 Surface structure and catalytic activity of electrodeposited Ni-Fe-Co-Mo alloy electrode by partially leaching Mo and Fe
Surface structure and catalytic activity of electrodeposited Ni-Fe-Co-Mo alloy electrode by partially leaching Mo and Fe
LUO Bei-ping(罗北平)1, 2, GONG Zhu-qing(龚竹青)3, REN Bi-ye(任碧野)2,
YANG Yu-fang(杨余芳)1 , CHEN Meng-jun(陈梦君)1
1. School of Metallurgical Science and Engineering, Central South University, Changsha 410083, China;
2. Department of Chemistry and Chemical Engineering, Hunan Institute of Science and Technology,
Yueyang 414000, China;
3. Research Institute of Materials Science, South China University of Technology,
Guangzhou 510640, China
Received 9 December 2005; accepted 10 April 2006
Abstract:
Ni-Fe-Mo-Co alloy electrode was prepared in a citrate solution by electrodeposition, and then Mo and Fe were partially leached out from the electrode in 30% KOH solution. The unique surface micromorphology of a hive-like structure was obtained with an average pore size of about 50 nm. The electrode has a very large real surface area and a stable structure. The effects of sodium molybdate concentration on the composition, surface morphology, and structure of electrodes were analyzed by EDS, SEM and XRD. The polarization curves of the different electrodes show that the catalytic activity of electrodes is strongly correlated with the mole fraction of alloy elements (Ni, Fe, Mo, Co), and the addition of cobalt element to Ni-Fe-Mo alloy improves the catalytic activity. The Ni35.63Fe24.67Mo23.52Co16.18 electrode has the best activity for hydrogen evolution reaction(HER), with an over-potential of 66.2 mV, in 30% KOH at 80 ℃ and 200 mA/cm2. The alloy maintains its good catalytic activity for HER during continuous or intermittent electrolysis. Its electrochemical activity and catalytic stability are much higher than the other iron-group with Mo alloy electrodes.
Key words:
Ni-Fe-Mo-Co alloy; catalytic activity; surface structure; hydrogen evolution reaction; electrode; electrodeposition;
1 Introduction
The chlorine alkaline industry and the production of hydrogen by electrolyzing water have disadvantages of high cost and energy consumption, so it is especially important to study and develop a high catalytic activity and good stability electrode material for hydrogen evolution reaction. In order to reduce hydrogen overpotential and increase catalytic activity, there have been a lot of investigations made in the past[1-10]. An effective way to increase the catalytic activity for hydrogen evolution electrode is to increase the true electrode area by increasing the electrode surface roughness and/or by changing the surface microstructure of alloy electrodes[2, 11].
The traditional porous electrode was obtained to increase the true electrode area by leaching out Zn or Al in alkaline media. In earlier studies, Raney-Ni alloy was prepared by leaching out Al or Zn in alkaline solution. Recently, Raney-Ni-Mo electrode[12] of submicrometric porous structure was obtained by pressing powders of Ni or NiMo with Al or Ni-Mo-Al powders deposited by vacuum plasma spraying, and then leaching in 30% KOH solution with K-Na tartrate at 70 ℃ for 24 h. Ni-Fe- Mo-Zn alloy[13] of high activity for hydrogen evolution reaction(HER) with a rough surface was electrodeposited, and the subsequent leaching of Zn was performed by immersion in a 28% KOH solution at 80 ℃for 4 h. Its overpotential was 83.1 mV in 6 mol/L KOH at 80 ℃ and 135 mA/cm2.
An interesting phenomenon that the Mo and Fe contents of Ni-Fe-Mo-Co electrode decrease after electrolysis in 30% KOH (mass fraction) solution was discovered while investigating the catalytic activity of the electrodeposited molybdenum-iron group alloy, a similar finding was also reported in Refs.[5, 12-14]. The results showed that Mo and Fe were partially leached from the electrode during the electrolytic process. It is noted that the surface microstructure of the Ni-Fe-Mo-Co alloy electrode should be changed because of partial leaching of Mo and Fe. Thus, it is possible for the surface microstructure of Mo-Fe group electrode to be redesigned using different method.
