
Improvement of surface corrosion resistance for magnesium alloy by combining thermal spray and cast-infiltration
ZHANG Zhong-li(张忠礼), DING Yong(丁 勇), WANG Xin(王 鑫),
YANG Guo-qiang(杨国强), SHEN Wei-wei(沈威威), HAN Hai-ling(韩海玲)
School of Materials Science and Engineering, Shenyang University of Technology, Shenyang 110870, China
Received 28 July 2008; accepted 22 January 2010
Abstract:
The present work focuses on a new method combining cast-infiltration with thermal spraying technology to improve the surface corrosion resistance of magnesium alloy. A zinc-based alloy layer was fabricated on the surface of AZ91D magnesium alloy. The microstructure of the layer was characterized by scanning electron microscopy equipped with an energy dispersive X-ray spectroscopy (EDS). The phase constituent of these alloys was identified by X-ray diffractometry (XRD). The analysis results reveal that a zinc-based alloy layer with a thickness of 700 μm can form on the surface of AZ91 alloy matrix. The layer is composed of Mg7Zn3, MgZn and a small amount of α-Mg solid solution. The results indicate that the corrosion-resistance of the specimen with a zinc-based alloy layer is much better than that of the specimen without the layer after being immersed in 5% NaCl solution for 240 h, and the layer is more protective for the AZ91 alloy.
Key words:
arc spraying; cast-infiltration; magnesium alloy; corrosion resistance;
1 Introduction
Magnesium is the lightest metal used for engineering structure. It is one of the most abundant elements on the earth, and has such advantages as low density, high strength, good machining ability and casting ability as well as easy recycling. Thus, magnesium alloys are increasingly used in many fields including automobile and computer parts, aerospace components, mobile phones, sporting goods, handheld tools and household equipments[1-2]. However, their applications are limited due to low corrosion resistance. Compared with other structural metal materials, magnesium has the low electrochemical potential, and the oxide film formed on the surface is very loose and porous, so it cannot offer effective resistance to corrosion[3-4]. In the past decades, many researches concerning the surface modification were conducted in order to improve the corrosion-resistance of magnesium alloys[5-6]. Some researches reveal that the addition of enough aluminum or zinc can greatly enhance the corrosion resistance of magnesium alloys[7]. And the technologies of depositing either aluminum layer or zinc layer on the surface of magnesium alloys were developed [8-11].
The goal of the present study is to develop an approach to obtain a zinc-alloyed layer with good corrosion resistance on the surface of magnesium alloy. In this work, thermal spraying technology is combined with cast-infiltration technology. The cast-infiltration is actually a technology of obtaining the surface metal matrix composite layer which forms on the surface of metal casting through the reaction and diffusion between the poured molten metal and metal layer or metal powder piece previously covered on the casting mold wall. The process of cast-infiltration fully utilizes the remaining heat generated during casting, which not only saves energy and simplifies the procedures, but also realizes the surface alloying and leads to the gradient distribution of alloying elements in the castings[12]. In the present work, a zinc coating was sprayed on the inner surface of ceramic mold by thermal spraying. Then, the ceramic mold was filled with molten metal. A composite layer was obtained on the surface of the casting after solidification.
2 Experimental
2.1 Materials
AZ91D magnesium alloy ingot was employed in this study. The chemical composition of the alloy is given in Table 1.
Table 1 Chemical composition of AZ91D alloy (mass fraction, %)

The dimension of specimens for infiltration casting was 100 mm×100 mm×30 mm. The casting mold was made of alumina powder and silica sol binder which were firstly mixed and then cast into the required form and finally sintered at 900 ℃. Then, a zinc coating was sprayed on the inner surface of the mold. All coating samples were sprayed using an arc-spraying system. Pure zinc (99.9% purity) wires with a diameter of 3 mm were used in the arc-spraying process. The thickness of the coatings sprayed on the inner surface of the mold was in the range from 0.5 to 2 mm. The arc spraying parameters are listed in Table 2.
Table 2 Arc spraying parameters

2.2 Cast-infiltration process
The schematic diagram of cast-infiltration apparatus used in the present study is given in Fig.1. The AZ91D alloy was melted at 640 ℃ with mixed SF6 and N2 as protective atmosphere. A vacuum casting apparatus was employed. The cast-infiltration parameters are important factors to achieve an expected result because they greatly affect the Mg-Zn alloy layer, and thus are modified according to the experimental results. The selected parameters in the cast-infiltration process are listed in Table 3.

