网络首发时间: 2017-03-29 13:56
生物可降解锌基合金的研究进展
摘 要:
Zn的标准电极电位介于Mg和Fe之间, 同时Zn也是人体必需的微量元素。所以Zn及Zn合金作为新型的生物可降解金属材料最近几年受到了广泛关注。针对Zn及Zn合金应用于心血管支架及骨组织修复材料, 近年来在体外和体内生物相容性、降解速率进行了较多的研究。本文总结了近几年新型可降解生物Zn合金的研究结果, 在降解速率、生物相容性和力学性能等三方面概述了研究进展, 明晰目前存在的问题与不足, 并展望了生物可降解Zn合金的未来发展方向。
关键词:
中图分类号: R318.08;TG146.13
作者简介:王利卿 (1988-) , 男, 山西朔州人, 博士, 研究方向:生物可降解锌合金制备与性能研究;E-mailwlq881120@163.com;;秦高梧, 教授;电话:024-83691565;E-mail:qingw@smm.neu.edu.cn;
收稿日期:2017-02-23
基金:国家自然科学基金项目 (51371046, 51525101);中央高校基本科研业务 (N141008001) 资助;
Research Progress of Zn-Based Alloys as Biodegradable Materials
Wang Liqing Ren Yuping Qin Gaowu
Key Laboratory for Anisotropy and Texture of Materials (Ministry of Education) , School of Materials Science and Engineering, Northeastern University
Abstract:
Zinc ( Zn) is an essential trace element in human body, whose standard electrode potential is between that of Mg and Fe.Therefore, Zn and Zn-based alloys have been developed as a new class of metallic biodegradable materials in the last few years. Based on the recent results on their biodegradable rates and biocompatibility in vitro or in vivo, Zn and Zn-based alloys have been considered as potential cardiovascular stents or bone tissue repair materials. This review paper