
Preparation of nano-Ag/TiO2 thin-film
PENG Bing(彭 兵), WANG Jia(王 佳), CHAI Li-yuan(柴立元),
MAO Ai-li(毛爱丽), WANG Yun-yan(王云燕)
School of Metallurgical Science and Engineering, Central South University, Changsha 410083, China
Received 10 May 2007; accepted 18 October 2007
Abstract:
Steady TiO2 water-sol was prepared by peptization and the effects of pH value, temperature, concentration of colloid and peptizator on sol were investigated. Laser grain analyzer was used to verify nano-particles in the sol. The photocatalytic degradation ratio and antibacterial property of nano-Ag/TiO2 thin-film on ceramics were used as the main index in addition to XRD analysis. The effect of film layers, embedding Ag+, annealing temperature and time on the degradation ratio and antibacterial property was studied. The temperature 30-80 ℃, pH 1.2-2.0, concentrations of 0.05-0.3 mol/L sol and 5% HNO3 would be the optimal parameters for the TiO2 water-sol preparation. The nano-Ag/TiO2 film of three layers with 3% AgNO3 embedded and treated at 350 ℃ for 2 h exhibits good performance. The elementary research on the kinetics of degradation shows that the reactions are on the first order kinetics equation.
Key words:
nano-Ag/TiO2; water-sol; film; thermal treatment;
1 Introduction
Ceramics with nano-TiO2 film immobilized on the glaze has not only decorating function but also capability to degrade organic compounds. These make the ceramics widely used in sanitation, medical treatment, fitment and civil or industrial building[1]. Sol-gel technique, which includes two steps of sol-preparation and thermal treatment, is a practical method to prepare nano-TiO2 film[2-4]. It involves two steps of dispersion and coacervation. The former is adapted to prepare inorganic water-sol, which includes the mechanical-dispersion, electric-dispersion, ultrasonic-dispersion and peptization process[5-6]. The process of mechanical-dispersion is simple but its dispersion mixtures are usually not composed of nano-grains[7]. The electric-dispersion is effective but the process is complicated and hard to control[8]. The ultrasonic-dispersion has above advantages but its sol is not steady enough[9-10]. The peptization uses chemicals to turn fresh deposition into sol and the process is simple. The time period for the deposition could be shortened by using mechanical-dispersion at one time and the sol is more steady[11]. The ceramics are put into the water-sol to load TiO2 humid film after steady nano-TiO2 water-sol is prepared, and then the thermal treatment is conducted. It was found that the crystalloid structure determined by the thermal treating parameters influences the particle size of TiO2 powder, photo-catalytic and antibacterial performances of the film[12]. Other factors such as film thickness, surface structure and embedding metal worked also on the performances.
In this study, steady TiO2 water-sol was prepared by peptization, and then photo-catalytic degradation rate of methyl orange and antibacterial property of nano- Ag/TiO2 film on ceramics were used as main index to optimize the preparation parameters.
2 Experimental
2.1 Preparation of TiO2 water-sol
5%-60% nitric acid or 3%-36% hydrochloric acid was used to peptize 0.05-0.4 mol/L H4TiO4 at certain pH value, then the mixture was agitated in water bath at 30-80 ℃ until it became translucent sol. Following reactions[13] took place in the procedure:
TiO(OH)2↓+H+→TiO(OH)++H2On (1)
TiO(OH)++H2On+H+→TiO2++2H2O (2)
2.2 Preparation of TiO2 film immobilized on ceramics glaze
The ceramics were cut into 50 mm×50 mm pieces and washed with 5% NaOH solution, alcohol and distilled water, then dried at room temperature. The treated ceramics were put into the TiO2 water-sol to load TiO2. After loading one layer of film, they were dried in an oven at 100 ℃. By repeating these operations, the films with certain thickness were prepared. Finally, they were heated in a Muffle furnace from 100 ℃ to appointed temperature at the rate of 4-5 ℃/min for several hours.
2.3 Measurements
1) Stability of water-sol
Transmissivity was detected by VIS-7220 UV- spectrophotometer. Zeta potential and stabilization time were used to characterize the stability. And grain-size distribution of the water-sol was detected by Laser grain analyzer.
