文章编号:1004-0609(2013)07-1805-07
Al对Mg-Cu-Y合金非晶形成能力及热稳定性的影响
宁向梅1, 2,黄金亮1,贾淑果1,殷 镖1,张兴渊1
(1. 河南科技大学 材料科学与工程学院,洛阳 471023;
2. 河南科技大学 河南省有色金属材料科学与加工技术重点实验室,洛阳 471023)
摘 要:
采用单辊快速凝固法制备出Mg80-xAlxCu15Y5(x=0,1)合金薄带,再将薄带在不同温度下进行退火处理,利用XRD、DSC和HRTEM分析Al元素对Mg80-xAlxCu15Y5(x=0,1)合金薄带的非晶形成能力及热稳定性的影响。结果表明:Al的加入使得Mg-Cu-Y合金的玻璃转变温度和初始结晶温度升高,过冷液相区宽度ΔTx因Al部分置换Mg而增大,约化玻璃转变温度Trg从0.616升至0.631,合金的非晶形成能力及热稳定性提高。随着退火温度的升高,Mg79Al1Cu15Y5合金的晶化率低于Mg80Cu15Y5合金的。当退火温度为523 K时Mg80-xAlxCu15Y5(x=0,1)合金均发生明显晶化,在非晶基体上弥散析出大量HCP-Mg和Mg2Cu纳米颗粒;当温度升高至573 K时,Mg79Al1Cu15Y5合金中有针状AlMg化合物相形成。
关键词:
中图分类号:TG146.2 文献标志码:A
Effect of Al on glass forming ability and thermal stability of Mg-Cu-Y alloys
NING Xiang-mei1, 2, HUANG Jin-liang1, JIA Shu-guo1, YIN Biao1, ZHANG Xing-yuan1
(1. School of Materials Science and Engineering, Henan University of Science and Technology, Luoyang 471023, China;
2. Henan Key Laboratory of Advanced Non-ferrous Metals, Henan University of Science and Technology, Luoyang 471023, China)
Abstract: Mg80-xAlxCu15Y5(x=0,1) alloy ribbons were prepared by single-roll rapidly solidification method. Then the samples were annealed at various temperatures. The effects of Al on the glass forming ability (GFA) and thermal stability of Mg80-xAlxCu15Y5(x=0,1) alloys were studied by XRD, DSC and HRTEM. The results show that Al addition enhances the glass transition temperature and the onset temperature of crystallization of Mg-Cu-Y alloy. The width of supercooled liquid region ΔTx is found to become larger with partial substitution of Mg by Al, and the reduced glass transition temperature Trg increases slightly from 0.616 to 0.631, which result in the improvement of the glass forming ability and thermal stability. With the increase of annealing temperature, the crystallization degree of Mg79Al1Cu15Y5 alloy is lower than that of Mg80Cu15Y5 alloy. The