- Abstract:
- 1 Introduction▲
- 2 Experimental▲
- 3 Results and discussion▲
- 4 Conclusions▲
- References
- Figure
- Fig.1 XRD pattern of C3A
- Fig.2 SEM image of C3A
- Fig.3 SEM image of CaCO3
- Fig.4 FTIR spectra of different hydration ages of C3A-CaCO3- H2O system: (a) C3A-0; (b) C3A-15; (c) C3A-25
- Fig.5 DSC patterns of C3A-CaCO3-H2O system at various hydration ages: (a) C3A-0; (b) C3A-15
- Fig.6 SEM images of pure C3A hydrated for different hydration ages: (a) 3 d; (b) 7 d; (c) 28 d
- Fig.7 SEM images of C3A-25 hydrated for different hydration ages: (a) 3 d; (b) 7 d; (c) 28 d
J. Cent. South Univ. Technol. (2010) 17: 918-923
DOI: 10.1007/s11771-010-0577-2
Effect of CaCO3 on hydration characteristics of C3A
XIAO Jia(肖佳), GOU Cheng-fu(勾成福), JIN Yong-gang(金勇刚), WANG Yong-he(王永和)
School of Civil Engineering and Architecture, Central South University, Changsha 410075, China
? Central South University Press and Springer-Verlag Berlin Heidelberg 2010
Abstract:
Hydration products and morphology characteristics of C3A (tricalcium aluminate)-CaCO3-H2O system were studied by means of XRD, DSC, FTIR spectrum analysis and SEM. The results indicate that, the new phases, i.e., C3A·0.5CaCO3·0.5Ca(OH)2·
Key words:
C3A; CaCO3; hydration; morphology;
1 Introduction
A large amount of limestone powder and particles are generated when breaking limestone and producing machine-made sand. If these byproducts are discarded, they will waste the nature resource and pollute the environment. Besides slag and fly ash, limestone powder has already been utilized as an admixture in the cement and a mineral blend in concrete. It can accelerate the hydration of cement, enhance the early strength of cement and improve the rheology of fresh concrete, etc. [1-6]. Thus it provides potential advantages in terms of technology, economy and ecosystem. Researches in Refs.[7-12] show that limestone powder is hydration- active and gives carboaluminate hydration products when reacting with tricalcium aluminate (C3A). However, different viewpoints are held over when carboaluminate is generated and what category it belongs to of is. The effects of limestone powder or CaCO3 on cement and concrete were studied at home and abroad [13-24], whereas little research work on the hydration products of C3A and its morphology characteristics was carried out. Therefore, in order to better utilize limestone powder in the cement and concrete industry, it is meaningful to research the influence of ternary C3A-CaCO3-H2O system produced by limestone powder or CaCO3 on the properties of cement and concrete. By means of XRD, DSC, FTIR spectra analysis and SEM observation, the ternary C3A-CaCO3-H2O hydration products and their morphology characteristics were analysed, and the influence of CaCO3 on the hydration properties of C3A
was investigated.
2 Experimental
2.1 Materials
Tricalcium aluminate was synthesized at high temperature in laboratory by using analytically pure aluminium oxide and calcium carbonate. Fig.1 shows the XRD pattern of C3A, of which specific surface (nitrogen adsorption) is 740 m2/kg. Table 1 shows the particle size distribution of C3A. As shown in Fig.2, the morphology characteristic of C3A particles observed by SEM is irregular, without uniform edge angles.
2.1.2 CaCO3 (chemically pure reagent)
The specific surface (nitrogen adsorption) of CaCO3
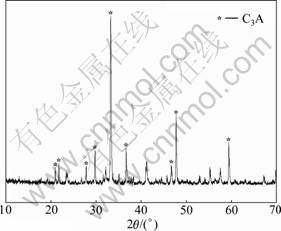
Fig.1 XRD pattern of C3A
is 4 580 m2/kg. Table 1 presents the particle size distribution of CaCO3. The morphology of CaCO3 observed by SEM is shown in Fig.3. As shown in Fig.3, it is clear that CaCO3 particles seem as thin flakes, and most of them seem as approximately long elliptical, whilst a few particles seem as irregular. These flake-like crystals pack together, exhibiting the appearance of CaCO3 conglomerate shape.