Fig.1 Schematic diagram of apparatus for cast-infiltration process
Table 3 Parameters for cast-infiltration process

2.3 Corrosion and electrochemical tests
The preparation of the specimens for immersion test was as follows. The specimens were cut into pieces with a size of 30 mm×20 mm×5 mm; all specimens were cleaned, successively polished with finer emery papers up to 1 000 grit; and then covered by a wax coating on the sides without zinc-based alloy layer; finally, the specimens were exposed in 5% NaCl solution for various times.
The electrochemical polarization experiments were carried out using an electrochemical measurement system. The applied electrodes were prepared by connecting a wire to one side of the specimen covered with cold setting resin. The opposite surface of the specimen was exposed to the solution. The exposed area was 1 cm2. The specimens were given a metallographic polishing prior to each experiment, and followed by washing with distilled water and acetone. The polarization measurements were carried out in a corrosion cell containing 150 mL of 5% NaCl solution using a standard three-electrode configuration, where the saturated calomel was used as reference and a platinum electrode as a counter. The specimens were immersed in the testing solution, and a polarization scan was conducted towards more noble values at a rate of 1 mV/s, after allowing a steady state potential to develop.
2.4 Microstructure analysis
The cross-section of the specimens was examined by optical microscopy, scanning electron microscopy (SEM) and energy-dispersive spectrometry (EDS) for analyzing the composition and thickness of the formed alloy layer. Moreover, X-ray diffraction (XRD) analysis was carried out to determine the phase constituent in the diffused layer. Two samples were employed for XRD analysis, the sampling position were 0.1 and 1.0 mm away from the surface, respectively.
3 Results and discussion
3.1 Microstructure and phase analyses
In the cast-infiltration process, the molten magnesium alloy is poured into the mold, the heat of which melts the zinc coating on the mold wall. Then, a zinc-based alloy layer forms on the casting surface after being cooled. Figs.2(a) and (b) respectively show the X-ray diffraction patterns of the two sampled positions. The XRD analysis results reveal that at the location of 1 mm from the surface, a normal AZ91D magnesium alloy microstructure is observed and it composes of α-Mg solid solution and minor β-Mg17Al12 phase (see Fig.2(a)). The pattern in Fig.2(b) shows that the top alloyed zone is composed of Mg7Zn3, MgZn and some α-Mg solid solution. The Mg7Zn3 and MgZn intermetallics are the main phases in the top layer, while the major peaks of α-Mg solid solution obviously decrease compared with those in Fig.2(a).

Fig.2 X-ray diffraction patterns of AZ91D (a) and cast- infiltration layer (b)
The transverse microstructures of the surface layer formed in the cast-infiltration process are shown in Fig.3. There are two areas with an obvious interface between them. The area shown in the lower part of the image is a normal AZ91D magnesium alloy microstructure, where the solid solution composes the matrix and some discontinuous intermetallic phases form at grain boundaries. The area shown in the upper part of the image is a zinc-based alloy layer which is characterized by microstructure composed of continuous matrix area (light gray) and leaf-shaped phase (dark gray). Through the chemical composition examination with EDS, it is known that the light gray phase as the matrix is composed of more zinc than the dark gray one. Moreover, the thickness of the zinc-based alloy layer is about 700 μm. Fig.3(b) shows the enlarged view of Fig.3(a), where the interface between zinc-based alloy layer and the substrate can be seen clearly.