remarkable crystallization behavior of Mg80-xAlxCu15Y5(x=0,1) alloys occurs after being annealed at 523 K, while the dispersed precipitation of HCP-Mg and Mg2Cu nanoparticles takes place in the amorphous phase matrix. The acicular-shaped AlMg compound phase is formed in Mg79Al1Cu15Y5 alloy annealed up to 573 K.
Key words: Mg-based amorphous alloys; glass forming ability; thermal stability; crystallization
自1960年DUWEZ等[1]采用快速凝固的方法成功制备出Au-Si非晶合金以来,非晶合金以其独特的性能迅速成为材料学界以及物理学界研究的热点。在过去的几十年里,人们相继开发出了Zr基、Ti基、Fe基、Co基、Pd基、Al基和Mg基等[2-7]非晶合金系。与其他非晶合金系相比,镁基非晶合金具有低密度、高强度、高弹性极限和储量丰富等优点,因此,作为一种极具潜力的轻质高比强度绿色环保工程结构材料,镁基非晶合金具有广阔的应用前景。
近20年来,镁基非晶合金的研究主要集中在Mg-Cu、Mg-Zn、Mg-Ca和Mg-Ni等二元系合金以及Mg-TM(过渡族元素)-Ln(稀土元素)三元系合金[8-11],其中的Mg-Cu-Y系三元合金更是以其较强的非晶形成能力受到研究者的广泛关注。在Mg-Cu-Y三元合金的基础上,采用元素替代法,即用Si[12-13]、Co[14]、Ag[15]、Zn[16]、 B[17] 和Ti[18]部分替代Cu;用Nd[19-20]、Nb[21]部分替代Y元素,或同时加入Ni+Nd[22]部分替代Cu和Y、Ag+Pd[19]部分替代Cu,发展了四元甚至五元合金。研究表明,通过加入第四及第五元素,利用合金多组元之间的交互作用不仅可以使Mg-Cu-Y系合金保持较高的非晶形成能力,还可以改善其热稳定性及力学性能。由此可见,采用元素替代法发展新型的多元Mg-Cu-Y系非晶合金将成为镁基非晶合金研究的重要方向之一。
目前,关于Al的加入对Mg-Cu-Y系合金非晶形成能力及热稳定性的影响研究甚少,尤其是对合金显微组织的影响未见报道,因此,本文作者在Mg80Cu15Y5非晶合金的基础上,选择Al部分替换Mg,形成Mg79Al1Cu15Y5四元合金系,采用单辊甩带法制备Mg80-xAlxCu15Y5(x=0,1)非晶合金薄带,并对其进行热处理,利用XRD、DSC及HRTEM分析探讨添加Al对Mg-Cu-Y非晶合金的非晶形成能力及晶化行为的影响,从而为今后采用快速凝固粉末冶金技术制备大尺寸的非晶合金及纳米晶/非晶复合材料提供基础。
1 实验
选择纯度高于99.9%(质量分数)的市售块状Mg、Cu、Y、Al纯金属为原料,按Mg80-xAlxCu15Y5(x=0,1)配比在高纯的流动氩气保护下经真空感应熔炼得到设计成分(摩尔分数)的母合金锭。采用快速凝固单辊甩带法制取镁合金薄带。具体步骤是将母合金锭破碎后放入下端带有喷嘴(喷嘴直径为10 mm)的石英管内,在SP009A半自动非晶态条带制造设备上经高频感应加热重熔后,在氩气压力下将合金熔体喷射至石英管下面高速旋转的铜辊(辊速为1600 r/min)上,制备出Mg80-xAlxCu15Y5快速凝固合金薄带。所制取的薄带厚度在40~60 μm,宽度在6~8 mm之间变化。将合金薄带放入SRJK-3-12管式电阻炉中,在氩气的保护下分别在523、573和623 K进行保温30 min的退火热处理。
利用D8型X射线衍射仪确定退火热处理前后快速凝固Mg80-xAlxCu15Y5(x=0,1)合金薄带的相结构;采用STA409PC差示扫描量热仪研究非晶合金薄带样品的玻璃转变、晶化和熔化行为,升温速度10 K/min,氩气保护;从所制备的退火薄带上截取样品,样品经离子减薄后在JEM-2100高分辨率透射电子显微镜上进行微观组织形貌观察。
2 结果与分析
2.1 非晶形成能力和热稳定性