2) Photo-catalytic degradation performance
U-2010 ultraviolet-visible spectra-photometer made in Japan was used to test absorbance of methyl orange at 465 nm wavelength. 100 mL methyl orange (5 mg/L) stirred by electromagnetic and a piece of ceramics were put into a glass dish with a ZF-2 ultraviolet lamp (wavelength 253.6 nm) lighting above at the distance of 14 cm. The absorbance was detected once per 30 min and water was compensated for evaporation in the process. Comparative trial was also conducted and the degradation rate(R0) was calculated in the following way[14]:
![]() (3)
(3)
where A0 and At are the absorbances at time of 0 and t, respectively.
3) Antibacterial performance
According to Ref.[15], antibacterial rates were tested by the method according to Japanese standards that was used by TOTO Co. and INAX Co. to test antibacterial efficiency of materials.
4) X-ray diffraction analysis
Rigaku D/max2550VB+ X-ray diffraction analyzer made in Japan was used to observe the phases of film after being heated at different temperature[16].
3 Results and discussion
3.1 Effect of pH on peptization
10% nitric acid and 6% hydrochloric acid were used respectively to peptize 0.1 mol/L H4TiO4 at corresponding pH values according to Table 1 and Table 2, then the mixture was stirred in water bath at 30 ℃ until it became translucent sol.
Table 1 Effect of pH on sol formation (10% HNO3)
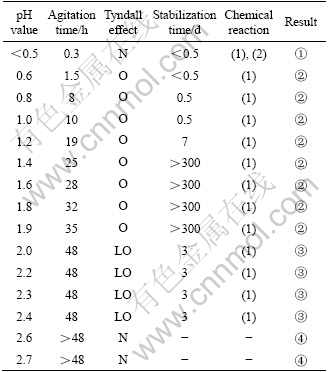
Tyndall effect: N—None; LO—Less obvious; O—Obvious.
Result: ① No sol formation; ② Blue and translucentsol; ③ Partial sol formation; ④ Colorless solution.
Table 2 Effect of pH value on sol formation (6% HCl)
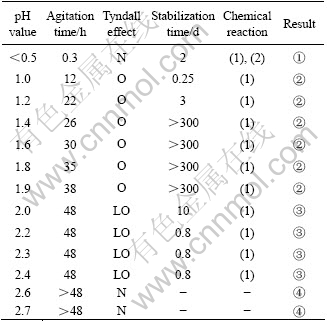
Tyndall effect: N—None; LO—Less obvious; O—Obvious
Result: ① No sol formation; ② Blue and translucentsol; ③ Partial sol formation; ④ Colorless solution
Table 1 and Table 2 indicate that peptization should be conducted at certain scope of pH: when pH<0.5, the mixture became translucent sol in reaction (1), but after a while it became lucid in reaction (2). The lucid solution was instable and the precipitate appeared a few days later. It is known that H4TiO4 dissolved under superfluous peptizer. When 0.5<pH<1.2, the mixturebecame translucent sol after 24 h agitation and Tyndall effect appeared, but it was not stable enough, and precipitate appeared after half a month aging. When 1.2<pH<2.0, the mixture became translucent sol after agitating for more than 24 h; the Tyndall effect is obvious and it could keep stable for at least 10 months. When 2.0<pH<2.5, the mixture could not become translucent sol even after agitating for more than 48 h; the Tyndall effect is faint and it became gel soon because of insufficient peptizer to make all of H4TiO4 grains separate. When pH>2.5, there was less peptizer added into the mixture so that repulsive force of double electric layers forming on the surface of grain could not conquer their attraction and the sol did not appear.
3.2 Effect of sol concentration on peptization
10% nitric acid was used to peptize H4TiO4 at corresponding concentration according to Table 3, then the mixture was stirred in water bath at 30 ℃, pH 1.4 until it became translucent sol.
Table 3 Effect of TiO2 concentration on formation and stability of Sb
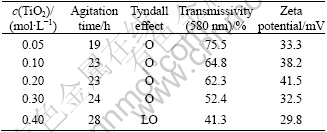
Tyndall effect: N—None; LO—Less obvious; O—Obvious.