Table 1 Particle size distribution of C3A and CaCO3

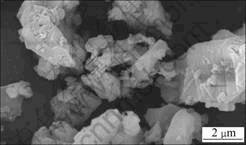
Fig.2 SEM image of C3A
2.2 Experimental methods
C3A was replaced with equivalent CaCO3. The mixing proportions are shown in Table 2. The net paste mixed according to Table 2 was cured in a standard chamber. Reaching the time of testing, the hydration processes of the samples were terminated by ethanol,samples were milled as powder and then passed through an 80 μm sieve. Finally, particles with diameters of 2- 5 mm were collected. The powder and particles were dried and sealed for XRD, DSC, FTIR spectra analysis and SEM observation.

Fig.3 SEM image of CaCO3
Table 2 Mixing proportions of C3A-CaCO3-H2O system
3 Results and discussion
3.1 Influence of CaCO3 on hydration products of C3A
3.1.1 XRD analysis
Table 3 shows the hydration products of C3A- CaCO3-H2O systems measured with XRD at the age of 1 h, 2 h, 1d, 3 d, 7 d and 28 d, respectively.
Table 3 Hydration products of C3A-CaCO3-H2O system (XRD analysis)
After 1 h hydration, pure C3A generated C3AH6 which was stable up to 28 d. After 1 d and 3 d hydration, C2AH8 and C4AH13 appeared respectively. After 7 d hydration, the ternary hydration products C2AH8, C3AH6 and C4AH13 coexisted, and the hexagonal lamellar crystals of C2AH8 and C4AH13 began to convert to cubic C3AH6 phase subsequently.
As shown in Table 3, the presence of CaCO3 inhibited the formation of hydration products C2AH8 and C4AH13. The ages of the presence of hydration product C3AH6 are both 1 h either for pure C3A or C3A containing 15% CaCO3, whereas C3A containing 25% CaCO3 generated C3AH6 till 1 d hydration. Therefore, it is concluded that the formation of C3AH6 has relation to the dosage of CaCO3, that is, when the dosage of CaCO3 exceeds a certain value, e.g., 25% in this experiment, the formation of C3AH6 is delayed. The researches of RAMACHANDRAN and ZHANG [25-26] suggested that the formation of carboaluminate hydrate cause CaCO3 to delay the formation of C3AH6 hydrated from C3A.
Both 15% CaCO3 or 25% CaCO3 could react with the hydration products of C3A and could form new calcium carboaluminate hydrate phase, and could give calcium hemicarboaluminate and calcium monocarbo- aluminate after 1 h hydration, and then the calcium hemicarboaluminate was converted to calcium monocarboaluminate and remained stable up to 28 d. According to the experimental results, it is demonstrated that calcium hemicarboaluminate is unstable while calcium monocarboaluminate is stable [14].
The particle diameters of C3A and CaCO3 are 16.09 and 5.81 μm, respectively, and the specific surfaces of them are 740 and 4580 m2/kg, respectively. So C3A particles are dispersed by the superfine CaCO3 particles. When C3A startes to hydrate, the activity of CaCO3 will make itself react with C3A and generate calcium carboaluminate hydrate. This process influenced the formation of products of pure C3A hydration, inhibited the formation of C2AH8 and C4AH13 and delayed the presence of C3AH6. Hydrating process is clearly shown in Table 3 from the XRD observation.
3.1.2 FTIR spectra analysis
Fig.4 shows the FTIR spectra of the C3A-CaCO3- H2O system at the hydration age of 1, 3, 7 and 28 d, respectively.
