Trans. Nonferrous Met. Soc. China 23(2013) 3167-3172
Grain refinement mechanism of Al-5C master alloy in AZ31 magnesium alloy
Ai-min ZHANG, Hai HAO, Xing-guo ZHANG
School of Materials Science and Engineering, Dalian University of Technology, Dalian 116023, China
Received 22 October 2012; accepted 8 January 2013
Abstract:
Al-5%C master alloy was prepared by powder in situ synthesis process, and its effects on grain refinement of AZ31 alloy and refining mechanism were investigated. The results indicate that the Al-5C master alloy consists of α(Al) and Al4C3 phases, and the size distribution of Al4C3 particles is controlled by sintering time. The Al-5C master alloy can remarkably reduce the grain size of AZ31 alloy, which decreases with the increasing addition amount of Al-5C master alloy when the addition amount is below 2%. The refining mechanism is attributed to the formation of new compounds of Al-C-Mn particles by Al4C3 and Mn, which might act as nucleating substrates for α-Mg grain.
Key words:
AZ31 magnesium alloy; grain refinement; Al-5C master alloy; Al-C-Mn compounds;
1 Introduction
As the lightest metallic structure materials, magnesium alloys have been widely used in automotive and aerospace industries for saving mass. The commercial Mg-Al alloys, such as AZ31 and AZ91 alloys, are the most commonly used magnesium alloys in industries. They are suitable for structural components in airplanes and cars, such as cylinder heads, airbag housings, steering wheels and seat flames [1]. In order to broaden the application fields of Mg-Al alloys, it is essential to improve their low strength and poor formability. It is well known that the grain refinement is an important technique for improving the mechanical properties of strength and ductility. Zr is the most promising grain refiner for Mg alloys which do not contain Al; however, it cannot be used in Mg-Al alloys due to immediate reaction with Al forming Al3Zr phase [2]. It is significant to find grain refiners which can be used for Mg-Al alloys.
Many potential methods have been developed for refining Mg-Al grain including superheating, carbon inoculation, the agitation methods, adding melt agents (Al4C3, AlN, SiC, TiB2) and micro-alloying with solute elements (Sr, Ca, Ce, Y) [3-10]. Among these methods, the addition of carbon-containing agents (C2Cl6) is known to be the most effective method and offers practical advantages because of the low operating temperature and the less fading with a long-time holding [11]. However, the problem of this method is the releasing of harmful chloride gas during refining. As other powder refiner agents (such as Al4C3 and SiC), MgCO3 [12] and MnCO3 [13] also have the problem of un-wetting with magnesium alloys and releasing CO2 gas. Therefore, a reliable, efficient and convenient commercial grain refiner has to be developed. According to the successful experiences of grain refinement in aluminum alloy, adding master alloy is an important method for its convenient operation and less pollution.
There are few investigations on the preparation of Al-C master alloy due to the bad wettability between graphite and aluminum melt. PAN et al [14] prepared the Al-1C master alloy through putting a certain amount of carbon containing preforms into the aluminum melt, but the particle size of the resultant Al4C3 is far away from the optimal size proposed by QUESTED and GREER [15]. HAN et al [16] fabricated the Al-2.5C master alloy by powder metallurgy, but it seems that the reaction of Al and C is not sufficient and the lamellar resultant with a large size is located at the grain boundaries of α(Al). It is also hardly to see the morphology of the formation particles of Al4C3 clearly. Therefore, it is critical to control the particle size distribution of the resultant Al4C3. Besides, the grain refinement mechanisms of Al4C3 are still unclear. In addition, it is still unclear what are the ideal volume fraction, particle size distribution for a given alloy composition. Hence, in the present work Al-5C master alloy was prepared by powder in situ synthesis process and its effects on grain refinement of AZ31 magnesium alloy were investigated, and the refining mechanisms were also discussed.
2 Experimental
For preparation of the Al-5C refiner, the mixture of aluminum powders and graphite powders (mass ratio 19:1) was milled in a planetary ball mill for 5 h, and then the mixed powders were pressed into a cylindrical preform with a diameter of 69 mm at 400 °C for 30 min under the pressure of 100 MPa. Subsequently, the cylindrical preform was sintered at 1000 °C for a given duration in vacuum condition and cooled down to room temperature in the furnace. The XRD and SEM analyses were employed for the analysis of phase identification and microstructures of the Al-5C master alloys.