Table 3 gives sol transmissivity at wavelength of 580 nm. It is indicated that with the increase of H4TiO4 concentration, agitation time became longer and the transmissivity decreased. This is because that the amount of peptizer was invariable, and it would need more time to disperse gains. When the amount of H4TiO4 increased heavily, there was not enough peptizer to turn them into sol so that the Tyndall effect was faint and transmissivity was low. Though low H4TiO4 concentration was of benefit to gain dispersion, it did nothing to the stability of sol and would lead to complicated coating process. The variation of Zeta potential indicates the same regularity. When the H4TiO4 concentration was 0.05-0.3 mol/L, the sol was stable.
3.3 Effect of peptizer concentration on peptization
Nitric acid and hydrochloric acid with corresponding concentration according to Table 4 were used respectively to peptize 0.1 mol/L H4TiO4 at pH=1.4, then the mixture was stirred in water bath at 30 ℃ until it became translucent sol.
Table 4 Effect of HNO3 and HCl concentration on sol formation
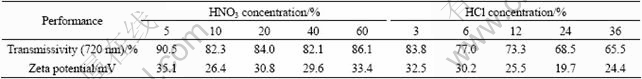
Table 4 gives the transmissivity and Zeta potential of sol peptized by two types of peptizers at wavelength of 720 nm. Fig.1 gives their transmissivity curves at different incidence wavelength. It is indicated that sol peptized by peptizer with low concentration had better effect than that peptized by peptizer with high concentration. The reason may be that the reaction TiO(OH)2(precipitate)+H+→TiO(OH)+·H2On progressed too fast, where the “n” value in resultant was not big enough and the sol was not stable. When the peptizer with low concentration was used, the reaction would have a suitable progressing speed, and the “n” value could be big and the sol was stable.
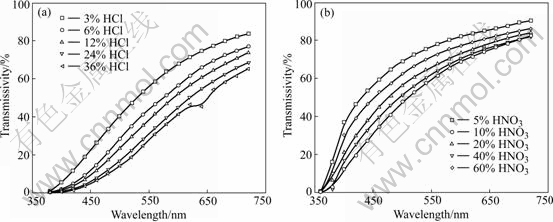
Fig.1 Transmissivity curves of TiO2 sol at different incidence wavelength
3.4 Effect of temperature on peptization
5% nitric acid and 3% hydrochloric acid were used respectively to peptize 0.1 mol/L H4TiO4 at pH=1.4, then the mixture was stirred in water bath at corresponding temperature according to Table 5 until it became translucent sol.
Table 5 Effect of temperature on sol formation
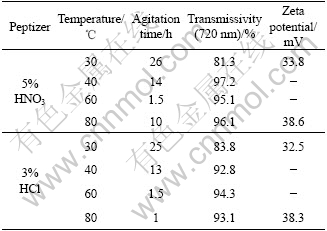
Table 5 gives the transmissivity of sol peptized by two types of peptizers at wavelength of 720 nm and their Zeta potential at 30 ℃ and 80 ℃. Fig.2 and Fig.3 give the transmissivity curves at different incidence wavelength. It is indicated that the sol stirred at high temperature had better peptization effect than that stirred at low temperature. With the increase of temperature, the time spent on grain dispersing became shorter. But the inordinate high temperature would make the grains move very fast and finally lead to agglomeration. The mixture never turned into sol but appeared white feculent when the temperature exceeded 80 ℃.