summarized the research results and development of biodegradable Zn-based alloys in recent years, mainly including degradation behaviors, biocompatibility and mechanical properties.Meanwhile, in view of the existing problems and shortcomings, the future research directions on this topic were proposed.
Keyword:
Zn-based alloys; biodegradable materials; biocompatibility; mechanical property;
Received: 2017-02-23
金属基生物材料以其良好的力学性能和加工性能, 而成为临床应用最广泛的植入材料。目前金属类植入材料包括钛合金、不锈钢以及Co-Cr-Mo合金等[1,2,3]。这些材料都具有良好的耐蚀性, 在体内能够长时间保持结构及性能稳定。但是, 作为短期植入器件, 上述生物材料需要进行二次手术取出[4,5]。为此, 人们提出可降解生物材料, 希望能够在治疗期间发挥其特定的功能, 并在生理环境中能够逐渐腐蚀降解并在达到治疗和修复组织与器官等效果之后完全降解, 而且材料本身及其降解产物能够被人体吸收或排出体外, 不会给人体带来其他伤害。目前可降解金属材料设计与开发主要是针对心血管支架、骨植入与内固定器械以及肠胃吻合器等而开展。上述植入器械在应用过程中需要同时具有适宜的降解速率、良好生物相容性和力学性能, 在维持足够的支撑或固定作用的同时兼顾材料降解速率与组织器官生长过程。因此, 从材料设计的角度而言, 需要综合考虑材料的功能性与结构性。
作为金属基可降解材料, Mg, Fe及其合金已进行了大量研究。然而, 两类材料存在明显的不足之处, 即Mg合金在人体环境中降解速率过快和降解不均匀性, 导致力学性能迅速下降无法提供足够的支撑或固定能力, 同时会生成大量气体并引起局部环境p H值升高[6,7];而Fe合金则由于降解产物具有良好的保护作用, 使其降解速率非常慢, 从而引起与不可降解材料相似的问题[8]。为此, 研究者们尝试了大量方法试图改善Mg, Fe及其合金降解速率方面的问题, 例如通过合金化设计具有高耐蚀性的Mg合金[9,10]、通过表面改性技术在Mg合金表面制备可降解的涂层[11,12]、通过合金化与热处理工艺调控Fe基合金微观组织以加速其降解速率等[13,14]。