For grain refinement tests, commercially pure Mg (>99.9%), Al (>99.9%), Zn (>99.9%) and Mg-4.5Mn master alloy were used for preparing AZ31 alloy in a magnesia crucible with 1% protecting flux. The flux (RJ-2) consists of 35%-45% KCl, 5%-8% CaF2, 40%-50% MgCl2, and 5%-8% (NaCl+CaCl2). Al-5C master alloy was added into the melt at 770-780 °C, the amount of Al in the Al-5C master alloy was carefully taken into consideration in order to exactly control Al content in the AZ31 alloy melt. The melt was held for 30 min and then poured into a preheated graphite mold with dimensions of d55 mm×70 mm×120 mm. The pouring processing was under protection of CO2 and SF6 mixed gas. All the grain refinement tests were repeated three times.
All specimens for the optical microscopy and SEM microscopy were sectioned, cold-mounted, polished and then etched in a solution of picric and acetic acid (solution of 10 mL acetic acid, 10 mL H2O, 4.2 g picric acid and 70 mL ethanol). Macrostructures were observed by MEF-3 optical microscope. The tensile tests were carried out at an initial engineering strain rate of 1 mm/min at room temperature. The Brinell-hardness tests were conducted with a load 245 N and the load time of 30 s.
3 Results and discussion
3.1 Characteristics of Al-5C master alloy
Figure 1 shows the characteristics of Al-5C master alloy. It can be seen from Fig. 1(a) that there are a lot of tiny particles distributed uniformly in the master alloy. Figure 1(b) shows the XRD pattern of Al-5C master alloy, it can be identified that the master alloy is composed of α(Al) phase and Al4C3 phase. According to the calculated results of LIU [17], the Gibbs free energy (ΔG) of the following reaction
Al(l)+C(s)→Al4C3(s) (1)
is negative when the temperature is higher than 933 K. So, sintering at 1000 °C can satisfy the thermo- dynamics conditions of the above reaction.
Figure 2 shows the sintering time as a function of the particle size of the resultant Al4C3. From Fig. 2(a), sintering for 1 h, the particles can be observed clearly under high magnification by SEM, and the further analyses of EDS confirm that the tiny particles (Area 1 in Fig. 2(a)) are Al4C3 particles (see Fig. 2(c)) with a size of 1-5 μm. However, when the sintering time is prolonged to 3 h, it can be found that the particle size of Al4C3 phase increases evidently. As shown in Fig. 2(b), the black rod-like particles with an average size of 15 μm turned out, which was further identified as Al4C3 phase in Fig. 2(d). It is concluded that the particle size distribution of Al4C3 is controlled by the sintering time strongly. In order to acquire the ideal particle size distribution, the sintering time must be controlled by no more than 1 h.
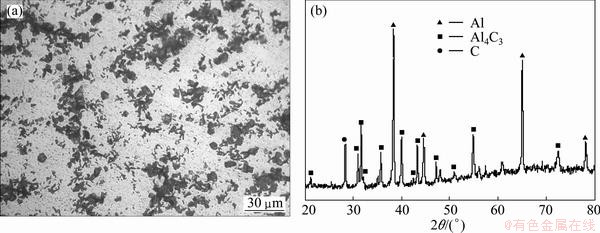
Fig. 1 Optical microstructure (a) and XRD pattern (b) of Al-5C master alloy

Fig. 2 SEM images (a, b) of Al-5C master alloy and corresponding EDS spectra of area 1 (c) and area 2 (d)

Fig. 3 Macrostructures of AZ31 alloys with different additions of Al-5C master alloy
3.2 Grain refinement of AZ31 by Al-5C master alloy
The macrostructures of as-cast AZ31 alloy with different adding amounts of Al-5C master alloy are presented in Fig. 3. When the adding amount of Al-5C master alloy is 0.5% (Fig. 3(b)), there is not apparent refinement of AZ31 alloy. With the addition amount increasing to 1% (Fig. 3(c)), the grain size of AZ31 alloy is remarkably refined compared with that observed in Fig. 3(a) for the base alloy, and the further grain refinement is attained as the adding amount of Al-5C master alloy is up to 2% (Fig. 3(d)). However, the grains coarsen again when adding 3% (Fig. 3(c)) Al-5C master alloy because of the aggregation of the redundant Al4C3 particles.