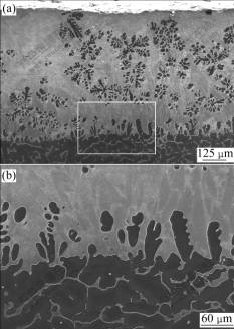
Fig.3 SEM images of layer formed in cast-infiltration process: (a) General cross sectional view; (b) Enlarged view
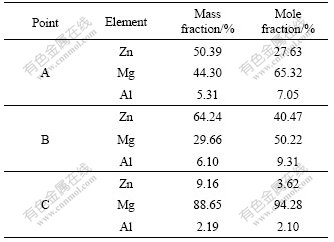
Fig.4 shows the SEM morphology of another zone in the zinc-based alloy layer. Three phases can be recognized clearly, a leaf-shaped phase (dark gray) and two different microstructural constituents in the matrix area (light gray). EDS analysis (shown in Table 4) and XRD patterns reveal that the light gray phase marked “A” is Mg7Zn3, another light gray phase marked “B” is MgZn and the dark phase marked “C” is α-Mg solid solution. The generation enthalpy of Mg-Zn alloy is negative through the whole ratio of Mg to Zn, which indicates that the compounds composed of Mg and Zn form easily and spontaneously. It was reported that there are five intermetallic compounds in Mg-Zn system, i.e., Mg7Zn3 (in-congruent melting at 340.9 ℃), MgZn (peritectic formation at 347 ℃), Mg2Zn3 (peritectic formation at 416 ℃), MgZn2 (congruent melting at 590 ℃) and Mg2Zn11 (peritectic formation at 381 ℃). At the eutectic transformation temperature, where L?Mg+ Mg7Zn3 (t=340 ℃), and the solubility of Zn in Mg is about 2.4%(mole fraction)[13]. Under the conditions used in this experiment, the diffusion of Zn and Mg at the temperatures above the melting point of zinc results in the formation of Mg-Zn alloy layer which is mainly composed of Mg7Zn3 and MgZn intermetallic phases. The intermetallic phases that distribute continuously at grain boundaries are resistant to corrosion, and thus impede the corrosion propagation[14]. Therefore, the zinc-based alloy layer of which the matrix consists of Mg7Zn3 and MgZn intermetallic phases, offers a good corrosion resistance.
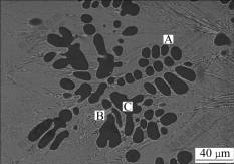
Fig.4 SEM image of zinc-alloyed layer
Table 4 EDS analysis of position in Fig.4
3.2 General corrosion and electrochemical behaviour
The exposing test results in 5% NaCl solution reveal that the specimens with a zinc-based alloy layer behave much better than the AZ91D alloy. Several corrosion pits appear on the surface of the AZ91D specimens, while there is no corrosion mark on that of the zinc-based alloy layer after being exposed for 24 h. With increasing the exposing time, more and more corrosion pits appear on the surface of the AZ91D alloy, and some pits finally are enlarged and connect with each other. Compared with the AZ91D alloy, there is no corrosion pit on the surface of the zinc-based alloy layer until the test is carried out for 240 h.
Fig.5 shows the potentiodynamic polarization curves for both AZ91D alloy and zinc-based alloy layer in 5% NaCl solution. It can be seen from the curves that the corrosion potential of the zinc-based alloy layer is 100 mV, higher than that of the AZ91D alloy, while the corrosion current is much less than that of the AZ91D alloy. This indicates that the corrosion resistance of the zinc-based alloy layer is much better than that of the AZ91D alloy.

Fig.5 Potentiodynamic polarization curves for AZ91D alloy and zinc-based alloy layer
The reason why magnesium or its alloy is easily corroded is that some impurities exist and result in the formation of galvanic corrosion couple in the system. In the environment with chloride ion, the pitting corrosion is the main corrosion mechanism[15]. It was reported[16] that with the addition of zinc, the strength and corrosion resistance of magnesium get reinforced. Addition of Zn element to Mg-Mn alloy stabilizes the passivation film, which mainly contributes to the low corrosion rate of magnesium alloy. However, when the Zn content is higher than 3%, the passivation film becomes unstable, which results in a relatively high corrosion rate[16]. However, with the addition of more zinc to the alloy, the major phases in the zinc-based alloy layer are intermetallic compounds, MgZn and Mg7Zn3, the potential of which is higher than that of magnesium matrix. Therefore, the continuous film on the surface of the Mg-Zn alloy is stable and dense. And thus, a good corrosion resistance to aggressive environment is attained.
4 Conclusions
1) It is possible to obtain a zinc-based alloy layer on the surface of the AZ91D alloy by combining cast-infiltration with thermal spraying technology.
2) The zinc-based alloy layer with a thickness of about 700 mm, is metallurgically bonded with the AZ91D substrate, and is composed of Mg7Zn3, MgZn and α-Mg solid solution.
3) The zinc-based alloy layer shows better corrosion resistance than the AZ91D alloy, and is more electrochemically cathodal than the AZ91D alloy in 5% NaCl solution.
References
[1] AGNEW S R. Wrought magnesium: A 21st century outlook [J]. JOM, 2004, 56(5): 20-21.
[2] KULEKCI M K. Magnesium and its alloys applications in automotive industry [J]. The International Journal of Advanced Manufacturing Technology, 2008, 39(9/10): 851-865.
[3] CHENG Ying-liang, QIN Ting-wei, WANG Hui-min, ZHANG Zhao. Comparison of corrosion behaviors of AZ31, AZ91, AM60 and ZK60 magnesium alloys [J]. Transactions of Nonferrous Metals Society of China, 2009, 19(3): 517-524.