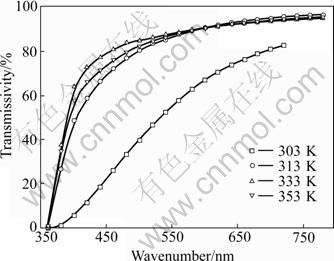
Fig.2 Transmissivity curves of TiO2 sol at different incidence wavelength (3% HCl used as peptizer)
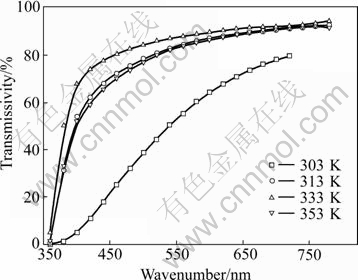
Fig.3 Transmissivity curves of TiO2 sol at different incidence wavelength (5% HNO3 used as peptizer)
3.5 Particle size of sol
5% nitric acid and 3% hydrochloric acid were used respectively to peptize 0.1 mol/L H4TiO4 at pH=1.4, then the mixture was stirred in water bath at 30 ℃ until it became translucent sol. The particle size distributions of TiO2 sol made in 5% HNO3 and 3% HCl are shown in Fig.4 and Fig.5, respectively.
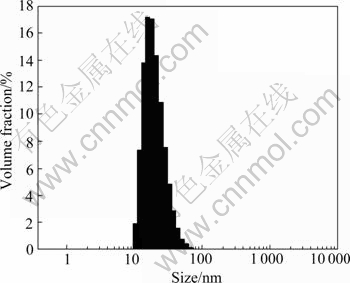
Fig.4 Size distribution of TiO2 sol particles (5% HNO3 used as peptizer)
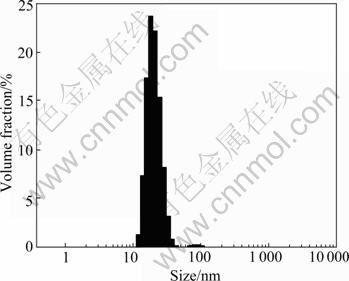
Fig.5 Size distribution of TiO2 sol particles (3% HCl used as peptizer)
The size of sol grains peptized by nitric acid is between 10 nm and 70 nm. The size of sol grains peptized by hydrochloric acid is between 10 nm and 45 nm or between 68 nm and 125 nm. This indicates that the sol grains are nano-particles and the sol peptized by nitric acid had better result.
3.6 Effect of Ag embedded on photo-catalytic and antibacterial performance
The TiO2 water-sol was prepared at 80 ℃, pH=1.4, c(sol)=0.1 mol/L, w(Peptizator of HNO3)=5% (and w(Peptizator of HCl)=3%) with some silver nitrate added. Then two types of ceramics with nano-Ag/TiO2 film were prepared after three layers of film were loaded and treated at 550 ℃ for 2 h.
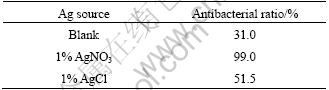
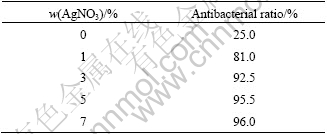
Fig.6 gives the methyl-orange photocatalytic degradation rate of the films embedded with different Ag+. Because Ag could restrain recombination of cavity and electron, the photocatalytic performance of film is improved. Besides that, degradation rate of the film embedded with AgNO3 is higer than that embedded with AgCl. It is because that the grain size of TiO2 in film prepared by HNO3 was smaller than that prepared by HCl. Based on the quantum size effect, the smaller the grain size of TiO2 in the film is, the wider the energy gap is. This makes oxidation reduction potential and photo-catalytic reaction driving force increased and the photocatalytic performance improved. Table 6 gives antibacterial property of the films embedded with different Ag+. It is indicated that antibacterial rate of the film embedded with AgNO3 is higher than that embedded with AgCl. In the film embedded with AgNO3, there are actually two types of Ag+, Ag2SO4 and AgNO3, either of which has higher solubility constant than AgCl. So there would be more Ag dissolving into bacterium solution. Besides that, it is easier for AgCl to turn into elementary substance or silver oxide under light, either of which has lower solubility constant than AgCl itself.