图1所示为快速凝固Mg80-xAlxCu15Y5(x=0,1)合金的XRD谱。由图1可见,在辊速为1600 r/min下快速凝固制备的Mg80-xAlxCu15Y5(x=0,1)合金薄带的XRD谱显示出非晶结构典型的漫散峰特征,不存在任何尖锐的晶体相衍射峰,表明这两种成分合金为完全非晶态。与Mg-Cu-Y三元合金系相比,Mg-Al-Cu-Y四元合金体系组成更复杂,原子间尺寸差异更大(Mg、Cu、Y和Al原子半径分别为0.160、0.128、0.182和0.143 nm),混合负热也更大(Mg-Y为-6 kJ/mol,Cu-Y为-22 kJ/mol,Mg-Cu为-3 kJ/mol,Al-Y为-38 kJ/mol [23]),从而有利于合金形成更为随机密排的原子堆垛结构,这种结构不仅增大液固界面能,而且提高了液态合金黏度,使得原子的扩散更加困难,降低了向晶态转变的驱动力,抑制晶体的形核及长大,促进非晶的形成。
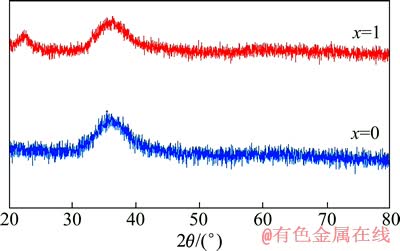
图1 快速凝固Mg80-xAlxCu15Y5(x=0,1)合金的XRD谱
Fig. 1 XRD patterns of rapidly solidified Mg80-xAlx- Cu15Y5(x=0,1) alloys
图2所示为快速凝固Mg80-xAlxCu15Y5(x=0,1)非晶合金在连续加热(加热速率为10 K/min)条件下的DSC曲线。由图2可以看出,两个合金样品的DSC曲线上都出现了明显的玻璃转变吸热峰和两个晶化转变放热峰(x=0的合金的晶化峰温度分别为470和540 K,x=1的合金的晶化峰温度分别为483和536 K),其中第二晶化放热峰明显较第一放热峰尖锐,且峰的面积也大,表明在第二放热峰温度范围合金中有大量稳定晶体相形核并长大,因此放热量大,由此也说明两个合金的晶化主要在第二放热峰阶段进行。
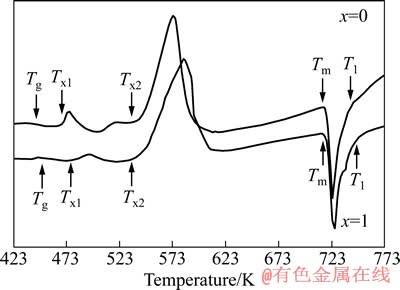
图2 Mg80-xAlxCu15Y5(x=0,1)非晶合金的DSC曲线
Fig. 2 DSC curves of Mg80-xAlxCu15Y5(x=0,1) amorphous alloys (heating rate of 10 K/min)
表1列出了Mg80-xAlxCu15Y5(x=0,1)非晶合金的玻璃化温度Tg、第一晶化峰起始温度Tx1、过冷液相区宽度ΔTx(ΔTx=Tx1-Tg)。由表1可以看出,Al的加入提高了Mg-Cu-Y非晶合金的玻璃化温度Tg及过冷液相区宽度ΔTx。通常认为玻璃化温度Tg的提高,意味着非晶合金组元原子间结合力的增加和更高的热稳定性[24]。由文献[25]可知,过冷液相区宽度ΔTx与合金组元间键参数(电负性差及原子尺寸参数)密切相关,组元间电负性差及原子尺寸参数越大,过冷液相区宽度ΔTx越大。利用该文献中电负性差及原子尺寸参数的计算公式算出,Mg79Al1Cu15Y5合金的组元间电负性差(Mg、Al、Cu及Y的电负性分别为1.31、2.00、1.22及1.61)及原子尺寸参数都比Mg80Cu15Y5合金的大,因此其过冷液相区宽度ΔTx也比Mg80Cu15Y5合金的大。而过冷液相区宽度ΔTx反映了非晶合金热稳定性的高低,即合金可以在较大的温度范围内存在过冷而不结晶形核并长大的能力高低,ΔTx越大,合金热稳定性越高。由此可知,1%Al的加入提高了Mg-Cu-Y非晶合金的热稳定性。
表1 Mg80-xAlxCu15Y5(x=0,1)非晶合金的热性能参数
Table 1 Thermal parameters of Mg80-xAlxCu15Y5 (x=0,1) amorphous alloys at heating rate of 10 K/min
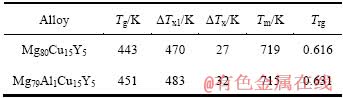