3.3 Mechanical properties
Table 1 lists the refinement effects of Al-5C on mechanical properties of AZ31 alloy. As can be seen from Table 1, the addition of Al-5C master alloy can improve the mechanical properties of the unrefined AZ31 alloy because of the grain refinement. Compared with the unrefined AZ31 alloy, the ultimate tensile strength, elongation and hardness of the AZ31 alloy refined by 2% Al-5C master alloy are improved from 136.9 MPa, 8.5% and HB 47.9 to 152.1 MPa, 8.7% and HB 50.7, respectively.
3.4 Grain refining mechanism
The most accepted refining mechanism of Mg-Al alloy by carbon inoculation is that Al4C3 or Al2OC particles can act as an excellent nucleating substrate for α-Mg phase [18-21]. In the present study, the notable grain refinement of the AZ31 alloy is obtained by adding 2% Al-5C master alloy, which should suppose to owe to Al4C3 particles in Al-5C master alloy. However, the Al4C3 or Al2OC particles have not been found in AZ31 alloy as expect. In recent years, it had been reported that carbon inoculation cannot refine the binary Mg-Al alloy containing more than 3% Al (no Mn element), but the commercial magnesium alloys such as AZ61 and AZ91 alloy (both containing about 0.3% Mn) can still be refined by carbon inoculation [14,22-24]. It is obviously inferred that Mn plays an important role in grain refinement of Mg-Al alloy inoculated by carbon bearing preforms. They found Al-C-O-Mn-Fe particles within α-Mg and proposed that it is the Al-C-O-Mn-Fe type particles responsible for the observed grain refinement rather than the Al4C3 or Al2OC type particles. However, they had the opposite opinion on the formation mechanism of the Al-C-O-Mn-Fe particles.
Table 1 Mechanical properties of AZ31 alloy treated by 2% Al-5C master alloy

LIU et al [22] assumed that the Al4C3 particles are absorbed on the surface of the Al-Mn-Fe-rich compounds and finally transformed into Al-C-O coating film. On the contrary, from the analysis results of the diffraction pattern, KIM et al [23] identified the polygonal structures of Al-C-O-Mn-Fe type particles as Al8(Mn, Fe)5 which nucleated on the surface of Al4C3, and proposed the duplex nucleation grain refining mechanism Al4C3→Al8(Mn, Fe)5→α-Mg.
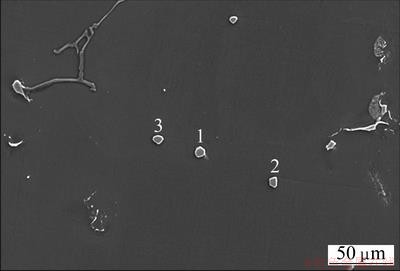
Fig. 4 SEM image of AZ31 alloy with addition of 2% Al-5C master alloy
Figure 4 shows the SEM image of alloy, from which we can see several polygonal particles with a size of about 5 μm within α-Mg grain. The EDS analysis results on the particles are shown in Fig. 5, and the element contents are also listed in Table 2. From Fig. 5 and Table 2, it can be seen that there are three main elements of Al, C, Mn and some tiny elements of O and Si in the three particles. The little oxygen element detected by EDS can be attributed to the slight oxidation of the sample during preparation [21]. As to silicon element, it is maybe introduced as impurities from Al-5C master alloy which contain certain of silicon. It is worthy to note that the carbon content in the three Al-C-Mn particles is much higher than that in the Al4C3 particles, from which it can be deduced that some of aluminum in the edge of Al4C3 particle maybe reacted with other elements. These observations are in good agreement with the current understanding of the grain refinement mechanism of Mg-Al alloys refined by Al4C3 proposed by NIMITYONGSKUL et al [24]. Therefore, it is reasonable to assume that the Al-C-Mn particle was formed by Al4C3 particle and manganese elements, and the Al4C3 particle rather than the Al-Mn particle is in the core of Al-C-Mn particle. It is also found that particles 2 and 3 do not contain iron elements, and the particle 1 contains only few iron, from which it is difficult to confirm whether iron element is necessary or not for grain refining of AZ31 inoculated with Al4C3. Further investigation should be carried out in the future.