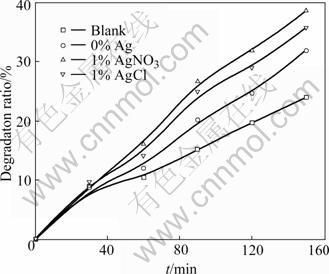
Fig.6 Effect of film embedded with different Ag+ on photo- catalytic degradation ratio of methyl orange
Table 6 Antibacterial property of film embedded with different Ag+
According to the method stated at the beginning, the amount of embedding Ag was changed, and the films with 1%, 3%, 5% AgNO3 were prepared. Fig.7 gives the degradation rate of the films embedded with different amount of Ag+. It is indicated that photocatalytic performance is improved with the increase of embedded Ag+ amount. This is because that the Ag+ assembling on surface forms a center to capture electrons, which could improve the separation probability of photogenerated electrons and cavity. Table 7 gives the antibacterial property of the films embedded with different amount of Ag+. From 1% to 3%, the more the amount of Ag embedded, the more the amount of Ag+ dissolving into bacterium solution. So the antibacterial rate is improved. Although the amount of Ag increased from 3% to 5% and even 7%, the antibacterial rate was not obviously improved. This is because that the concentration of Ag dissolving into bacterium solution tends to be a constant.
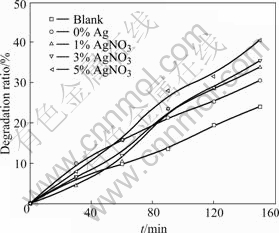
Fig.7 Effect of film embedded with different amount of Ag+ on photo-catalytic degradation ratio of methyl orange
Table 7 Antibacterial property of film embedded with different amount of Ag+
3.7 Effect of film thickness on photocatalytic and antibacterial performance
The TiO2 water-sol was prepared at 80 ℃, pH=1.4, c(Sol)=0.1 mol/L and 0.2 mol/L, w(Peptizator of HNO3) =5%. Several types of ceramics with nano-TiO2 thin film were prepared after 1, 3, 5 layers of humid film were respectively loaded and heated at 550 ℃ for 2 h.
Fig.8(a) gives the degradation ratio of films with different layers prepared by 0.1 mol/L sol. Layer increasing means that the amount and surface area of TiO2 increase, and it would lead to the increase of electron and cavity concentration, so that the photocatalytic performance of the film is improved. Fig.8(b) gives the degradation ratio of films with different layers prepared by 0.2 mol/L sol. It is indicated that the degradation ratio increases from 1 to 3 layers but declines from 3 to 7 layers. The superposition of the network structure of TiO2 caused by superabundant TiO2 loaded on the glaze reduces the surface area of TiO2. Table 8 gives the actual degradation ratio tested at 150 min of the films prepared by 0.1 mol/L and 0.2 mol/L sol. It is indicated that when the sol concentration is high, the film with fewer layers exhibits better performance; when the sol concentration is low, the film with more layers exhibits better performance.
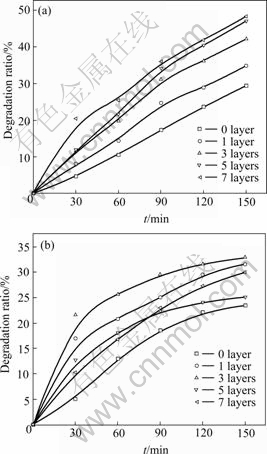
Fig.8 Effect of different film layers on photocatalytic degradation ratio of methyl orange: (a) 0.1 mol/L sol; (b) 0.2 mol/L sol
Table 8 Photocatalytic properties of 0.1 mol/L sol film and 0.2 mol/L sol film (150 min)

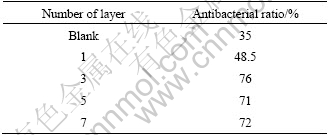
According to the method stated at the beginning, 1% AgNO3 was added into 0.1 mol/L sol to prepare Ag/TiO2 films with 1, 3, 5 layers. Table 9 gives the antibacterial property of the films embedded with 1% AgNO3 with different layers. With the layers increasing from 1 to 3, the amount of Ag embedded in films also increases so that its antibacterial performance is improved. But antibacterial ratio of the film with 5 and 7 layers is lower than that with 3 layers. It’s because that superposition of the network structure of TiO2 prevents the Ag+ in the bottom films dissolving into the bacteria solution.