TURNBULL[26]提出的“约化玻璃转变温度”Trg(Trg=Tg/Tl)是评价合金非晶形成能力的一个重要参数。通常认为,具有较大Trg的合金体系具有较强的非晶形成能力(通常Trg在0.56~0.69范围内非晶形成能力较高)。影响合金非晶形成能力最关键的因素是合金成分,随着合金组元类型或浓度的变化,其非晶形成能力也将发生变化。合金成分位于共晶点或共晶点附近是合金具有大的非晶形成能力的必要条件[27]。由图2可见,Mg80Cu15Y5和Mg79Al1Cu15Y5合金的熔化过程均表现为单一的吸热峰。它们的Tm及Tl依次分别为719和741 K及715和747 K。Mg79Al1Cu15Y5合金的熔化温度区间比Mg80Cu15Y5的22 K稍宽,为32 K,表明两种合金的成分都位于合金系共晶点附近,所以取合金的熔化起始温度Tm作为Tl代入Trg=Tg/Tl式中,由此得到的Trg值如表1所列。由表1可知,Mg79Al1Cu15Y5合金的Trg为0.631,较Mg80Cu15Y5 (Trg=0.616)有所提高,说明Mg79Al1Cu15Y5合金的非晶形成能力稍高于Mg80Cu15Y5合金的。
图3所示为快速凝固Mg80-xAlxCu15Y5(x=0,1)合金经不同温度退火处理后的XRD谱。由图3可以看出,当退火温度为523 K时,在非晶漫散峰的基底上出现了尖锐的晶体相衍射峰,表明合金均发生了晶化。通过计算得知,在该温度Mg79Al1Cu15Y5合金的晶化率为54.87%,而未加入Al的Mg80Cu15Y5合金的晶化率为67.45%,即Al的加入减慢了合金的晶化速度,提高了合金的热稳定性,这与前面的DSC曲线分析结果相一致。经分析两种合金中形成的晶体相主要为HCP-Mg和金属间化合物Mg2Cu,可见在523 K加热后,合金已由加热前单一的非晶相转变为HCP-Mg、Mg2Cu晶体相和残余非晶相的三相混合物。温度升高至573 K时,XRD谱中的晶体相衍射峰的数目及衍射强度增加,说明合金的晶化率提高(其中Mg79Al1Cu15Y5合金为79.37%,Mg80Cu15Y5合金为81.35%)。由分析可知,加入Al的合金中除了HCP-Mg和金属间化合物Mg2Cu晶体相外,还产生了AlMg化合物新相。当温度达到623 K时,漫散峰几乎完全消失,两种合金的晶化率接近100%,非晶合金已转变为由HCP-Mg和一些金属间化合物(Mg80Cu15Y5合金为Mg2Cu相,Mg79Al1Cu15Y5合金为Mg2Cu和AlMg相)组成的多相混合物结构。
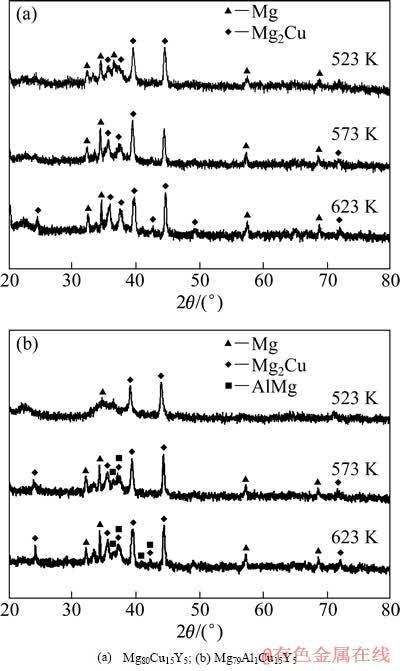
图3 不同温度退火后的快速凝固Mg80-xAlxCu15Y5(x=0,1)合金的XRD谱
Fig. 3 XRD patterns of rapidly solidified Mg80-xAlxCu15Y5 alloys (x=0,1) annealed at various temperatures
2.2 显微组织
图4所示为在523 K退火30 min后快速凝固Mg80-xAlxCu15Y5(x=0,1)合金的明场像及其相应的SAED花样。由图4的明场像可以看出,在合金基体中弥散析出大量尺寸约为5~15 nm的纳米颗粒,这些颗粒取向随机,除少数颗粒相互接触外,大多处于相互独立的状态,并且Mg79Al1Cu15Y5合金(见图4(b))中析出的颗粒较Mg80Cu15Y5合金(见图4(a))尺寸小且数目少。它们相应的SAED像(见图4(a)和(b)右上角)中出现了一些模糊不清的不完整的环状花样以及许多散乱分布的亮点状的斑点,表明这两种成分的非晶合金加热至523 K时均出现晶化,同时衍射花样中重叠在内侧的宽化的德拜环(由非晶散射产生)证明合金中非晶的存在。
在523 K退火30 min后的Mg80-xAlxCu15Y5(x=0,1)合金HRTEM像如图5所示。图5(a)和(b)中各种取向的晶格条纹区的出现证明非晶合金中晶体相的形成。每种取向的条纹区(由彼此平行的等间距直条纹所组成)代表一个纳米晶粒,在这些条纹区的周围仍保留有非晶结构特有的“无序点状特征”(无规则的弯曲条与亮斑点组成的斑纹状图案),即纳米晶是被非晶所包围的。由合金的HRTEM图像可以清楚地看出在523 K加热30 min后两种成分的非晶合金均已转变为纳米晶体相与非晶相复合的组织结构。与Mg80Cu15Y5合金(见图5(a))相比,Mg79Al1Cu15Y5合金(见图5(b))中非晶相(无序点状区域)所占的比例更大;另外Mg80Cu15Y5合金晶格条纹区不仅数目多、尺寸大,而且条纹也比Mg79Al1Cu15Y5合金清晰且连续,说明其形成的晶体点阵较为完整,原子呈现规则性排列;而Mg79Al1Cu15Y5合金的部分条纹区条纹强度分布非常不均匀,原子排列处于断断续续的状态,存在很多晶体缺陷(如空位、层错),晶体点阵相当不完整,仍保留有原来非晶结构的痕迹。以上分析说明在523 K加热30 min后Mg79Al1Cu15Y5合金的晶化程度低于Mg80Cu15Y5合金的,即加入1%Al提高了Mg-Cu-Y非晶合金的热稳定性,这与前面图3的XRD分析结果相一致。