Fig. 5 SEM images and EDS analysis results of particles shown in Fig. 4
Table 2 EDS results of particles in AZ31 alloy treated by 2% Al-5C master alloy

4 Conclusions
1) The Al-5C master alloy consists of α(Al) phase and Al4C3 phase, and the Al4C3 particle size distribution is controlled by the sintering time.
2) The addition of 2% Al-5C master alloy can successfully reduce the grain size of AZ31 alloy, the tensile strength, elongation and macro-hardness of AZ31 alloy are improved.
3) The refining mechanism of AZ31 alloy refined by Al-5C master alloy is attributed to the new Al-C-Mn particle acting as heterogeneous nuclei for primary α-Mg grain.
References
[1] KIM B H, PARK K C, PARK Y H, PARK I M. Microstructure and creep properties of Mg-4Al-2Sn-1(Ca,Sr) alloy [J]. Transactions of Nonferrous Metals Society of China, 2010, 20(7): 1184-1191.
[2] DAHLE A K, LEE Y C, NAVE M D, SCHAFFER P L, STJOHN D H. Development of the as-cast microstructure in magnesium- aluminium alloys [J]. Light Metals, 2001, 1: 61-72.
[3] DU Jun, YANG Jian, KUWABARA M, LI Wen-fang, PENG Ji-hua. Effect of strontium on the grain refining efficiency of Mg-3Al alloy refined by carbon inoculation [J]. Journal of Alloys and Compounds, 2009, 470: 228-232.
[4] FU H M, ZHANG M X, QIU D. Grain refinement by AlN particles in Mg-Al based alloys [J]. Journal of Alloys and Compounds, 2009, 478(1-2): 809-812.
[5] CHEN T J, JIANG X D, MA Y, LI Y D, HAO Y. Grain refinement of AZ91D magnesium alloy by SiC [J]. Journal of Alloys and Compounds, 2010, 496: 218-225.
[6] LIU Sheng-fa, ZHANG Yuan, HAN Hui, LI Bo. Effect of Mg-TiB2 master alloy on grain refinement of AZ91D magnesium alloy [J]. Journal of Alloys and Compounds, 2009, 487(1-2): 202-205.
[7] CHEN Gang, PENG Xiao-dong, FAN Pei-geng, XIE Wei-dong, WEI Qun-yi, MA Hong, YANG Yan. Effects of Sr and Y on microstructure and corrosion resistance of AZ31 magnesium alloy [J]. Transactions of Nonferrous Metals Society of China, 2011, 21: 725-731.
[8] WANG Feng, WANG Yue, MAO Ping-li, YU Bao-yi, GUO Quan-ying. Effects of combined addition of Y and Ca on microstructure and mechanical properties of die casting AZ91 alloy [J].Transactions of Nonferrous Metals Society of China, 2010, 20(S2): s311-s317.
[9] WANG Ming-xing, ZHOU Hong, WANG Lin. Effect of yttrium and cerium addition on microstructure and mechanical properties of AM50 magnesium alloy [J]. Journal of Rare Earths, 2007, 25: 233-237.
[10] YANG Ming-bo, PAN Fu-sheng, CHENG Ren-ju, TANG Ai-tao. Effects of Al-10Sr master alloys on grain refinement of AZ31 magnesium alloy [J]. Transactions of Nonferrous Metals Society of China, 2008, 18: 52-58.
[11] STJOHN D H, MA Q, MARK A E, CAO P. Grain refinement of magnesium alloys [J]. Metallurgical and Materials Transactions A, 2005, 36(7): 1669-1679.
[12] WANG Ling, KIM Young-Min, LEE Je-Hyun, YOU Bong-Sun. Effect of magnesium carbonate on microstructure and rolling behaviors of AZ31 alloy [J]. Materials Science and Engineering A, 2011, 528: 1485-1490.
[13] KIM Young-Min, WANG Ling, YOU Bong-Sun. Grain refinement of Mg-Al cast alloy by the addition of manganese carbonate [J]. Journal of Alloys and Compounds, 2010, 490: 695-699.
[14] PAN Yi-chuan, LIU Xiang-fa, YANG Hua. Role of C and Fe in grain refinement of an AZ63B magnesium alloy by Al-C master alloy [J]. Journal of Material Science and Technology, 2005, 21(6): 822-826.