Table 9 Antibacterial property of film with different layers
3.8 Effect of annealing temperature on photocatalytic and antibacterial performance
The TiO2 water-sol was prepared at 80 ℃, pH=1.4, c(Sol)=0.1 mol/L, w(Peptizator of HNO3)=5%. Several types of ceramics with nano-TiO2 thin film were prepared after 3 layers of humid film was loaded and heated at 350 ℃, 450 ℃, 550 ℃, 650 ℃ respectively for 2 h.
Fig.9 gives XDR pattern of the films heated at different temperature. TiO2 in the film heated at 350 ℃ begins to turn to anatase at 2θ=25.3?. The amorphous phase and anatase TiO2 coexist. The indication that there is not rutile but anatase appearing in the film at 450 ℃ and both of them coexist in the film at 550 ℃ accords with the report in Ref.[17] that TiO2 begins to turn to rutile at 500 ℃. With the annealing temperature approaching 650 ℃, the amount of rutile TiO2 increases, and there is still some anatase TiO2. Because the film on the glaze is very thin, the diffraction peaks of rutile and anatase TiO2 are weak. The zirconium salt and SiO2 are main components of the ceramics glaze. According to Scherre Formula[18], the average diameter of TiO2 crystal grain is 29.8 nm, and it is a nano-thin film.
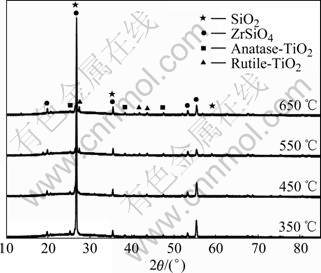
Fig.9 X-ray diffraction patterns of TiO2 thin film at different annealing temperatures
Fig.10 gives the degradation rate of the films heated at different temperature. The order of degradation rate from maximum to minimum is at 350, 450, 650 and 550 ℃. Generally speaking, anatase TiO2 has more active photocatalytic performance than the rutile. When the annealing temperature goes up, crystal grains of the film grow larger, its surface area reduces and adsorption capability becomes weaker. Meanwhile, the amount of the rutile increases. All of these lead to the decline of photocatalytic performance. But it could be improved as if there is a few of rutile coexsiting[19]. This is the reason why the film heated at 650 ℃ has higher degradation rate than that heated at 550 ℃. This may be the same reason why the film with amorphous and anatase TiO2 coexisting has more active photocatalytic performance than that with pure anatase TiO2.
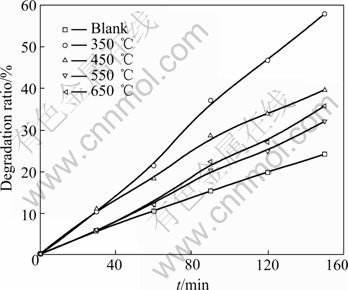
Fig.10 Effect of annealing temperature on photocatalytic degradation ratio of methyl orange
According to the method stated at the beginning, 1% AgNO3 was added into the sol to prepare Ag/TiO2 films heated at different temperature.
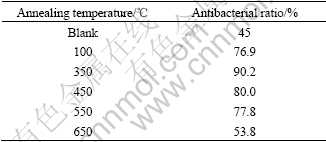
Table 10 gives the antibacterial property of the nano-Ag/TiO2 films heated at different temperatures. The antibacterial ratio of the film goes up with the annealing temperature rising from 100 ℃ to 350 ℃. The film heated at 100 ℃ is not firmly attached on the glaze, but falls off easily by finger scraping. When the annealing temperature keeps on rising from 350 ℃ to 650 ℃, AgNO3 in the film turns into elementary substance or silver oxide and the antibacterial ratio declines.
Table 10 Antibacterial property of film at different annealing temperature
3.9 Effect of annealing time on photocatalytic and antibacterial performance
The TiO2 water-sol was prepared at 80 ℃, pH=1.4, c(Sol)=0.1 mol/L, w(Peptizator of HNO3)=5%. Several types of ceramics with nano-TiO2 thin film immobilized on glaze were prepared after 3 layers of humid film were loaded and heated for 0, 0.5, 1, 2 and 3 h respectively at 550 ℃.
