
Preparation of LiNi1/3Co1/3Mn1/3O2 cathode materials from spent Li-ion batteries
LI Jian-gang(李建刚)1, ZHANG Qian(张 倩)1, HE Xiang-ming(何向明)2
1. School of Chemical Engineering, Beijing Institute of Petrochemical Technology, Beijing 102617, China;
2. Institute of Nuclear and New Energy Technology, Tsinghua University, Beijing100084, China
Received 15 July 2007; accepted 10 September 2007
Abstract:
A recycling process including separation of electrode materials by ultrasonic treatment, acid leaching, Fe-removing, precipitation of cobalt, nickel, manganese and lithium has been applied successfully to recycle spent lithium-ion batteries and to synthesize LiNi1/3Co1/3Mn1/3O2. When ultrasonic treatment with 2-nitroso-4-methylphenol(NMP) at 40 ℃ for 15 min, the electrode materials are separated completely. Above 99% of Co, Ni, Mn and Li, 95% of Fe in the separated electrodes are acid-leached in the optimized conditions of 2 mol/L H2SO4, 1?2 H2O2?H2SO4 (molar ratio), 70 ℃, 1?10 initial S?L ratio, and 1 h. 99.5% of Fe and less than 1% of Co, Ni, Mn in the leaching solution can be removed in the conditions of initial pH value 2.0-2.5 adjusted by adding 18% Na2CO3, 90 ℃ and stirring time 3 h. After adjusted to be equal by adding NiSO4, CoSO4 and MnSO4 solution, 97.1% of Ni, Co, Mn in the Fe-removing surplus leaching solution can be recovered as Ni1/3Co1/3Mn1/3(OH)2. 94.5% of Li in the surplus filtrate after the deposition of Co, Ni and Mn can be recovered as Li2CO3. The LiNi1/3Co1/3Mn1/3O2, prepared from the recovered compounds, is found to have good characteristics of the layered structure and elecrtochemical performance.
Key words:
recycling process; spent Li-ion batteries; cathode material; LiNi1/3Co1/3Mn1/3O2;
1 Introduction
Lithium-ion batteries(LIBs) are widely used as electrochemical power sources in many portable apparatus. Because LIBs contain lots of valuable metals such as cobalt, lithium and aluminum, the spent LIBs increases the metal-containing hazardous waste. Therefore, the recycling of major components from spent LIBs is considered to be a beneficial way to prevent environmental pollution and as alternative resources of valuable metal.
Up to now, some processes for the recycling of spent LIBs have been proposed. Because LiCoO2/C batteries are widely used, most of the reported processes concentrate mainly on the recycling and reuse of Co and Li. Hydrometallurgical and pyrometallurgical routes, such as chemical deposition[1-2], solvent extraction [3-4], ion-exchange[5] or electrolysis[6] have been widely used to recover valuable metals from the spent LIBs. In addition, several procedures for the direct regeneration of lithium cobalt oxide electrode from waste LIBs have published by KIM et al[7] and RA et al[8].
At the moment, LiCoO2 is the most used active cathode material, however, the use of new cathode materials such as LiMn2O4, LiFePO4, LiCoxNi1-xO2 and LiNixCo1-2xMnxO2 have increased in the last few years due to cost and safety. Coexistence of Li, Fe, Co, Ni and Mn ions increases the difficulty of recycling valuable metals. Recently, LUPI et al[9] had reported a process for recycling Co and Ni from the spent LIBs by electrochemical process. NAN et al[10] had published a novel recycling process for the valuable metal from a mixture of spent LIBs and nickel-metal hydride batteries.
In this work, we present a new process for recycling the valuable metals from a mixture of spent LIBs, in which LiCoO2, LiNi0.4Co0.2Mn0.4O2, LiFePO4 and LiMn2O4 are used as cathode matrerials respectively. In view of the better electrochemical performance of LiNi1/3Co1/3Mn1/3O2, the new recycling process of spent LIBs bases on the preparation of LiNi1/3Co1/3Mn1/3O2 from the spent LIBs.
