal of Refractory Metals and Hard Materials, 1993-1994, 12(3): 115-119.
[12] ANDR?N H ?, ROLANDER U, LINDAHL P. Phase composition in cermeted carbides and cermets[J]. International Journal of Refractory Metals and Hard Materials, 1993-1994, 12(3): 107-113.
[13] KINOSHITA S, UEKI M, SUZUKI H. Cutting performance of titanium carbide based cermet nitrified during the course of sintering[J]. Journal of the Japan Society of Powder and Powder Metallurgy, 1994, 41(2): 152--155.
[14] ZACKRISSON J, ROLANDER U, WEINL G, ANDREN H O. Microstructure of the surface zone in a heat-treat cermet material[J]. International Journal of Refractory Metals and Hard Materials, 1998, 16: 315-322.
[15] ETTMAYER P, KOLASKA H, DREYER K. Effect of the sintering atmosphere on the properties of cermets[J]. Powder Metall Int, 1991, 23(4): 224-229.
[16] CAO We-bin, LI Jiang-tao, GE Chang-chun. Design and fabrication of TiC-based symmetrically compositional functional graded materials[J]. Materials and Design, 2001, 22(1): 45-51.
Foundation item: Project(2002AA331090) supported by the Hi-tech Research and Development Program of China; Project(06D073) supported by
Scientific Research Fund of Education Department of Hunan Province
Received date: 2006-05-21; Accepted date: 2006-07-27
Corresponding author: ZHOU Shu-zhu, Tel: +86-733-2889446; Fax: 86-733-2887888; E-mail: zhoushuzhu@126.com
(Edited by YUAN Sai-quan)
Abstract: The effect of the sintering atmospheres (vacuum, N2, Ar) on the microstructures and properties of the TiC based cermets was studied using XRD, SEM/BSE and energy dispersive spectrometer. Compared with the alloy sintered in vacuum, the carbon content of the specimen sintered in N2 and Ar is lower by 0.5%; and the nitrogen content is higher by 0.3% when sintered in nitrogen. The central part of the ring structure may be carbide with either a high W or Ti content. The ring structures are (Ti, W, Ta, Mo, Co, Ni)C solid solutions with different metallic elements and distributions. The composition of the binder phase is (Co, Ni) solid solution with different Ti, W, Ta, Mo, C contents. The structures are uniform for the cermets sintered in vacuum and the properties are the best. When sintered in Ar or N2, the O2 and N2 in the atmosphere take part in the sintering reaction to break the carbon balance in the cermets to form a shell structure and defects, which results in poor density, microhardness (HV) and transverse rupture strength (TRS).