As shown in Fig.4, 535-541, 670-675 and 874- 876 cm-1 are characteristic wavenumbers of calcium monocarboaluminate (3CaO×Al2O3×CaCO3×11H2O). Comparing Fig.4(a) without CaCO3(C3A-0) with Fig.4(b) with 15%CaCO3(C3A-15) and Fig.4(c) with 25%CaCO3 (C3A-25). FTIR spectra analyses prove that the hydration
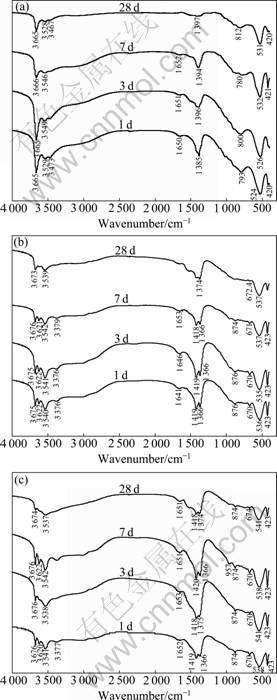
Fig.4 FTIR spectra of different hydration ages of C3A-CaCO3- H2O system: (a) C3A-0; (b) C3A-15; (c) C3A-25
products of C3A containing CaCO3 react with CaCO3, giving calcium carboaluminate hydrate, and calcium monocarboaluminate is stable up to 28 d.
3.1.3 DSC analysis
Fig.5 shows the DSC analysis results of the C3A-CaCO3-H2O system at the hydration ages of 1, 3, 7 and 28 d, respectively.
As shown in the C3A-0 DSC patterns (Fig.5(a)), the endothermic peak between 65 and 75 ℃ is generated by losing bond water and absorbed water, and that between
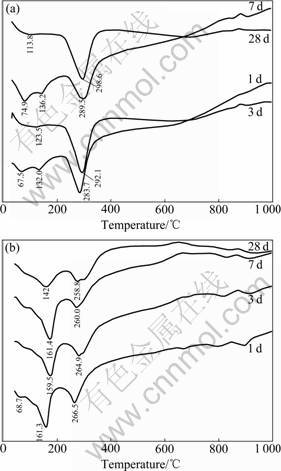
Fig.5 DSC patterns of C3A-CaCO3-H2O system at various hydration ages: (a) C3A-0; (b) C3A-15
110 and 140 ℃ indicates the hydration of C2AH8 and C4AH13, while that between 280 and 300 ℃ denotes the hydration of C3AH6. At the age of 28 d, the small endothermic peak at 113.8 ℃ suggests that there exist a small amount of hexagonal lamellar hydrates.
Fig.5(b) shows that DSC patterns will be changed if C3A contains 15% CaCO3. At each hydration age, distinct endothermic peaks appear between 140 and 165 ℃, which result from the dehydration of calcium carboaluminate hydrate, while the endothermic peaks between 258 and 267 ℃ are the results of the dehydration of C3AH6. Therefore, it is confirmed from the DSC analysis that the hydration products of C3A containing CaCO3 react with CaCO3, which generates calcium carboaluminate hydrate, and the dehydration temperature of C3AH6 is reduced at approximately 20 ℃ due to the admixture of CaCO3.
3.2 Morphology characteristics of C3A-CaCO3-H2O system hydrates
Figs.6(a), (b) and (c) show SEM images of pure C3A at different hydration ages of 3, 7 and 28 d, respectively.
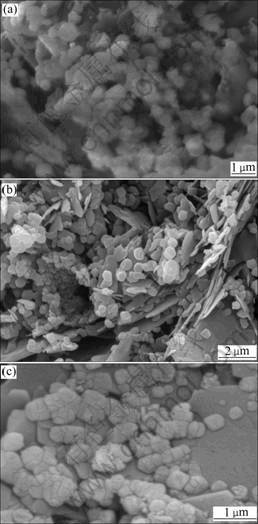
Fig.6 SEM images of pure C3A hydrated for different hydration ages: (a) 3 d; (b) 7 d; (c) 28 d
There is a great deal of C3AH6 of cubic phase hydrated for 3 d, along with a small amount of hexagonal lamellar crystals. At hydration age of 7 d (Fig.6(b)), cubic and hexagonal lamellar crystals appear, and then the cubic crystals grow, crowd and accumulate together up to the hydration age of 28 d (Fig.6(c)), which gradually alters and increases the shape and the volume of these crystals. From Fig.6(c), the hexagonal lamellar hydrate still exists even as pure C3A hydrated for 28 d, which confirms the analysis results of DSC.