2 ExperimentalCommercial LIBs used in this study were kindly provided by Mike Battery Co. Ltd., in which LiCoO2, LiNi0.4Co0.2Mn0.4O2, LiFePO4, LiMn2O4 were used as cathode matrerials respectively, graphite active materials as anode materials, carbon as conducting additives, and polyvinylidence fluoride(PVDF) as binder, Al and Cu foil as current collector, Celgard 2400 as separator. The batteries were opened by simply cutting their cases and extracting the internal rolls. The rolls were submitted to a series of tests defining their recycling and treating process. Finally, an overall recycling process, as shown in Fig.1, was designed and studied. The determination of the concentration of lithium, cobalt, nickel, manganese, iron in aqueous solutions were carried out by inductively coupled plasma atomic emission spectroscopy (ICP- AES) using a Perkin Elmer Optima 2100DV spectro- photometer. Powder X-ray diffraction was used to characterize the structure of the LiNi1/3Co1/3Mn1/3O2 powders. The charge-discharge performance of the synthesized materials was tested in a half-cell type of corn cell by using a computer controlled battery test system.
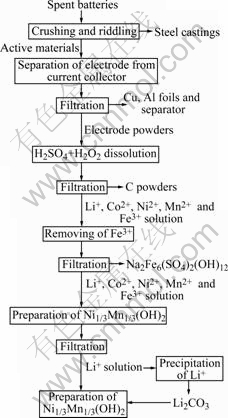
Fig.1 Flow-sheet of recycling process
3 Results and discussion
Fig.1 shows the flow-sheet of the process designed in this work, the various step operations are described below.
3.1 Separation of electrode materials
To simplify the recycling process, separation of electrode materials from the current collector is necessary. The ultrasonic vibration is applied in this study to assist this treatment and improve the effect of separation. Fig.2 shows the effect of organic solvents on the separation. The results indicate that the effect of organic solvent on the separation decreases in the order of 2-nitroso-4-methylphenol(NMP)>N, N-dimethyl formamide(DMF)>Dimethyl sulfoxide(DMSO)?acetone. In view of good solvent for PVDF and high boiling point, NMP is a good choice for liberating the active materials from current collectors. The optimized process is ultrasonic treatment with NMP at 40 ℃ for 15 min. The separation temperature and time are respectively lower and lesser than those reported by CONTESTABILE et al[2]. To identify if ultrasonic vibration improves the separation, we compare the treatment effect by ultrasonic vibration with those by magnetic stirring in DMF and NMP respectively. As shown in Fig.3, the separation effect by ultrasonic vibration is better than that by magnetic stirring. Ultrasonic treatment lowers the temperature of organic solvent for dissolution of PVDF, and shortens the separation times.

Fig.2 Effect of organic solvent on liberation of electrode from current collector at 30 ℃ (a) and 40 ℃ (b), respectively
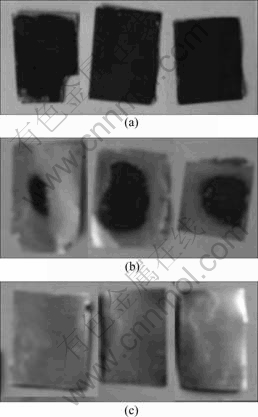
Fig.3 Appearance of electrode after magnetic stirring in DMF for 60 min at 30 ℃ by ultrasonic vibration (a), in DMF for 60 min at 30 ℃ by ultrasonic vibration (b) and in NMP for 15 min at 40 ℃ (c)
After the seperation of electrode materials, the recovery of both Al and Cu foil in their metallic form are carried out by simply filtrating them out from the NMP solution. The electrode powders, such as carbon additive and active materials settling on the bottom, are separated and recovered by solution decantation. The decanted NMP can be reused for many times owing to its high solubility for PVDF.
3.2 Dissolution of separated electrode powders
Then H2SO4+H2O2 is chosen as acid leaching reagent to dissolve Co, Fe, Ni, Mn and Li from the separated electrode powders, in which some iron can precipitate as FePO4 and be filtrated out. Based on several leaching experiments results, the optimum conditions are determined to be 2 mol/L H2SO4, 1?2 H2O2?H2SO4 molar ratio, 70 ℃, 1?10 initial S?L ratio, and 1h. Above 99% of Co, Ni, Mn and Li, 95% of Fe are leached under this condition.