Fig.11 gives the degradation ratio of the films heated for different time. The effect of annealing time on degradation ratio is unconspicuous, but there is still a max value at 0.5 h. After crystal grains of the film grow larger, the amount of rutile also increases with the extending of annealing time. All of these lead to poor photocatalytic performance.
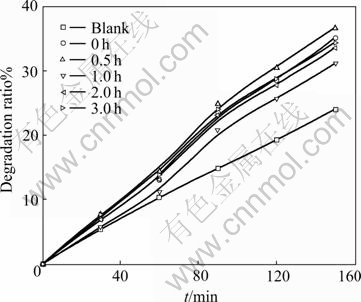
Fig.11 Effect of annealing time on photocatalytic degradation ratio of methyl orange
According to the method stated at the beginning, 1% AgNO3 was added into the sol to prepare Ag/TiO2 films heated for different time.
Fig.12 gives antibacterial property of the nano- Ag/TiO2 films heated for different time. Antibacterial ratio rises within 0-2 h and keeps steady within 1-2 h, but it goes down markedly within 2-3 h. That’s because more and more AgNO3 in the film gradually turns into elementary substance or silver oxide. So good antibacterial performance film could not be obtained if the annealing time lasts too long or too short.
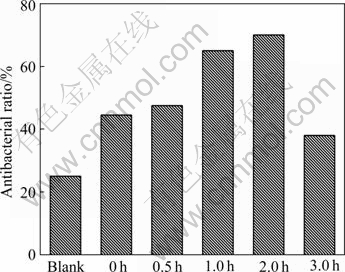
Fig.12 Antibacterial property of film at different annealing time
3.10 Methyl-orange photocatalytic degradation kineticsof nano-Ag/TiO2 film
The photocatalytic reaction in this study is a kind of heterogeneous reaction taking place on the solid/liquid interface and could be described by the Langmuir- Hinshelwood(L-H) equation[20-22]:
![]() (4)
(4)
where KA is the adsorption equilibrium constant of A on the TiO2 surface, CA is the concentration of A. 1/r has linear relationship with 1/CA. Besides that, according to Eqn.(4), the ln(C0/CA) has linear relationship with t, which accords with the first order equation, when A has a very low concentration and KACA<< 1.
Because the concentration of methyl-orange used in these experiments is about 5 mg/L, the process of its photocatalytic-degradation reaction could be considered as the first order kinetics equation, as shown in Fig.13. The first order kinetics equation is
ln(C0/C)=-0.003 05+0.003 31 t (R=0.997 91) (5)
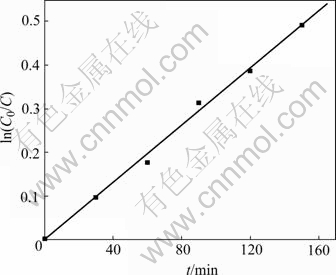
Fig.13 First order kinetics of methyl orange photocatalyst- degradation
4 Conclusions
1) By optimizing the preparation process, TiO2 water-sol prepared at 30-80 ℃, pH 1.2-2.0, c(sol) 0.05-0.3 mol/L, w(Peptizator of HNO3) 5% would be more steady.
2) The nano-Ag/TiO2 film of three layers with 3% AgNO3 embedded and treated at 350 ℃ for 2 h would exhibit good performance. The elementary research on the kinetics of degradation shows that the degradation reactions are on the first order kinetics equation.
References
[1] ZENG Ling-ke, ZHANG Hai-wen, WANG Hui, ZHANG Ming, SHI Lin-lin, CHEN Yong-jie. Antibacterial ceramics [J]. Jiangsu Ceramics, 2001, 34(4): 8-10. (in Chinese)
[2] CUI Xiao-li, JIANG Zhi-yu. Studies on the preparation and characteristics of titanium dioxide nano thin film [J]. Electroplating & Finishing, 2002, 21(5): 17-21.
[3] CHEN Xiao-bing, CHENG Xiao-ling, YU Shuang-ping, DEN Shu-hua, HUANG Hui-ming. Development in preparation of TiO2 thin films [J]. Advances in Fine Fetrochemicals, 2004, 5(2): 23-28.
[4] WANG Liang-xian. Pilot and prospect of anti bacteria building and sanitary ceramics [J]. Shandong Ceramics, 2000, 23(4): 5-9.