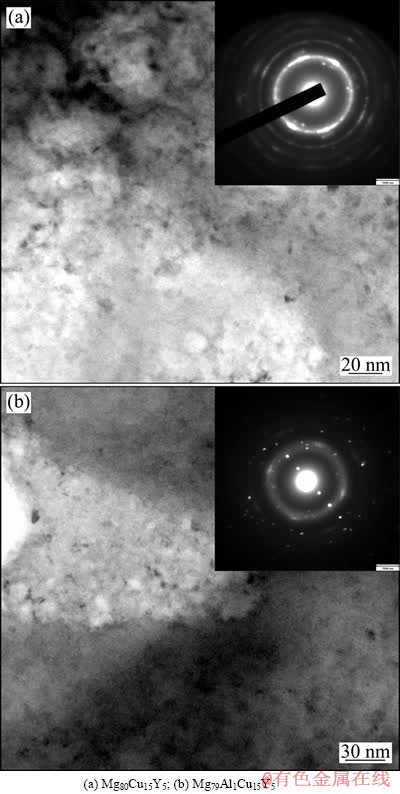
图4 在523 K退火30 min后的快速凝固Mg80-xAlx- Cu15Y5(x=0,1)合金薄带的TEM明场像及相应的SAED花样
Fig. 4 TEM bright field images and corresponding selected area electron diffraction (SAED) patterns of rapidly solidified Mg80-xAlxCu15Y5(x=0,1) alloy ribbons annealed at 523 K for 30 min
图6所示为在623 K退火30 min后的快速凝固Mg80-xAlxCu15Y5(x=0,1)合金薄带的组织形貌。与图4相比,随着退火温度的升高,原先在低温时形成的纳米颗粒明显长大,且数目减少,如图6 (a)和(b)所示;而且Mg79Al1Cu15Y5合金中晶粒的长大速度要比Mg80Cu15Y5合金的快,这可能与低温时其晶粒较Mg80Cu15Y5合金晶粒更为细小有关。另外, Mg79Al1Cu15Y5合金中出现了大量尺寸不一的针状新相(见图6(b)),该相均匀分布在原先的纳米颗粒和基体中,表明它们是从Mg2Cu、Mg晶粒中脱溶析出。根据图3(b)所示XRD分析结果可知,该针状相应为AlMg化合物相。
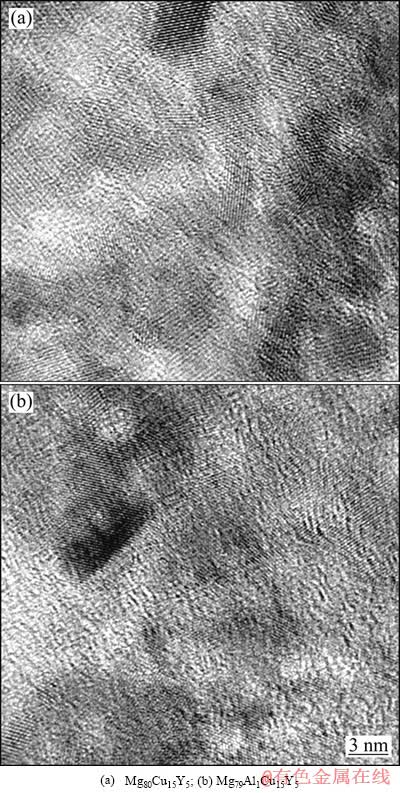
图5 在523 K退火30 min的快速凝固Mg80-xAlx- Cu15Y5(x=0,1)合金薄带的HRTEM像
Fig. 5 HRTEM images of rapidly solidified Mg80-xAlx- Cu15Y5(x=0,1) alloy ribbons annealed at 523 K for 30 min
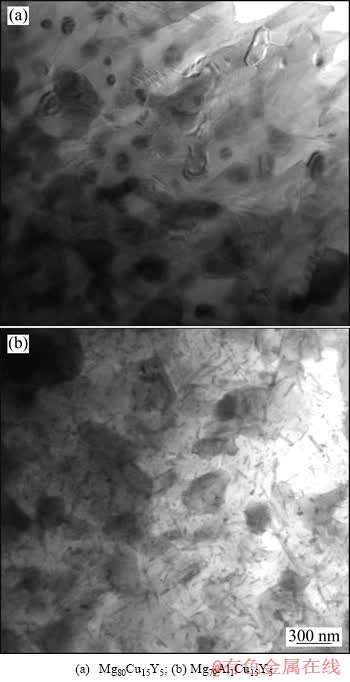
图6 在623 K退火30 min的快速凝固Mg80-xAlxCu15Y5 (x=0,1)合金薄带的显微组织
Fig. 6 Microstructures of rapidly solidified Mg80-xAlx- Cu15Y5(x=0,1)alloy ribbons annealed at 623 K for 30 min
3 结论
1) Mg79Al1Cu15Y5非晶合金的过冷液相区宽度ΔTx约为32 K,比Mg80Cu15Y5非晶合金的ΔTx值27 K高,约化玻璃转变温度Trg从0.616升至0.631,说明Mg79Al1Cu15Y5非晶合金的热稳定性及非晶形成能力均高于Mg80Cu15Y5非晶合金的。
2) 在退火温度为523 K时,Mg80-xAlxCu15Y5(x=0,1)合金均由HCP-Mg、Mg2Cu和残余非晶相组成,但Mg79Al1Cu15Y5非晶合金的晶化率低于Mg80Cu15Y5非晶合金。当温度超过573 K时,Mg79Al1Cu15Y5非晶合金中有AlMg化合物新相形成;至623 K时Mg80-xAlxCu15Y5(x=0,1)合金则完全转变为由HCP-Mg和一些金属间化合物(Mg80Cu15Y5合金为Mg2Cu相, Mg79Al1Cu15Y5合金为Mg2Cu和AlMg相)组成的多相混合物结构。
3) 在523 K加热后,Mg80-xAlxCu15Y5(x=0,1)合金组织为在非晶基体上均匀弥散分布着大量的纳米颗粒;当加热温度升高到573 K时,Mg79Al1Cu15Y5合金中有针状AlMg相形成。
REFERENCES
[1] CLEMENT W, WILLENS R H, DUWEZ P. Non-crystalline structure in solidified gold-silicon alloys[J]. Nature, 1960, 187: 869-870.