3.3 Separation of iron from leaching solution
Because Fe(OH)3 precipitates at lower pH values of about 2-4, Fe ions must be separated from the leaching solution before the Ni, Co, Mn ions are recycled as hydroxide precipitates. Separation of iron in the form of jarosite, goethite, and Fe(OH)3 is widely used[11-12], and separation of iron by extraction method also has been reported[13]. Based on several Fe-removing experiments, separation of iron in the form of jarosite is chosen in this work because of enough amounts of ![]() in the leaching solution and easy filtration of the formed precipitates Na2Fe6(SO4)4(OH)12. The procedure is as follows: adjusting the initial pH of the leaching solution by adding 18% (mass fraction) Na2CO3 aqueous solution, and then stirring for 0.5-3.0 h at a stable temperature. After that, the solution is readjusted to pH 2.5-3.1 by adding 18% (mass fraction) Na2CO3 aqueous solution, and the formed precipitates are filtrated out. The total reaction is
in the leaching solution and easy filtration of the formed precipitates Na2Fe6(SO4)4(OH)12. The procedure is as follows: adjusting the initial pH of the leaching solution by adding 18% (mass fraction) Na2CO3 aqueous solution, and then stirring for 0.5-3.0 h at a stable temperature. After that, the solution is readjusted to pH 2.5-3.1 by adding 18% (mass fraction) Na2CO3 aqueous solution, and the formed precipitates are filtrated out. The total reaction is
3Fe2(SO4)3+6Na2CO3+6H2O=Na2Fe6(SO4)4(OH)12↓+ 5Na2SO4+6CO2↑
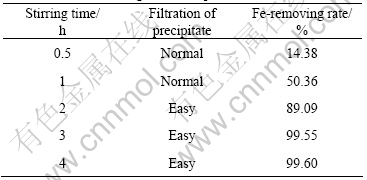
Table 1 lists the effect of initial pH value on the separation of iron in the condition of 90 ℃ and stirring time 4 h. The Fe-removing rate enhances with increasing the initial pH value. However, filtrating out the precipitates becomes difficult while pH>2.5 because small amounts of Fe3+ precipitate in the form of Fe(OH)3. Table 2 lists the effect of temperature on the separation of iron in the condition of pH 2.0 and stirring time 4 h. The effect of stirring time on the separation of iron in the condition of pH 2.0 and 90 ℃ is also investigated, as listed in Table 3. Based on the results mentioned above, the separation condition is optimized to be initial pH value 2.0-2.5, temperature 90 ℃ and stirring time 3 h. Under this condition, above 99.5% of iron is removed and the loss of Co, Ni, Mn is less than 1%, respectively.
Table 1 Effect of initial pH value on separation of iron

Table 2 Effect of temperature on separation of iron
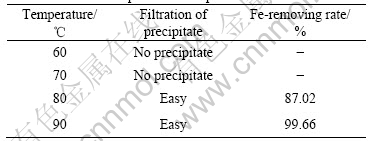
Table 3 Effect of stirring time on separation of iron
3.4 Preparation of Ni1/3Co1/3Mn1/3(OH)2
After filtrating out Na2Fe6(SO4)4(OH)12, the content of Ni, Co, Mn in the surplus leaching solution is determined, and adjusted to be equal by adding NiSO4, CoSO4 and MnSO4 solution. Following that, the spherical Ni1/3Co1/3Mn1/3(OH)2 is prepared by controlled precipitation with NaOH solution under N2 atmosphere. The precipitation condition is optimized to be pH 10.5 (±0.2), 55 ℃, and 6 h of reaction time under stirring intensely. The recovery of Ni, Co, Mn in the adjusted leaching solution reaches 97.1% under this condition.
3.5 Recovery of lithium
Lithium is left in the surplus filtrate after the removal of Ni1/3Co1/3Mn1/3(OH)2. The filtrate is concentrated to be about 3 mol/L of Li, and then treated with a saturated Na2CO3 solution. The amount of Na2CO3 on the recovery of Li is investigated, the results indicate that the recovery of Li can reach 94.5% when adding 100% excess saturated Na2CO3 solution.