The purpose of this work is to prepare a Ni-Fe-Mo-Co alloy electrode by electrode position from citrate bath by introducing Co element to enhance the activity for HER. In order to alter the surface microstructure of electrode, the electrode is immersed in 30% KOH solution and partially leached out Mo and Fe. The surface microstructure and composition of the different Ni-Fe-Mo-Co alloy electrodes are then analyzed before and after electrolysis using SEM, EDS and XRD techniques. Their electrocatalytic activity and chemical stability are examined in 30% KOH solution under various electrolytic conditions.
2 Experimental
2.1 Electrodeposition of alloys
Ni-Fe-Mo-Co alloys were prepared on copper foil (99.9%) substrates. The copper foils were cut into 1 cm×3 cm strips, then polished to a mirror finish with 4# and 6# fine emery papers. After that, the foils were cleaned with acetone. Finally, the foils were dipped into 10% HCl solution for 1 min followed by a wash with distilled water. The size of high-density graphite plate anode was two times larger than that of the cathode.
The plating bath consisted of Ni2SO4?6H2O 120 g/L, Ni2Cl?6H2O 40 g/L, FeSO4?7H2O 10 g/L, Na2MoO4? 2H2O 5-20 g/L, Na3cite 90 g/L, saccharin 3 g/L, H3BO3 30 g/L, LGLA 25 g/L, LGLB 200 mg/L.
The pH value of the solution was adjusted to 6.0 using 10% NaOH solution. The cathode current density was 6 A/dm2. The temperature was maintained at 30 ℃. The deposition time was 120 min.
2.2 Physical characterization
The surface morphology of the alloy electrodes was analyzed with FEI(USA) Sirion 200 field emission SEM. The microstructure was examined with X-ray diffraction(XRD) (Rikagu D/Max 2500 diffractometer, Japan). The compositions of the electrodes were examined by EDS(GENESIS60S, EDAX Inc, USA).
2.3 Electrochemical measurements
The electrochemical measurements were performed in a conventional three-electrode glass cell using an PAR(EG&G) mode 2273(USA) linked to a micro- computer. A Ni-Fe-Mo-Co alloy was used as the working electrode (WE). All potentials were referenced to the Hg/HgO, OH- electrode. The reference electrode was positioned in a luggin capillary filled with 30% solution. The counter electrode(CE) was a platinum foil with a large surface. The alkaline solution was prepared from analytical grade KOH in triply distilled water.
3 Results and discussion
3.1 Characterization of alloy electrodes
The Ni-Fe-Mo-Co alloys were obtained by electrodeposition at different contents of sodium molybdate (with other conditions constant), and then the compositions of the alloy were measured by EDS. The results are presented in Table 1. The data in Table 1 show that the molybdenum content of the alloys rapidly increases at first and then slows down with the increase of sodium molybdate content. The iron and cobalt contents in the alloys increase at first and subsequently decrease. The content of Ni in the alloys first decreases and then increases slowly.
Table 1 Chemical composition of alloy prepared at different contents of sodium molybdate
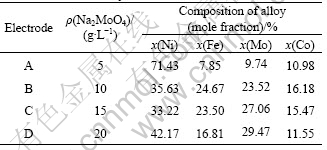
Fig.1 shows the SEM micrographs of the Ni-Fe-Mo-Co alloy electrodes by electrodeposition different concentrations of sodium molybdate. The surface morphology of the electrodes was obviously different. Fig.1(a) shows the SEM micrograph of the surface morphology for electrode A. The electrode surface has a grain shape similar to a cauliflower. The cauliflower-shape particle is made of coagulate nano-size particle.
Fig.1(b) shows the SEM micrograph of the surface morphology of electrode B. There are two different size particles on the electrode surface. The very exiguous crystalline grain is nano-size, the lager particle is the globular particle which is made of very tiny grain. The larger globular particle is more dispersive than the tiny particle. The tiny particles coagulate without a pattern. In addition, it can be seen that there are many voids on the surface.