[2] 张宝庆, 曹国华, 郎庆斌, 张 志, 战再吉, 刘日平, 王文魁. Zr47Cu44Al9大块非晶合金的制备及其力学性能[J]. 中国有色金属学报, 2010,20(1): 112-117.
ZHANG Bao-qing, CAO Guo-hua, LANG Qing-bin, ZHANG Zhi, ZHAN Zai-ji, LIU Ri-ping, WANG Wen-kui. Preparation and mechanical properties of Zr47Cu44Al9 bulk metallic glass[J]. The Chinese Journal of Nonferrous Metals, 2010,20(1): 112-117.
[3] 袁子洲, 包石磊, 佟亚东, 姚 林. 晶化对Co43Fe20Ta5.5B31.5非晶合金磁性能的影响[J]. 稀有金属材料与工程, 2009, 38(5): 851-856.
YUAN Zi-zhou, BAO Shi-lei, TONG Ya-dong, YAO Lin. Effect of crystallization on magnetic properties of Co43Fe20Ta5.5B31.5 amorphous alloy[J]. Rare Metal Materials and Engineering, 2009, 38(5): 851-856.
[4] 王建强, 马长松, 张 甲, 侯万良, 常新春. Al基合金体系非晶形成能力的相关理论进展[J].材料研究学报, 2008, 22(2): 113-119.
WANG Jian-qiang, MA Chang-song, ZHANG Jia, HOU Wan-liang, CHANG Xin-chun. Progress in research on glass forming ability of Al-based metallic glasses[J]. Chinese Journal of Materials Research, 2008, 22(2): 113-119.
[5] 王敬丰, 柳 林, 邹 辉, 蒲 健, 肖建中. 大块金属玻璃Pd40Ni10Cu30P20结构弛豫的研究[J]. 稀有金属材料与工程, 2005, 34(1): 98-101.
WANG Jing-feng, LIU Lin, ZOU Hui, PU Jian, XIAO Jian-zhong. Study on the structure relaxation of Pd40Ni10Cu30P20 bulk metallic glass[J]. Rare Metal Materials and Engineering, 2005, 34(1): 98-101.
[6] 白 琴, 满 华, 唐永军, 郝彦斐, 徐 晖, 夏 爽, 胡业旻. 铜模吸铸法制备Fe-Nd-Al-B-Dy合金的结构和磁性能[J]. 中国有色金属学报, 2011,21(5): 1118-1122.
BAI Qin, MAN Hua, TANG Yong-jun, HAO Yan-pei, XU Hui, XIA Shuang, HU Ye-min. Structure and magnetic properties of Fe-Nd-Al-B-Dy alloys by suction casting[J]. The Chinese Journal of Nonferrous Metals, 2011, 21(5): 1118-1122.
[7] HUANG K, CHEN G,ZHAO Y,WANG G,SHAO Y. Crystallization microstructure of Mg65Cu25Y10 bulk amorphous alloy[J]. Transactions of Nonferrous Metals Society of China, 2012, 22(4): 831-836.
[8] MATSUURA M, KONNO K,YOSHIDA M, NISHIJIMA M,HIRAGA K. Amorphous particles embedded in hcp Mg grains of melt-quenched Mg98Cu1Gd1 alloys[J]. Materials Transactions, 2008, 49(2): 387-389.
[9] BAE G T, LEE S B , KIM N J. Effect of alloying elements on the crystallization kinetics of Mg-Cu-(Y,Gd) bulk amorphous alloys[J]. Materials Science and Engineering A, 2007, 449/451: 489-492.