3.6 Synthesis of LiNi1/3Co1/3Mn1/3O2
The Ni1/3Co1/3Mn1/3(OH)2 precipitates are filtrated out, heated at 600 ℃ for 5 h to transform to metal oxides, then mixed with Li2CO3 in a molar ratio of Li?(Ni+Co+Mn)=1.03?1.00 and annealed at 850 ℃ in air for 10 h to prepare LiNi1/3Co1/3Mn1/3O2 materials, successively. As shown in Fig.4, the XRD pattern of as-prepared LiNi1/3Co1/3Mn1/3O2 reveals that the sample presents the typical layered α-NaFeO2 structure. No impurity-related reflection peaks can be observed in this XRD pattern. The clear splitting of the lines assigned to the Miller indices (006, 102) and (108, 110) in Fig.4 indicates that the sample presents good characteristics of the layered structure. The ratio I003/I104 of 1.62 is obtained, indicating no undesirable cation mixing[14]. The lattice parameters a and c of the sample are determined to be 0.286 0 nm and 1.423 7 nm, respective- ly. These data match well with the values observed by Ohzuku and Makimura (a=0.286 7 nm and c=1.424 6 nm) [15] and SHAJU et al[16](a=0.286 4 nm and c=1.423 3 nm).
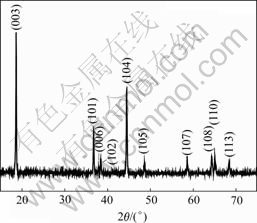
Fig.4 XRD pattern of as-prepared LiNi1/3Co1/3Mn1/3O2
The charge-discharge characteristic of as-prepared LiNi1/3Co1/3Mn1/3O2 is tested in a corn cell, which is consisted of a cathode with the composition of 88% (mass fraction, the same below) LiNi1/3Co1/3Mn1/3O2, 6% carbon black, and 6% PVDF, a lithium metal anode separated by a Celguard 2400 microporous film, and electrolyte composed of 1 mol/L LiPF6/EC+DEC+DMC (1?1?1 by volume). Fig.5 shows that LiNi1/3Co1/3Mn1/3O2 synthesized from the recovered compounds of spent LIBs presents a specific capacity of 161.1 mA·h/g and good cycleabilty at 0.3C over 2.5-4.4 V range.
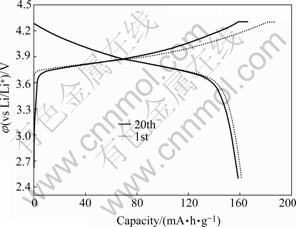
Fig.5 Charge-discharge curves of as-prepared LiNi1/3Co1/3- Mn1/3O2
4 Conclusions1) A sequential recycling process including separation of electrode materials by ultrasonic treatment, acid leaching, Fe-removing, and precipitation of cobalt, nickel, manganese and lithium has been applied successfully to recycle spent lithium-ion batteries and to synthesize LiNi1/3Co1/3Mn1/3O2.
2) Ultrasonic treatment can lower the temperature of organic solvent for dissolution of PVDF, and shorten the separation times, which can lessen harm of organic solvent to people. The optimized process of separating electrode from current collector is ultrasonic treatment with NMP at 40 ℃ for 15 min.
3) The optimum leaching conditions for the separated electrode materials are 2 mol/L H2SO4, 1?2 H2O2?H2SO4 molar ratio, 70 ℃, 1?10 initial S?L ratio, and 1h. Above 99% of Co, Ni, Mn and Li, 95% of iron are leached in this condition.
4) The Fe-removing condition is optimized to be initial pH value 2.0-2.5 adjusted by adding 18% Na2CO3 solution, temperature 90 ℃ and stirring time 3h. In this condition, above 99.5% of iron is removed and the loss of Co, Ni, Mn is less than 1%, respectively.