[5] SHAM Yang-zhen. Physical chemistry and gel chemistry [M]. Chengdu: Science and Technology Publication of Sichuan, 1986: 335.
[6] Jilin University, Sichuan University. Physical chemistry and gel chemistry [M]. Beijing: Education Publication of People, 1980: 415-423.
[7] ZHAO Xiao-bing, CHEN Zhi-gang. The summary of nanocomposite and its preparation technology [J]. Journal of Jiangsu University of Science and Technology, 2002, 23(4): 52-53.
[8] WANG Xiang-tian, HU Li-ming, GU Da, HU Ying. The analysis of dispersing process for tiny grains [J]. Chemistry, 1995(5): 13-14.
[9] XIAO Feng, YE Jian-dong, WANG Ying-jun. Application of ultrasonic technique in the processing and synthesis of inorganic materials [J]. Journal of the Chinese Ceramic Society, 2002, 30(5): 615-616.
[10] MASON T J. Sonochemistry: The uses of ultrasound in chemistry [M]. Cambrige: The Royal Society of Chemistry, 1990: 66-69.
[11] YAO Min-qi, WEI Ying-hui, HU Lan-qing, XU Bing-she. Fabrication of nanometer powders by the sol-gel method [J]. Rare Metal Materials and Engineering, 2002, 31(5): 325-329.
[12] FAN Chong-zheng, XIAO Jian-ping, DING Yan-wei. Stduy on nano-TiO2 and photocatalytic reaction [J]. Bulletin of Science, 2001, 46 (4): 265-273.
[13] ZHANG Yun, ZHAO Lang, YIN Guang-fu, ZHOU Da-li, XU Xiu-juan. Preparation and characterization of nanometer TiO2 film by sol method from H3TiO4 [J]. Chinese Journal of Inorganic Chemistry, 2004, 20(8): 991-995.
[14] LI Hong, ZHAO Gao-ling, LIU Qin-hua, WENG Wen-jian, DU Pi-yi, SHEN Ge, HAN Gao-rong. Influence of silicon doping and siliconvanadium co-doping on photocatalyst property of TiO2 thin films [J]. Journal of the Chinese Ceramic Society, 2005, 33(6): 784-788.
[15] WANG Jing, JIN Zong-zhe, LIANG Jin-sheng, JI Zhi-jiang, YAN Xue-wu. Study on the antibacterial test method for ceramics [J]. Jiangsu Ceramics, 2001, 34(4): 11-17.
[16] WU Guang-ming, MA Jian-hua, WEI Jian-dong, ATTIA S M. Effect of heat treatment on properties of TiO2 films prepared with sol-gel method [J]. Journal of Tongji University, 2003, 1(34): 73-75.
[17] BALACHANDRAN U, EROR N G. Raman spectrum of titanium dioxide [J]. J Solid State Chem, 1982, 42(2): 276-278.
[18] ZHOU Gong-du, DUAN Lian-yun. Structural chemistry [M]. Beijing: Education Publication of Peking University, 2002: 295-296. (in Chinese)
[19] BALACHANDRAN U, EROR N G. Raman spectrum of titanium dioxide [J]. Solid State Chem, 1982, 42(2): 276-278.
[20] FOX M A, DULAY M T. Heterogeneous photocatalysis [J]. Chemical Review, 1993, 93: 341-357.
[21] HASHIMOTO S, MIYATA T, WASHINO M, KAWAKAMI W. A liquid chromatographic study on the radiolysis of phenol in aqueous solution [J]. Environ Sci Technol, 1979, 13(1): 71-75.
[22] HASHIMOTO A, MIYATA T, KAWAKAMI W. Radiation induced decomposition of phenol in low system [J]. Radiat Phys Chem, 1980, 16: 59-65.
Foundation item: Projects(04GK2007, 2007CK3075 ) supported by Industrial Key Project of Science and Technology of Hunan Province, China
Corresponding author: PENG Bing; Tel: +86-731-8830875; E-mail: pb@mail.csu.edu.cn
(Edited by YANG Bing)