近年来, 由于Zn的标准电极电位 (-0.763 V) 处于Mg (-2.372 V) 与Fe (-0.447 V) 之间[15], 因此, 从耐蚀性的角度, Zn及Zn合金有望表现出适宜的降解速率。其次, Zn参与人体细胞发育生长、基因表达、免疫系统和神经系统等大量生理反应过程[16,17,18]。Zn在正常成年人体内总量可以达到2 g左右[19], 每天摄入量在5~20 mg之间。因此, Zn及Zn合金作为生物可降解材料引起了广大研究者的极大兴趣。值得注意的是, 尽管过量的Zn2+可以通过肾脏排出体外, 但是长期过量摄入Zn2+则会对人体肾、肝、脾、大脑、心脏等重要器官产生损伤[20,21]。所以, 在可降解Zn基合金开发过程中应当对生物相容性和降解速率给予足够的关注与评价。同时, 鉴于纯Zn的力学性能差, 近年来以Mg, Ca, Sr, Mn, Cu, Fe, Li以及RE作为合金元素通过挤压和轧制的方法开发了新型可降解Zn基合金[22,23,24,25,26,27,28,29,30,31,32,33,34,35,36,37,38,39,40], 并对相关性能进行了调控与评价。因此, 本文将从生物相容性、降解速率与力学性能的评价及调控方面总结了国内外可降解Zn基合金的研究进展, 并对该类合金的未来发展方向进行了展望。
1 生物可降解Zn基合金的降解速率及机制
Zn相对于标准氢电极的电位为-0.763 V, 故Zn在溶液中具有逐渐腐蚀溶解的倾向, 这使其作为可降解材料而受到生物材料工作者的关注。但是, 作为可降解材料, 更重要的是要关注其降解速率的大小。理想的生物可降解材料需要具有适宜的降解速率和均匀的降解过程, 使材料的降解过程与植入后相邻人体组织的恢复过程相匹配。
可降解材料降解速率可以通过体外模拟生理环境来测定, 也可以植入到动物体内测量。体外测量降解速率通常是在模拟人体体液中浸泡一段时间根据质量或体积变化计算, 或者是通过电化学工作站测定合金极化曲线根据自腐蚀电流密度计算。为保证植入器械发挥其功能, 可降解材料的降解速率应维持在合理的范围内。骨修复器械通常要求能够维持3~6个月的服役期, 那么其体外降解速率应小于0.5 mm·a-1[41];而作为心血管支架材料则需要维持3~6个月力学性能完整性, 并在1~2年内完全降解, 据此, 降解速率应该不超过0.02 mm·a-1[22]。表1总结了目前不同方法获得的生物Zn基合金在体内外降解速率的结果。可见, 不同Zn及Zn合金通过浸泡实验得到降解速率都非常接近, 基本维持在0.1 mm·a-1左右;而电化学测量数据则略高于浸泡测量数据, 且不同合金之间相差不大。这意味着不同合金元素以及含量对Zn基合金耐蚀性影响较小, 其体外降解速率均维持在0.3 mm·a-1以下。同时, Zn及Zn合金体外降解产物多为含Ca, Zn的磷酸盐, 并推测其为 (Ca, Zn) 3 (PO4) 2 (H2O) 4化合物。