5) After adjusted to be equal by adding NiSO4, CoSO4 and MnSO4 solution, 97.1% of Ni, Co, Mn in the Fe-removing surplus leaching solution can be recovered as Ni1/3Co1/3Mn1/3(OH)2. 94.5% of Li in the surplus filtrate after the deposition of Co, Ni and Mn can be recovered as Li2CO3. The LiNi1/3Co1/3Mn1/3O2, prepared from the recovered compounds, is found to be having good characteristics of the layered structure and elecrto- chemical performance.
References[1] CASTILLO S, ANSART F, LABERTY-ROBERT C, et al. Advances in the recovering of spent lithium battery compounds [J]. J Power Sources, 2002, 112(1): 247-254.
[2] CONTESTABILE M, PANERO S, SCROSATI B. A laboratory-scale lithium-ion battery recycling process [J]. J Power Sources, 2001, 92(1): 65-69.
[3] ZHANG Ping-wei, YOKOYAMA T, ITABASHI O, et al. Hydrometallurgical process for recovery of metal values from spent lithium-ion secondary batteries [J]. Hydrometallurgy, 1998, 47: 259-271.
[4] NAN Jun-min, HAN Dong-mei, ZUO Xiao-xi. Recovery of metal values from spent lithium-ion batteries with chemical deposition and solvent extraction [J]. J Power Sources, 2005, 152(1): 278-284.
[5] WANG Xiao-feng, KONG Xiang-hua, ZHAO Zeng-ying. Recovery of noble metal lithium ion batteries [J]. Battery Bimonthly, 2001, 31(1): 14-15. (in Chinese)
[6] SHEN Yong-feng. Recovery cobalt from discarded lithium ion cells [J]. Nonferrous Metals, 2002, 54(4): 69-71. (in Chinese)
[7] KIM D S, SOHN J S, LEE C K, et al. Simultaneous separation and renovation of lithium cobalt oxide from the cathode of spent lithium ion rechargeable batteries [J]. J Power Sources,2004, 132(1/2): 145-149.
[8] RA D, HAN K S. Used lithium ion rechargeable battery recycling using Etoile-Rebatt technology [J]. J Power Sources, 2006, 163(1): 284-288.
[9] LUPI C, PASQUALI M. Electrolytic nickel recovery from lithium-ion batteries [J]. Minerals Engineering, 2003, 16(6): 537-542.
[10] NAN Jun-min, HAN Dong-mei, YANG Min-jie, CUI Ming, HOU Xian-lu. Recovery of metal values from a mixture of spent lithium-ion batteries and nickel-metal hydrid batteries [J]. Hydrometallurgy, 2006, 84: 75-80.
[11] XIE Fu-biao. Practice on comprehensive recovery of valuable metals from cobalt-bearing wastes [J]. Mining & Metallurgy, 2001, 10(3): 61-64. (in Chinese)
[12] WANG Yan, ZHOU Chun-shan. Study on the separation of cobalt, iron and manganese from the leach solution of sulphated calcined cobalt residue [J]. Chemical World, 2001(6): 289-305. (in Chinese)
[13] XIAO Jin-e. Quality control for production of cobalt oxide [J]. Non-ferrous Smelting, 1994(2): 26-29. (in Chinese)
[14] OHZUKU T, UEDA A, NAGAYAMA M. Electrochemistry and structural chemistry of LiNiO2 (R3m) for 4 volt secondary lithium cells [J]. J Electrochem Soc, 1993, 140(7): 1862-1870.
[15] OHZUKU T, MAKIMURA Y. Layered lithium insertion material of LiNi1/3Co1/3Mn1/3O2 for lithium ion batteries [J]. Chem Lett, 2001, 642-644.
[16] SHAJU K M, SUBBA RAO G V, CHOWDARI B V R. Performance of layered LiNi1/3Co1/3Mn1/3O2 as cathode Li-ion batteries [J]. Electrochim Acta, 2002, 48(2): 145-151.
(Edited by LAI Hai-hui)
Foundation item: Project(20051D0500403) supported by the Cultivation of Excellent Talents Program of Beijing Municipal Ministry of Organization, China
Corresponding author: LI Jian-gang; Tel: +86-13681208073; E-mail: lijiangang@bipt.edu.cn