Fig.2 shows the XRD patterns of the Ni-Fe-Mo-Co alloy electrode electrodeposited at different sodium molybdate concentrations. The intensity of the electrodes decreases with increasing the concentration of sodium molybdate in the solution. It can be seen that there is a distinct diffraction peak for electrodes A and B at 2θ≈44?, but for electrodes C and D the diffraction peaks reveal the diffusive peaks. The results indicate that electrodes A and B are crystalline structure, electrodes C and D are amorphous structure. Electrodes A and B show one main broader peak, and seem to correspond to an alloy with a partially-amorphous structure or with a crystalline structure of nanometric crystalline site[15]. According to Scherrer’s formula, the grain size of electrode A is 7.5 nm, and that of electrode B is 3.5 nm.
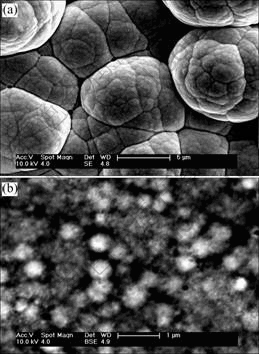
Fig.1 SEM morphologies of surface of Ni-Fe-Mo-Co alloy electrodes at different concentrations of sodium molybdate: (a) (Na2MoO4·2H2O)=5 g/L; (b) (Na2MoO4·2H2O)=10 g/L
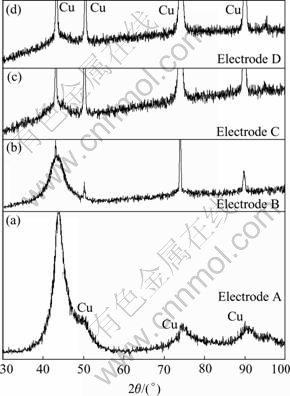
Fig.2 XRD patterns of Ni-Fe-Mo-Co alloy electrodes at different concentrations of sodium molybdate
3.2 Electrocatalytic activity of alloy electrodes
Fig.3 shows the polarization curves for HER on the different electrodes in 30% KOH(mass fraction) solution at 30 ℃. As shown in Fig.3 and Table 1, it can be concluded that the electrode activity strongly increases with increasing the Mo content of the electrode at first. However, further increasing the Mo content of the electrode will cause the activity slightly drop. For a molybdate concentration in the bath equal to 10 g/L, the alloy electrode shows the maximum catalytic activity at the Mo content 23.52% (mole fraction). The electrode activity increases with increasing the Fe or Co content of the alloy. The electrode B which has a composition of Ni35.63Fe24.67Mo23.52Co16.18 exhibits the best activity compared with the other electrodes. It is clearly shown in Fig.3 and Table 1 that the catalytic activity of the electrode is strongly dependent on the atomic fraction of elements for the molybdenum-iron group alloy, the introduction of Co considerably improves the cathode performance in comparison with the other electrodes.
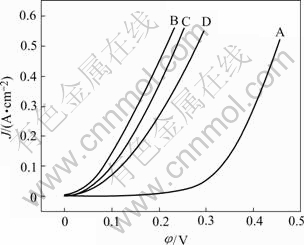
Fig.3 Polarization curves of Ni-Fe-Mo-Co alloy electrodes in 30% KOH solution
Fig.4 shows the polarization curves of electrode B for HER in 30% KOH solution at different temperatures.
The complete set of electrochemical parameters for the HER on electrode B in 30% KOH solution at different temperatures is presented in Table 2, where the value η200 (overpotential corresponding to a current density of 200 mA/cm2) is also included for a performance comparison in a region unaffected for ohmic drop. Both Fig.4 and Table 2 show that electrode B possesses very low overpotential and higher exchange current density in 30% KOH solution. The overpotential of electrode B is decreased with temperature increasing, while the exchange current density is increased. The Tafel slope and exchange current density increase with increasing temperature in low current density region. The Tafel slope drops with increasing temperature, but the exchange current density and transfer coefficient rise in high current density region.