另一方面, 体内降解速率由于植入部位不同导致相互之间存在较大差距。Bowen等[22]将纯Zn丝植入到老鼠血管内, 其降解速率由植入后45 d的0.012 mm·a-1增加到180 d的0.05 mm·a-1。通过对植入的Zn丝形貌的观察, 发现Zn呈均匀降解的特点, 其降解产物主要是Zn O和含Zn的碳酸盐。Li等[26]将Zn合金植入到老鼠股骨髓腔内, 经过0~8周的观察, 通过μ-CT结果计算其降解速率随植入时间变化分别为0.17, 0.19和0.22 mm·a-1。而大部分生物Mg合金的降解速率集中在0.2~1.5 mm·a-1之间[19], 明显高于表1中Zn及Zn合金降解速率。因此, 现有的Zn合金体内、体外降解速率测试结果表明, Zn合金能够满足骨修复植入材料的要求。而作为心血管支架材料, Zn合金的降解速率略高于0.02 mm·a-1[22]。虽然在植入初期尚能满足要求, 但是随着植入时间延长, 能否保持足够的性能并保持均匀降解仍需要进一步系统的研究。
对Zn及Zn合金体外降解过程及机制也进行了深入研究。根据Zn在体外降解过程的研究结果
表1 可降解Zn基合金在体内、体外降解速率Table 1 In vitro and in vivo degradation rates of biodegradable Zn-based alloys 下载原图
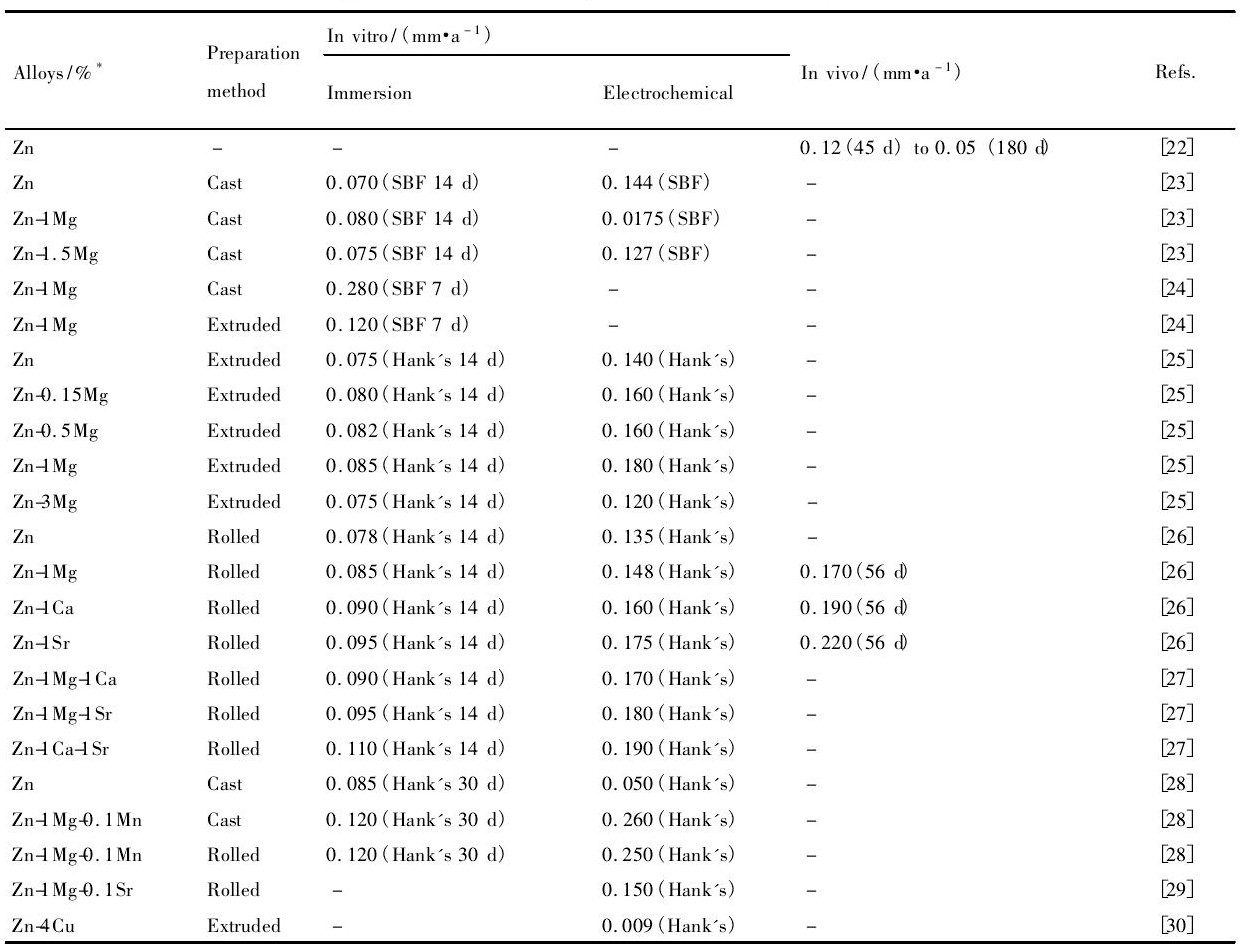
*:Unit of alloys content being mass fraction, except the other explaining
表1 可降解Zn基合金在体内、体外降解速率Table 1 In vitro and in vivo degradation rates of biodegradable Zn-based alloys
显示, Zn在降解过程中不产生氢气, 其反应过程表示为[31]:
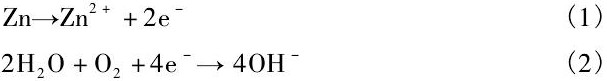
反应生成的Zn2+与OH-反应生成不溶于水的Zn (OH) 2:
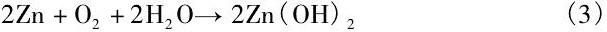
而溶液中Cl-离子则会使不溶于水的Zn (OH) 2溶解:
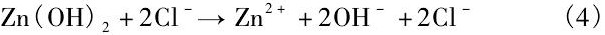
并最终与溶液中HPO42-, Na+或K+反应生成不溶于水的磷酸盐
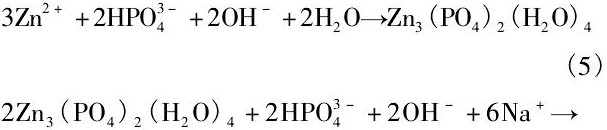

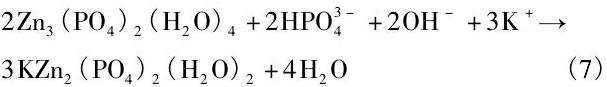
Chen等[31]指出, 在降解初期, Zn合金基体遵循方程 (1) ~ (3) 的反应过程, 呈现出均匀降解特征。同时降解产物Zn (OH) 2表现出良好的保护作用, 使得降解过程逐渐减慢。但随着浸泡时间延长, Cl-与Zn (OH) 2之间的反应 (4) 逐渐增强, 破坏了表面的保护膜导致局部腐蚀的发生。这意味着从长期植入降解的角度而言, Zn合金同样存在着局部降解的可能性。而且在体内降解过程中还应该考虑到体内氧含量、细胞、蛋白质及其他各种生物分子的粘附对降解速率和行为的影响, 因此, 生物Zn合金在生物体内的降解速率和降解行为仍需重点关注。