[10] LIU L Y, TEHG X Y, WANG Y J, LIU T. Glass-forming ability and crystallization of Mg-Ni amorphous alloys with Y addition[J]. Rare Metals, 2012, 31(3): 244-249.
[11] 邱克强, 王 猛, 张洪兵, 柏笑君, 任英磊, 张 涛. Cu对Mg-Zn-Ca合金非晶形成能力与力学性能的影响[J]. 中国有色金属学报, 2009,19(4): 677-681.
QIU Ke-qiang, WANG Meng, ZHANG Hong-bing, BAI Xiao-jun, REN Ying-lei, ZHANG Tao. Effect of Cu on glass forming ability and mechanical properties of Mg-Zn-Ca alloys[J]. The Chinese Journal of Nonferrous Metals, 2009,19(4): 677-681.
[12] LIU K M, ZHOU H T,YANG B,LU D P,ATRENS A. Influence of Si on glass forming ability and properties of the bulk amorphous alloy Mg60Cu30Y10[J]. Materials Science and Engineering A, 2010, 527(29/30): 7475-7479.
[13] 刘克明, 杨 滨, PRYDS Nini. Mg-Cu-Y-Si合金玻璃形成能力及力学性能[J]. 北京科技大学学报, 2008, 30(2): 141-143.
LIU Ke-ming, YANG Bin, PRYDS N. Glass-forming ability and mechanical properties of Mg-Cu-Y-Si alloy[J]. Journal of University of Science and Technology Beijing, 2008, 30(2): 141-143.
[14] 门 华, 杨明川, 徐 坚. 添加Co对Mg65Cu25Y10合金玻璃形成能力的影响[J]. 材料研究学报, 2002, 16(4): 379-384.
MEN Hua, YANG Ming-chuan, XU Jian. The effect of Co addition on glass-forming ability of Mg65Cu25Y10 alloy[J]. Chinese Journal of Materials Research, 2002, 16(4): 379-384.
[15] WANG Xiao-jun,XIA Tian-dong, CHEN Xue-ding. Kinetics study on non-isothermal crystallization of amorphous alloy Mg65Cu15Ag10Y10[J]. Advanced Materials Research: Materials Science and Engineering Application II, 2012, 413: 432-438.
[16] 门 华, 徐 坚. Mg-Cu-Zn-Y块体金属玻璃的形成[J]. 金属学报, 2001, 37(12): 1243-1246.
MEN Hua, XU Jian. Formation of Mg-Cu-Zn-Y bulk metallic glass[J]. Acta Metallurgica Sinica, 2001, 37(12): 1243-1246.
[17] CHENG Y T, HUNG T H, HUANG J C, HSIEH P J,JANG J S C. Thermal stability and crystallization kinetics of Mg-Cu-Y-B quaternary alloys[J]. Materials Science and Engineering A, 2007, 449/451: 501-505.
[18] LIU G B, GAO P, XUE Z, YANG S Q, ZHANG M L. Study on the formation of new Mg-Cu-Ti-Y quaternary bulk metallic glasses with high mechanical strength[J]. Journal of Non-Crystalline Solids, 2012, 358(23): 3084-3088.
[19] 吴树森, 刘 伟, 毛有武, 安 萍. Nd对块体Mg-Cu-Y-Nd非晶合金玻璃形成能力的影响[J]. 中国有色金属学报, 2007, 17(6): 852-857.
WU Shu-sen, LIU Wei, MAO You-wu, AN Ping. Effect of Nd on glass forming ability of bulk amorphous Mg-Cu-Y-Nd alloys[J]. The Chinese Journal of Nonferrous Metals, 2007, 17(6): 852-857.
[20] ZHENG Q, MA H, MA E, XU J. Mg-Cu-(Y, Nd) pseudo-ternary bulk metallic glasses: The effects of Nd on glass-forming ability and plasticity[J]. Scripta Materialia, 2006, 55(6): 541-544.
[21] ZHANG C M, HUI X, Li Z G, CHEN G L. Improving the strength and the toughness of Mg-Cu-(Y,Gd) bulk metallic glass by minor addition of Nb[J]. Journal of Alloys and Compounds, 2009, 467(1/2): 241-245.
[22] LI Q F, QIU K Q, YANG X, YUAN X G, ZHANG T. Glass forming ability and reliability in fracture stress for Mg-Cu-Ni-Nd-Y bulk metallic glasses[J]. Materials Science and Engineering A, 2008, 491(1/2): 420-424.
[23] TAKEUCHI A, INOUE A. Classification of bulk metallic glasses by atomic size difference, heat of mixing and period of constituent elements and its application to characterization of the main alloying element[J]. Materials Transactions, 2005, 46(12): 2817-2829.