Figs.7(a), (b) and (c) present SEM images of C3A-25 hydrated for 3, 7 and 28 d, respectively. It can be observed that plenty of irregular long thick crystal flakes overlap when hydrated for 3 d (Fig.7(a)), and some cubic crystals can be observed but no hexagonal lamellar ones. These long thick crystal flakes can even be observed at the hydration age of 7 d (Fig.7(b)), however, the shapes of crystals are gradually changed to long rod but irregular appearance. Then, at the hydration age of 28 d
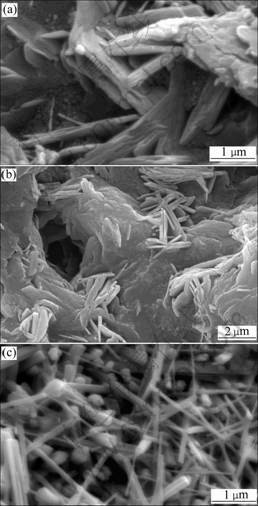
Fig.7 SEM images of C3A-25 hydrated for different hydration ages: (a) 3 d; (b) 7 d; (c) 28 d
these crystals convert into fine-needle shape from the previous long rod appearance (Fig.7(c)). The XRD patterns of hydrated C3A-25 samples show that the hydration products of C3A containing CaCO3 are C3AH6 of cubic phase and calcium monocarboaluminate, which determines that the crystals changing with time are calcium monocarboaluminate. Therefore, it can be concluded that the hydration product in the C3A-CaCO3-H2O system, i.e., calcium monocarbo- aluminate, originally appears with irregular long thick shape and then changes to long rod shape at the hydration age of 3 d, and finally changes to fine-needle appearance at hydrate age of 28 d.
4 Conclusions
(1) New phases, i.e., calcium hemicarbo-aluminate and calcium monocarboaluminate are found in this system due to the activity of CaCO3.
(2) The formation of some hydration products, such as C2AH8 and C4AH13, is inhibited, and C3AH6 is delayed at early age.
(3) Calcium hemicarboaluminate appears at the initial hydration age and then changes completely within 24 h. Calcium monocarboaluminate exists stably during 1 h hydration up to 28 d hydration; its appearance changes from early thick flake to long rod shape, and to finally fine-needle shape after hydrated for 28 d.
References
[1] LI Bu-xin,CHEN Feng. Study on the mechanical property of Portland limestone cement [J]. Journal of Building Materials, 1998, 1(2): 186-191. (in Chinese)
[2] CHEN Jian-xiong, CUI Hong-tao, CHEN Han-bin, XIAO Fei. Study on performance of concrete mixed with ultra-fine limestone flour [J]. Construction Technology, 2004, 33(4): 39-41. (in Chinese)
[3] VOGLIS N, KAKALI G, CHANIOTAKIS E, TSIVILIS S. Portland- limestone cements: Their properties and hydration compared to those of other composite cements [J]. Cem Concr Compo, 2005, 27(2): 191-196.
[4] CARRASCO M F, MENENDEZ G, BONAVETTI V, IRASSAR E F. Strength optimization of “tailor- made cement” with limestone filler and blast furnace slag [J]. Cem Concr Res, 2005, 35(7): 1324-1331.
[5] TIAN Qian. The mineral admixture of self-compacting high performance concrete [J]. China Concrete and Cement Products, 2000(5): 18-20 (in Chinese)
[6] CAO Peng-fei, QIN Hong-gen, PANG Chao-ming. Study on performance of self-compacting concrete mixed with limestone-powder [J]. Construction Technology, 2005, 34(S1): 35-37, 43. (in Chinese)
[7] PE?RA J, HUSSON S, GUILHOT B. Influence of finely ground limestone on cement hydration [J]. Cem Concr Compos, 1999, 21(2): 99-105.
[8] TSIVILIS S, KAKALI G, CHANIOTAKIS E, SOUVARIDOU A. A study on the hydration of Portland limestone cement by means of TGA [J]. J Therm Anal, 1998, 52(4): 863-870
[9] BONAVETTI V L, RAHHAL V F, IRASSAR E F. Studies on the carboaluminate formation in limestone filler-blended cements [J]. Cem Concr Res, 2001, 31(6): 853-859.
[10] KAKALI G, TSIVILIS S, AGGELI E, BATI M. Hydration products of C3A, C3S and Portland cement in the presence of CaCO3 [J]. Cem Concr Res, 2000, 30(7): 1073-1077.