2 生物可降解Zn基合金的生物相容性
Zn是人体必需的微量元素, 尽管这可以作为Zn合金具有良好生物相容性的依据, 但作为植入器件, 仍需考虑Zn对其周围细胞、血液、组织、器官等的影响。
作为潜在的心血管支架用Zn及Zn合金与血管接触可能会对周围细胞、血液产生不同的影响, 所以相关的细胞毒性试验与溶血试验成为主要的评价内容。Schaffer等[42]研究表明, 人体动脉内皮细胞与平滑肌细胞在Zn2+浓度达到340和330μmol·L-1时保持50%的活性。Ma等[43]发现, 当Zn2+浓度为20μmol·L-1时能够促进冠状动脉内皮细胞增殖, 而当浓度为100μm时能够抑制其增殖。Shearier等[44]对比人血管内皮细胞、人动脉平滑肌细胞、人皮肤成纤维细胞活性达到50%时对应的Zn2+浓度分别为265, 70, 50μmol·L-1;但是当这些细胞直接接触Zn合金表面进行培养时, 初期细胞能够很好的附着, 但是很快就出现凋亡, 而对Zn表面进过一定的表面处理之后细胞可以实现很好的生长。Bowen等[45]将纯Zn丝植入到老鼠血管内, 结果表明Zn以及Zn降解产物不会引起炎症反应, 同时能够抑制平滑肌细胞增殖、抑制血管再狭窄, 实验过程中动物并未表现出全身或局部的毒性反应。同时, 对Zn及不同Zn基合金的溶血试验则表明其溶血率均低于5%, 血小板很好地吸附在Zn合金表面, 说明Zn及Zn合金不会导致血栓形成, 具有良好的血液相容性[27,28,29]。
除了作为心血管支架材料, Zn及合金作为骨固定材料也受到了同样的关注。Murni等[32]指出, 在Zn-3Mg合金浸提液 (Zn2+浓度约8μmol·L-1) 中人原代成骨细胞活性降低到30%~50%;同时没有检测到常见的炎症标志物, 说明Zn2+具有明显的抵抗炎症的作用。Kubasek等[33]研究表明, Zn2+对老鼠成纤维细胞和人骨肉瘤细胞最大安全浓度为80和120μmol·L-1;Zn-0.8Mg合金浸提液对这两种细胞并未表现出基因毒性。Li等[26]在纯Zn和Zn-1X (X=Mg, Ca, Sr) 合金浸提液中测试了Zn2+浓度对人成骨肉瘤细胞 (MG63) 、人脐静脉内皮细胞 (ECV304) 、鼠血管平滑肌细胞 (VSMC) 3种细胞活性的影响。结果表明, 上述合金元素添加能够提高ECV304细胞的活性, 降低MG63细胞的活性, 而对VSMC细胞无显著影响, 3种细胞活性均保持在60%以上。3种细胞在纯Zn及Zn合金表面直接培养则发现:3种细胞均不能在纯Zn表面正常生长;ECV304和MG63细胞则可以在Zn-1X合金表面正常生长, 而VSMC无法在Zn-1X合金表面粘附、生长。同时, 将上述Zn-1X合金植入到老鼠股骨髓腔内, 经过1~8周观察结果表明Zn-1X合金具有促进新骨形成的作用[26]。