[24] 袁广银, 尹 健, 丁文江. Mg-Cu-Ni-Gd非晶合金结构弛豫及力学性能[J]. 金属学报, 2008, 44(2): 222-226.
YUAN Guang-yin, YIN jian, DING Wen-jiang. Structural relaxation and mechanical properties of Mg-Cu-Ni-Gd amorphous alloys[J]. Acta Metallurgica Sinica, 2008, 44(2): 222-226.
[25] FANG Shou-shi, XIAO Xue-shan, XIA Lei, LI Wei-huo, DONG Yuan-da. Relationship between the widths of supercooled liquid regions and bond parameters of Mg-based bulk metallic glasses[J]. Journal of Non-Crystalline Solids, 2003, 321(1/2): 120-125.
[26] TURNBULL D. Under what conditions can a glass be formed[J]. Contemporary Physics, 1969, 10(5): 473-488.
[27] BUSCH R, LIU W, JOPHNSON W L. Thermodynamics and kinetics of the Mg65Cu25Y10 bulk metallic glass forming liquid[J]. Journal of Applied Physics, 1998, 83(8): 4134-4142.
(编辑 何学锋)
基金项目:河南省杰出青年基金资助项目(074100510008);河南科技大学青年科学研究基金资助项目(2006QN028)
收稿日期:2012-10-09;修订日期:2013-03-11
通信作者:宁向梅,副教授;电话:15838802816;E-mail:nxm168@163.com
摘 要:采用单辊快速凝固法制备出Mg80-xAlxCu15Y5(x=0,1)合金薄带,再将薄带在不同温度下进行退火处理,利用XRD、DSC和HRTEM分析Al元素对Mg80-xAlxCu15Y5(x=0,1)合金薄带的非晶形成能力及热稳定性的影响。结果表明:Al的加入使得Mg-Cu-Y合金的玻璃转变温度和初始结晶温度升高,过冷液相区宽度ΔTx因Al部分置换Mg而增大,约化玻璃转变温度Trg从0.616升至0.631,合金的非晶形成能力及热稳定性提高。随着退火温度的升高,Mg79Al1Cu15Y5合金的晶化率低于Mg80Cu15Y5合金的。当退火温度为523 K时Mg80-xAlxCu15Y5(x=0,1)合金均发生明显晶化,在非晶基体上弥散析出大量HCP-Mg和Mg2Cu纳米颗粒;当温度升高至573 K时,Mg79Al1Cu15Y5合金中有针状AlMg化合物相形成。
[2] 张宝庆, 曹国华, 郎庆斌, 张 志, 战再吉, 刘日平, 王文魁. Zr47Cu44Al9大块非晶合金的制备及其力学性能[J]. 中国有色金属学报, 2010,20(1): 112-117.
[3] 袁子洲, 包石磊, 佟亚东, 姚 林. 晶化对Co43Fe20Ta5.5B31.5非晶合金磁性能的影响[J]. 稀有金属材料与工程, 2009, 38(5): 851-856.
[4] 王建强, 马长松, 张 甲, 侯万良, 常新春. Al基合金体系非晶形成能力的相关理论进展[J].材料研究学报, 2008, 22(2): 113-119.
[5] 王敬丰, 柳 林, 邹 辉, 蒲 健, 肖建中. 大块金属玻璃Pd40Ni10Cu30P20结构弛豫的研究[J]. 稀有金属材料与工程, 2005, 34(1): 98-101.
[11] 邱克强, 王 猛, 张洪兵, 柏笑君, 任英磊, 张 涛. Cu对Mg-Zn-Ca合金非晶形成能力与力学性能的影响[J]. 中国有色金属学报, 2009,19(4): 677-681.
[13] 刘克明, 杨 滨, PRYDS Nini. Mg-Cu-Y-Si合金玻璃形成能力及力学性能[J]. 北京科技大学学报, 2008, 30(2): 141-143.
[14] 门 华, 杨明川, 徐 坚. 添加Co对Mg65Cu25Y10合金玻璃形成能力的影响[J]. 材料研究学报, 2002, 16(4): 379-384.
[16] 门 华, 徐 坚. Mg-Cu-Zn-Y块体金属玻璃的形成[J]. 金属学报, 2001, 37(12): 1243-1246.
[19] 吴树森, 刘 伟, 毛有武, 安 萍. Nd对块体Mg-Cu-Y-Nd非晶合金玻璃形成能力的影响[J]. 中国有色金属学报, 2007, 17(6): 852-857.
[24] 袁广银, 尹 健, 丁文江. Mg-Cu-Ni-Gd非晶合金结构弛豫及力学性能[J]. 金属学报, 2008, 44(2): 222-226.