[11] ZHANG Yong-juan, ZHANG Xiong. Research on effect of limestone and gypsum on C3A, C3S and PC clinker system [J]. Construction and Building Materials, 2007, 22(8): 1634-1642. (in Chinese)
[12] YANG Hua-shan, FANG Kun-he, TU Sheng-jin, YANG Hui-feng. The effect and its mechanism of calcium carbonate on the cement based materials [J]. Concrete, 2006(6): 32-35. (in Chinese)
[13] LIU Shu-hua, YAN Pei-yu. Hydration properties of limestone powder in complex binding material [J]. Journal of the Chinese Ceramic Society, 2008, 36(10): 1401-1405. (in Chinese)
[14] LI Yue, DING Qing-jun, HU Shu-guang. Utilization of limestone as mineral admixture in cement and concrete [J]. Journal of Wuhan University of Technology, 2007, 29(3): 35-37, 41. (in Chinese)
[15] CHEN Jian-xiong, LI Hong-fang, CHEN Peng, ZHANG Lan-fang. Study on super early-strength, high-strength and high-performance concrete containing limestone powder composite admixture [J]. Bulletin of the Chinese Ceramic Society, 2007, 26(1): 190-193. (in Chinese)
[16] CHEN Jian-xiong, LI Hong-fang, CHEN Han-bin, LI Wen-ting, WEN He. Study of super high strength concrete containing super-fine limestone powder and titanium slag powder [J]. Journal of Building Materials, 2005, 8(6): 672-676. (in Chinese)
[17] XIAO Jia, WANG Yong-he, DENG De-hua, CHEN Lei. Chloride diffusivities of high strength concrete with fly ash and ground limestone [J]. Journal of Building Materials, 2008, 11(2): 212-216. (in Chinese)
[18] XIAO Jia, DENG De-hua, TANG Xian-yan, CHEN Feng, CHEN Lei. Experiment on the anti-chloride ion permeability of concrete mixed with slag and limestone powder [J]. Industrial Construction, 2007, 37(10): 73-75, 87. (in Chinese)
[19] GUO Yu-xia, GONG Jin-xin, LI Jing. Influence of mass fractions of limestone powder on mechanical property and durability of concrete [J]. Journal of Building Materials, 2009, 12(3): 266-271. (in Chinese)
[20] MA Ye-hong. Study on limestone powder used as mineral admixture of concrete [D]. Guangzhou: South China University of Technology, 2007. (in Chinese)
[21] GONZA?LEZ M A, IRASSAR E F. Effect of limestone filler on the sulfate resistance of low C3A Portland cement [J]. Cem Concr Res, 1998, 28(11): 1655-1667.
[22] BONAVETTI V, DONZA H, RAHHAL V, IRASSAR E. Influence of initial curing on the properties of concrete containing limestone blended cement [J]. Cem Concr Res, 2000, 30(5): 703-708.
[23] ZELIC J, KRSTULOVIC R, TKALCEC E, KROLO P. The properties of Portland cement-limestone-silica fume mortars [J]. Cem Concr Res, 2000, 30(1): 145-152.
[24] BOSILJKOV V B. SCC mixes with poorly graded aggregate and high volume of limestone filler [J]. Cem Concr Res, 2003, 33(9): 1279-1286.
[25] RAMACHANDRAN V S, ZHANG C M. Hydration kinetics and microstructural development in the 3CaO·Al2O3-CaSO4·2H2O- CaCO3-H2O system [J]. Materiaux et Constructions, 1986, 19: 437-444.
[26] RAMACHANDRAN V S, ZHANG C M. Thermal analysis of the 3CaO·Al2O3-CaSO4·2H2O-CaCO3-H2O system [J]. Thermochimica Acta, 1986, 106: 273-282.
Foundation item: Project(50678177) supported by by the National Natural Science Foundation of China
Received date: 2010-03-31; Accepted date: 2010-07-06
Corresponding author: XIAO Jia, PhD, Professor; Tel: +86-13974842678; E-mail: jiaxiao@mail.csu.edu.cn
- Effect of CaCO3 on hydration characteristics of C3A