上述研究结果说明, 不同细胞类型对于Zn2+的活性反应不同。随着Zn2+浓度增加, 细胞的活性均不同程度减小。这就意味着Zn及Zn基合金作为植入材料, 对其降解速率的控制是至关重要的。尽管现有的动物植入实验结果表明, Zn及Zn合金植入动物体内并未显示出局部或全身毒性反应[26,45]。但是, Zn及Zn合金降解过程中会导致局部Zn2+浓度增加, 如此Zn合金植入材料是否存在对病变组织以及正常组织产生不利影响的风险, 还需要在未来做进一步的研究。通过表面处理来改善Zn合金表面状态或者降解速率, 可能会消除此种潜在的风险。然而到目前为止, Zn及Zn基合金植入动物体不同部位后生物相容性相关的试验数据仍然是相当匮乏的。所以, 亟待进行动物植入试验, 以对Zn及Zn合金的生物相容性进行深入系统地评价。
3 Zn基生物合金的力学性能
植入器械, 例如骨钉、骨板、心血管支架以及吻合器等, 植入人体后均需要起到一定的固定或支撑的作用, 因此作为植入材料均需要具有足够的力学性能。鉴于植入器械不同的服役条件, 其对植入材料的力学性能要求也不同。例如, 作为可降解骨固定材料其室温屈服强度大于200 MPa, 延伸率大于15%[41];而作为心血管支架其力学性能要满足屈服强度大于200 MPa, 抗拉强度大于300MPa, 延伸率大于15%[22]。
铸态纯Zn的强度和塑性均很低, 很难满足植入器械对力学性能的基本要求, 合金化或变形处理成为首选的解决方案。合金元素的选择需要考虑元素的生物安全性, 通常选择人体自身含有的金属元素或者对人体无毒副作用的元素, 同时也可以参考可降解生物Mg合金常用的合金化元素。因此, Mg, Ca, Sr, Mn等元素成为了可降解Zn基合金重点研究对象。表2列出了目前不同制备方法所获得Zn基合金的力学性能。尽管铸态Zn基合金强度较纯Zn显著提高, 但延伸率仍然与纯Zn接近, 不超过3%。经过变形处理之后的Zn基合金, 其力学性能均具有显著地提高。挤压态Zn-0.8Mg合金的屈服强度、抗拉强度和延伸率分别为
203, 301 MPa和15%[33]。轧制态Zn-1Mg-0.1Mn和Zn-1Mg-0.1Sr合金的屈服强度、抗拉强度和延伸率分别达到195, 300 MPa和22%或以上[28,29]。挤压态Zn-4Cu[30]合金屈服强度、抗拉强度和延伸率分别为250, 270 MPa和51%。同时, Zn- (0~3%) Mg二元合金经过14 d浸泡后合金的压缩屈服强度减小了8%~20%, 低于同条件下AZ31镁合金的45%[25]。这意味着随着降解过程的进行, Zn基合金的力学性能仍能保持较高的完整性。此外, Zn-5Mg-1Fe管材经过拉拔后屈服强度可以达到176~187 MPa, 延伸率可以达到22%~26%[34]。