Table 2 Kinetic parameters for HER on Ni-Fe-Mo-Co alloy electrode in 30% KOH at different temperatures
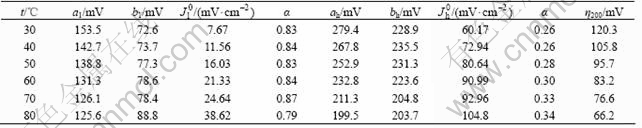
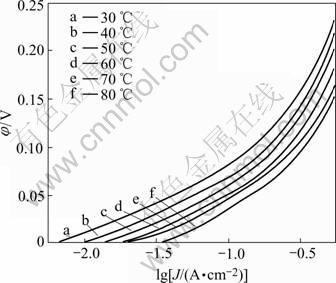
Fig.4. Polarization curves of Ni-Fe-Mo-Co alloy electrode in 30% KOH solution at different temperatures
As for the electrochemical activity of the electrodes on HER, electrode B shows an overpotential value of 66.2 mV for a current density of 200 mA/cm2 at 80℃.
3.3 Continuous electrolysis and interrupt current test
To assess the stability of the Ni-Fe-Mo-Co alloy electrode under continuous operation condition, a continuous electrolysis was carried out in 30% KOH solution at 60 ℃ with a constant current density of 200 mA/cm2 for 250 h. This was to simulate the conditions that the electrodes were under industrial use, which requires not only good durability, but also good stability against current interruption.
The electrode electrolytic operation was performed for 250 h in 30% KOH solution at 60 ℃, with system shut-down for 2 h at 100 h operation and for 4 h again after 175 h operation.
Fig.5 shows the electrode overpotential variations with time (without IR compensation) during continuous electrolysis and current interruption. It can be observed that the value of the overpotential varied for HER on electrode B at first stage is obviously larger than the values at the subsequent two stages, which is around 20 mV. The overpotential value varies within 5 mV range and tends to stabilize at later two stages. This displays that the Ni-Fe-Mo-Co alloy electrode has very good activity durability and stability even after 250 h of elec- trolysis.
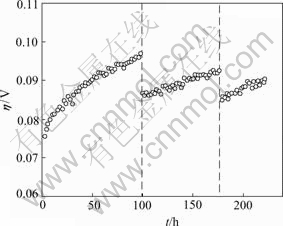
Fig.5 Overpotential variations for Ni-Fe-Mo-Co alloy electrodes with time during continuous electrolysis and current interrupts(J=200 mA/cm2, 30% KOH, 60 ℃)
The results show that the catalytic activity of the electrode gradually increases in the electrolytic process, so does the catalytic stability. The diagram also shows that the initial overpotential drops back to original level after its shut-down. This indicates that the electrode activity does not get reduced and the surface structure of the electrode is not damaged during the continuous and interrupted current test.
This phenomenon is probably caused by the overpotential variation, which is generated by some of the electrolytic resistance, the activity resumes when the resistance disappears after current interruption.
This conclusion is different and better compared with the results reported in Refs.[3,12,13,16]. The difference may be related with the surface structure of electrode.
3.4 Alteration of electrode composition and surface structure
The average compositions of the electrodes after electrolysis by EDS analysis are listed in Table 3. As shown in Table 1 and Table 3, the composition change of electrode A is smaller. But the change of electrode B is many times larger before and after electrolysis. Its Mo content decreases from 23.52% to 9.98%, and the Fe content from 24.67% to 18.01%, and the Ni content increases from 35.63% to 52.30%. Mo and Fe are dissolved and leached from the electrode during this process. These results confirmed our prediction.