[4] SINGH RAMAN R K. The role of microstructure in localized corrosion of magnesium alloys [J]. Metallurgical and Materials Transactions A, 2004, 35(8): 2525-2531.
[5] CHENG Y L, CHEN Z H, WANG H M. Progress in the research of corrosion and protection of magnesium alloys [J]. Materials for Mechanical Engineering, 2005, 29(5): 1-4.
[6] TSUBAKINO H, YAMAMOTO A, SUGAHARA K, FUKUMOTO S. Corrosion resistance in magnesium alloys and deposition coated magnesium alloy [J]. Materials Science Forum, 2003, 419/422: 915-920.
[7] ZHU Li-qun, LIU Hui-cong, LI Wei-ping, SONG Guang-ling. Zinc alloyed coating on AZ91D magnesium alloy [J]. Journal of Beijing University of Aeronautics and Astronautics, 2005, 31(1): 8-12. (in Chinese)
[8] SHIGEMATSU I, NAKAMURAL M, SAITOU N, SHIMOJIMA K. Surface treatment of AZ91D magnesium alloy by aluminum diffusion coating [J]. Journal of Materials Science Letters, 2000, 19: 473-475.
[9] MA You-ping, XU Ke-wei, PAN Xi-de. Effect of solid diffusion metallic cementation on properties of ZM5 magnesium alloy [J]. Rare Metal Materials and Engineering, 2005, 34(3): 433-435. (in Chinese)
[10] ZHANG Xin-ping, ZHAO Zheng-peng, WU Feng-ming, WANG You-le,WU Jie. Corrosion and wear resistance of AZ91D magnesium alloy with and without microarc oxidation coating in Hank’s solution [J]. Journal of Materials Science, 2007, 42(20): 8523-8528.
[11] WIELAGE B, PODLESAK H, GRUNDL T, WANK A. Interfacial microstructure of cold gas spraying coated Al and Mg alloy substrates [J]. Microchimica Acta, 2006, 152(1/2): 73-76.
[12] LI Zu-lai, JIANG Ye-hua, ZHOU Rong. Using cast-penetrating process to obtain metal matrix wear resistant surface composites [J]. Journal of Kunming University of Science and Technology (Science and Technology), 2003, 28(5): 56-59. (in Chinese)
[13] HUANG M L, LI HX, DING H, REN Y P, HAO S M. Isothermal section of Mg-Zn-La system in Mg rich corner at 350 ℃ [J]. Acta Metallurgica Sinica (English Letters), 2008, 21(5): 329-335.
[14] POPOV I, STAROSVETSKY D, SHECHTNAN D. Initial stages of corrosion within Mg-Zn-Y-Zr alloy in 1 g/L NaCl solution [J]. Journal of Materials Science, 2000, 35(1): 1-8.
[15] BARIL G, PEBERE N. The corrosion of pure magnesium in aerated and deaerated sodium sulphate solutions [J]. Corrosion Science, 2001, 43(3): 471-484.
[16] YIN Dong-song, ZHANG Er-lin, ZENG Song-yan. Effect of Zn on mechanical properties and corrosion properties of as-cast Mg-Mn alloy [J]. The Chinese Journal of Nonferrous Metals, 2008, 18(3): 388-393. (in Chinese)
Corresponding author: ZHANG Zhong-li; Tel: +86-24-25496301; E-mail: zhonglil@sina.com
DOI: 10.1016/S1003-6326(09)60247-8
(Edited by FANG Jing-hua)
Abstract: The present work focuses on a new method combining cast-infiltration with thermal spraying technology to improve the surface corrosion resistance of magnesium alloy. A zinc-based alloy layer was fabricated on the surface of AZ91D magnesium alloy. The microstructure of the layer was characterized by scanning electron microscopy equipped with an energy dispersive X-ray spectroscopy (EDS). The phase constituent of these alloys was identified by X-ray diffractometry (XRD). The analysis results reveal that a zinc-based alloy layer with a thickness of 700 μm can form on the surface of AZ91 alloy matrix. The layer is composed of Mg7Zn3, MgZn and a small amount of α-Mg solid solution. The results indicate that the corrosion-resistance of the specimen with a zinc-based alloy layer is much better than that of the specimen without the layer after being immersed in 5% NaCl solution for 240 h, and the layer is more protective for the AZ91 alloy.