[15] QUESTED T E, GREER A L. The effect of the size distribution of inoculant particles on as-cast grain size in aluminum alloys [J]. Acta Materialia, 2004, 52: 3859-3868.
[16] HAN Guang, LIU Xiang-fa, DING Hai-min. Grain refinement of Mg-Al based alloys by a new Al-C master alloy [J]. Journal of Alloys and Compounds, 2009, 467: 202-207.
[17] LIU Sheng-fa, LI Bo, HAN Hui, KANG Liu-gen, WANG Xiao-hu. Refinement effect and mechanism of Mg-Al4C3 master alloy in AZ91D Mg alloy [J]. The Chinese Journal of Nonferrous Metals, 2009, 19(1): 32-37. (in Chinese)
[18] MA Q, CAO P. Discussions of grain refinement of magnesium alloys by carbon inoculation [J]. Scripta Materialia,2005, 52(5): 415-419.
[19] LU L, DAHLE A K, STJOHN D H. Heterogeneous nucleation of Mg-Al alloys [J]. Scripta Materialia, 2006, 54: 2197-2201.
[20] CAO P, MA Q, STJOHN D H. Native grain refinement of magnesium alloys [J]. Scripta Materialia, 2005, 53: 841-844.
[21] LU L, DAHLE A K, STJOHN D H. Grain refinement efficiency and mechanism of aluminum carbide in Mg-Al alloys [J]. Scripta Materialia, 2005, 53: 517-522.
[22] LIU Sheng-fa, ZHANG Yuan, HAN Hui. Role of manganese on the grain refining efficiency of AZ91D magnesium alloy refined by Al4C3 [J]. Journal of Alloys and Compounds, 2010, 491: 325-329.
[23] KIM Young-Min, YIM Chang-Dong, YOU Bong-Sun. Grain refining mechanism in Mg-Al alloys with carbon addition [J]. Scripta Materialia, 2007, 57: 691-694.
[24] NIMITYONGSKUL S, JONES M, CHOI H. Grain refining mechanisms in Mg-Al alloys with Al4C3 microparticles [J]. Materials Science and Engineering A, 2010, 527: 2104-2111.
Al-5C中间合金对AZ31镁合金的细化机理
张爱民,郝 海,张兴国
大连理工大学 材料科学与工程学院,大连 116023
摘 要:采用粉末原位合成工艺制备Al-5C中间合金,研究Al-5C中间合金对AZ31镁合金晶粒细化的影响及细化机理。结果表明:Al-5C中间合金由α(Al) 和 Al4C3 两相组成,Al4C3 颗粒的尺寸分布由烧结时间控制。Al-5C中间合金能显著地细化AZ31镁合金晶粒尺寸,当Al-5C中间合金添加量低于2%时,随着Al-5C中间合金添加量的增加,AZ31镁合金晶粒尺寸减小。晶粒细化机理是由于 Al4C3 与 Mn 反应生成的Al-C-Mn 颗粒能作为初生α-Mg晶粒的异质形核基底,从而细化晶粒。
关键词:AZ31 镁合金;晶粒细化;Al-5C中间合金;Al-C-Mn化合物
(Edited by Xiang-qun LI)
Foundation item: Project (2011921065) supported by Liaoning BaiQianWan Talents Program, China; Project (DUT11ZD115) supported by the Fundamental Research Funds for the Central Universities, China
Corresponding author: Hai HAO; Tel/Fax: +86-411-84709458; E-mail: haohai@dlut.edu.cn
DOI: 10.1016/S1003-6326(13)62848-4
Abstract: Al-5%C master alloy was prepared by powder in situ synthesis process, and its effects on grain refinement of AZ31 alloy and refining mechanism were investigated. The results indicate that the Al-5C master alloy consists of α(Al) and Al4C3 phases, and the size distribution of Al4C3 particles is controlled by sintering time. The Al-5C master alloy can remarkably reduce the grain size of AZ31 alloy, which decreases with the increasing addition amount of Al-5C master alloy when the addition amount is below 2%. The refining mechanism is attributed to the formation of new compounds of Al-C-Mn particles by Al4C3 and Mn, which might act as nucleating substrates for α-Mg grain.