Table 3 Chemical composition of Ni-Fe-Mo-Co alloy electrodes after electrolysis in 30% KOH (mole fraction, %)

Fig.6 shows the SEM micrographs of the surface morphology of electrodes A and B after electrolysis. The surface morphology has gone through an obvious change compared with Fig.1. Furthermore, there is a big difference of surface microstructure between Figs.6(a) and (b). In Fig.6(a), there is no cauliflower shape of particles on the surface any more. Its crystalline grain surface is dissolved. In Fig.6 (b), a net and pore hive-like structure is formed on the surface. The average pore size is around 50 nm. The formation of the unique structure is due to the leaching of Mo and Fe on the surface of electrode during electrode electrolysis.
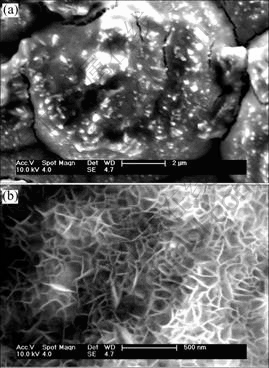
Fig.6 SEM images of surface morphology of Ni-Fe-Mo-Co alloy electrode after electrolysis: (a) (Na2MoO4·2H2O)=5 g/L; (b) (Na2MoO4·2H2O)=10 g/L
For leaching or electrolysis, the electrode surface morphology has considerably changed, for example, the sub-micrometric pore[12], porous structure[5] and rough/coarse surface[13], but the electrode surface structure at this study is very different compared with the other electrode materials. This net and pore hive-like structure has much larger real surface area and better structure stability than the other catalytic materials. Its surface structure is not easily damaged and its electrocatalytic activity maintains for prolonged electrolysis and intermittent current interruption tests. These results further confirm our previous conclusions about polarization curves, stability and durability test.
The surface structure difference between electrode A and electrode B is concerned with the disparity of atomic fraction in the alloy and the microcrystalline structure by electrodeposition.
4 Conclusions
1) The Ni-Fe-Mo-Co quaternary alloy is electro- deposited in citrate bath. A unique net and pore hive-like microstructure is obtained using a new method by which Mo and Fe are leached out from the electrode in 30% KOH solution. The average pore size of the structure is about 50 nm.
2) The alloy electrode has much larger real surface area and much higher stability than the electrodes reported in the literature.
3) The catalytic activity and the surface micro- structure of Ni-Fe-Mo-Co alloy electrode are strongly correlated with the mole fraction of alloy elements. The Ni35.63Fe24.67Mo23.52Co16.18 electrode has the best activity for HER and maintains good activity and stability under prolonged or current interruption tests. The Ni35.63 Fe24.67Mo23.52Co16.18 electrode has the highest electrochemical activity and highest catalytic stability among the molybdenum-iron alloy groups.
References
[1] ZOU Qun, LOU Tai-fang, WANG Yin-ping, WU Jun, HUANG Qing-an. Investigation of high catalytic activity electrodes for hydrogen evolution [J]. Materials Protection, 2002, 35(3): 11-14.(in Chinese)
[2] GAO Cheng-hui. Plating Amorphous alloy and property of deposition [M]. Beijing: Science Press, 2004. 376-398.(in Chinese)
[3] Ramesh L, Sheshadri B S, Mayanna S M. Electrolytic preparation and characterization of Ni-Mo-Fe alloys: cathode materials for alkaline water electrolysis [J]. International Journal of Energy Research, 1999, 23(10): 919-924.
[4] XIE Zhi-hua, QIU Yu-bing, WU Lou-tao. Preparation and characterization of a new type active cathode material [J]. Chinese Journal of Materials Research, 2003, 17(1): 83-86.(in Chinese)
[5] HU Wei-kang. Electrocatalytic properties of new electrocatalysts for hydrogen evolution in alkaline water electrolysis [J]. International Journal of Hydrogen Energy, 2000, 25(2): 111-118.
[6] MA Jie, LIU Shun-cheng, JIANG Lin-cai, JIANG Xiong. Effects of molybdenum content of nanocrystalline Ni-Mo alloy composite coating on hydrogen evolution reaction [J]. Acta Chemica Sinica, 1997, 55(4): 363-369.(in Chinese)
[7] CHANG Yu-chi, FAN Kuang-cheng, LIN Chun I. Structural and electrocatalytic characteristics of electrodeposited nickel- molybdenum alloys [J]. Journal of the Chinese Institute of Chemical Engineers, 2002, 33(5): 499-507.