表2 生物可降解Zn基合金力学性能Table 2 Mechanical properties of biodegradable Zn-based alloys 下载原图
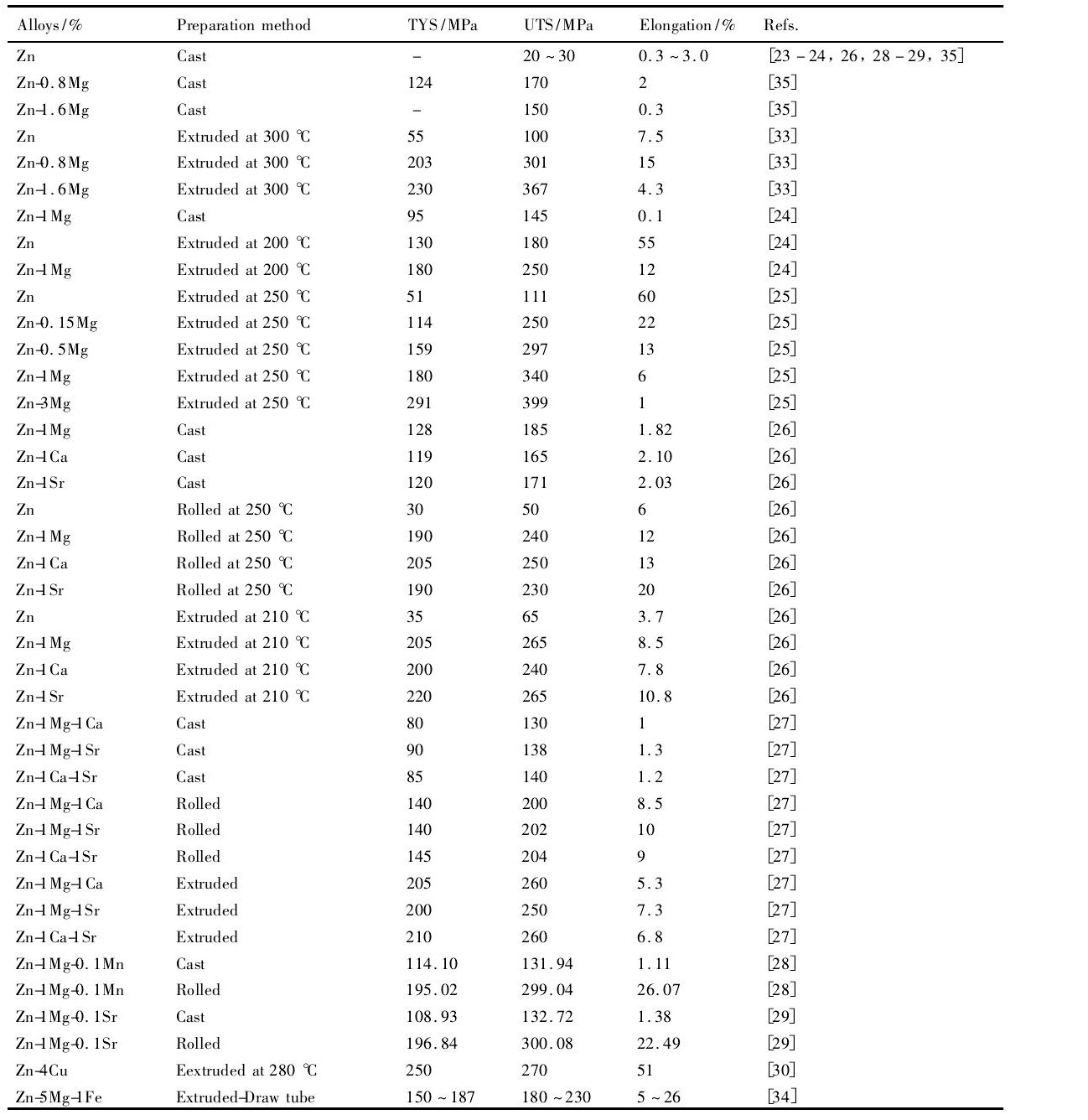
表2 生物可降解Zn基合金力学性能Table 2 Mechanical properties of biodegradable Zn-based alloys
由于强度和塑性没有良好的匹配, 上述开发的可降解Zn合金很难满足骨修复植入器件和心血管支架对力学性能的要求。值得注意的是, 合金化为改善金属材料力学性能的基本手段, 微量Mn和Sr的添加, 可以显著地提高变形态Zn-1Mg的综合力学性能[28,29]。同时, Cu的合金化效果显著地要好于其他元素的作用 (表2) 。另一方面, 变形后的组织得到细化, 使得综合力学性能明显优于铸态锌合金 (表2) 。然而, 由于当前对Zn合金的研究主要关注其降解行为和生物相容性, 对其力学性能改善的组织机制并没有引起研究者的广泛关注。例如, Zn-1Mg合金在200~250℃进行挤压时, 其力学性能明显地不同, 但在现有研究结果中并没有对此进行系统地分析。因此, 为更好地理解锌合金中成分-组织-工艺-力学性能的关系, 需要系统地研究合金化元素及成形过程中锌合金的组织演化机制, 从而为制备出满足医用植入锌合金材料的成分及工艺优化提供依据。
4 总结与展望
Zn合金是继Mg, Fe合金之后又一种新型潜在的生物可降解材料。相比于镁合金, 锌合金的降解速率明显降低, 且随着降解过程, 其力学性能衰减较小。然而, 作为一个服役期较长的植入物, 应该还要关注其在生理环境下的疲劳性能。同时, 在生物相容性方面, Zn2+浓度对不同细胞的影响结果存在一个临近值。超过此阀值, 其对细胞会产生毒性。因此对锌及锌合金在体内植入时的生物相容性仍需要进行全面、深入和系统的研究。另一方面, 为满足植入器件对其力学性能的要求, 合金化与变形改善锌合金力学性能的组织演化机制研究也是一项紧迫而必要的任务。同时, 需要关注锌合金不同的微观组织对降解速率的影响。
参考文献
[17] Rink L.Zinc in Human Health[M].Amsterdam:IOS Press, 2011.195.
[20] Nriagu J.Zinc toxicity in humans[J].Encyclopedia of Environmental Health, 2011, 20 (4) :801.
[21] Fosmire G J.Zinc toxicity[J].American Journal of Clinical Nutrition, 1990, 51 (2) :225.