[8] Toshio s, Hideyuki t, Eiichiro m, ATSUSHI M. Local atomic structure and catalytic activities in electrodeposited Mo-Ni alloys [J]. Materials Transactions, 2002, 43(7): 1525-1529.
[9] HAN Qing, CHEN Jian-she, LIU Kui-ren, LI Xin, WEI Xu-jun. Hydrogen evolution reaction of the electrodeposited amorphous Ni-S-Co alloy in alkaline medium [J]. Acta Metallurgica Sinica, 2004, 40(3): 331-336.(in Chinese)
[10] HU C C, WENG C Y. Hydrogen evolution activity on nickel-molybdenum deposits using experimental strategies [J]. Journal of Applied Electrochemistry, 2000, 30(4): 499-506.
[11] HUANG Ling, YANG Fang-zu, SUN Shi-gang, XU Shu-kai. Studies on structure and electrocatalytic hydrogen evolution of nanocrystalline Ni-Mo-Fe alloy electrodeposit electrodes [J]. Chinese Journal of Chemistry, 2003, 21(4): 382-386.
[12] BIRRY L, LASIA A. Studies of the hydrogen evolution reaction on Raney nickel-molybdenum electrodes [J]. Journal of Applied Electrochemistry, 2004, 32(1): 1-15.
[13] Crnkovic F C, Machado S A S, Avaca L A. Electrochemical and morphological studies of electrodeposited Ni-Fe-Mo-Zn alloys tailored for water electrolysis [J]. International Journal of Hydrogen Energy, 2004, 29(3): 249-254.
[14] HU Wei-kang, ZHANG Yun-shi, SONG De-ying, ZHOU Zuo-xiang, WANG Yun. Electrode properties of amorphous nickel-iron- molybdenum alloy as a hydrogen electrocatalyst in alkaline solution [J]. Materials Chemistry Physics, 1995, 41(2): 141-145.
[15] DOLATI A G, GHORBANI M, AFSHAR A. The electrodeposition of quaternary Fe-Cr-Ni-Mo alloys from the choride-complexing agents electrolyte (Part I. Processing) [J]. Surface and Coatings Technology, 2003, 166(2-3): 105-110.
[16] Arul R I, Vasu K I. Transition metal-based cathodes or hydrogen evolution in alkaline solution: electrocatalysis on nickel-based ternary electrolytic codeposits [J]. Journal of Applied Electro- chemistry, 1992, 22(5): 471-477.
Foundation item: Project(20374021) supported by the National Natural Science Foundation of China
Corresponding author: LUO Bei-ping; Tel: +86-13017226075; Fax: +86-730-8640122; E-mail: luobeiping 2006 @126.com
(Edited by YUAN Sai-qian)
Abstract: Ni-Fe-Mo-Co alloy electrode was prepared in a citrate solution by electrodeposition, and then Mo and Fe were partially leached out from the electrode in 30% KOH solution. The unique surface micromorphology of a hive-like structure was obtained with an average pore size of about 50 nm. The electrode has a very large real surface area and a stable structure. The effects of sodium molybdate concentration on the composition, surface morphology, and structure of electrodes were analyzed by EDS, SEM and XRD. The polarization curves of the different electrodes show that the catalytic activity of electrodes is strongly correlated with the mole fraction of alloy elements (Ni, Fe, Mo, Co), and the addition of cobalt element to Ni-Fe-Mo alloy improves the catalytic activity. The Ni35.63Fe24.67Mo23.52Co16.18 electrode has the best activity for hydrogen evolution reaction(HER), with an over-potential of 66.2 mV, in 30% KOH at 80 ℃ and 200 mA/cm2. The alloy maintains its good catalytic activity for HER during continuous or intermittent electrolysis. Its electrochemical activity and catalytic stability are much higher than the other iron-group with Mo alloy electrodes.


