Trans. Nonferrous Met. Soc. China 29(2019) 840-848
Roasting oxidation behaviors of ReS2 and MoS2 in powdery rhenium-bearing, low-grade molybdenum concentrate
Xiao-hui FAN, Qiong DENG, Min GAN, Xu-ling CHEN
School of Minerals Processing and Bioengineering, Central South University, Changsha 410083, China
Received 21 June 2018; accepted 24 December 2018
Abstract:
The oxidation roasting process of molybdenum concentrate has significant advantages in industrial applications. However, utilization of low-grade mineral has many problems because it is more complex than the standard concentrate. In this study, the oxidation behaviors of powdery rhenium-bearing low-grade molybdenum concentrate were investigated through thermodynamic calculation, roasting experiments, thermogravimetric analysis, and phase analysis. The results obtained show that oxidation of MoS2 begins at 450 °C, and MoO3 reacts with metal-oxide forming molybdate at 600 °C. Finally, MoO3 can be dissolved in ammonia with a maximum content of approximately 80%. The volatile speed of Re was considerably slower than the oxidation speed of MoS2 because the nonvolatile products ReO2 and ReO3 were generated in reactions among MoS2, SO2, and Re2O7. The final volatilization rate of Re was almost 70%. This study determined the problems related to the roasting of low-grade molybdenum concentrate, which lays the scientific foundations for subsequent enhancement of molybdenum and rhenium extraction.
Key words:
low-grade molybdenum; rhenium; oxidation roasting; thermodynamics; phase evolution;
1 Introduction
Molybdenum is a rare and refractory metal [1-5] and China’s molybdenum reserve ranks second in the world [6]. However, over 64% of the molybdenum deposits in China exist in the form of complex paragenetic mines [7,8] and are characterized as low-grade, which results in processing difficulties. As rhenium is usually associated with molybdenum ore in the form of ReS2, molybdenum and rhenium generally become enriched in the molybdenum concentrate during mineral processing [9]. Rhenium is a rare and precious metal characterized by high temperature resistance, corrosion resistance, a special electronic configuration, and excellent catalytic activity. These characteristics make it efficient for use in spaceflight [10-12], medicine [13], and catalyst [14,15] applications. Because of its widespread commercial value and prospects for development, the recovery of rhenium has attracted substantial research attention. Thus, extracting rhenium during the recovery of molybdenum has great economic value.
The main oxidation methods for rhenium-bearing molybdenum concentrate are hydrometallurgy [16-22] and pyrometallurgy. The hydrometallurgical route can avoid the emission of SO2 but requires high-quality facilities, huge investment, and large costs because of the high price of the autoclave (one with a processing capacity of 1000 L costs at least $150000) and the specific requirements of a waterproof, acid proof, alkaline proof, and anticorrosion workshop.
At present, pyrometallurgy processing is a potentially attractive method of oxidizing molybdenum concentrate that is widely used in China. It includes the oxidizing roasting process, alkali fusing method [23], and lime-roasting process [24,25]. The oxidizing roasting process has greater advantages because it can effectively separate the oxidized products of MoO3 and Re2O7. In the oxidation process, MoS2 and ReS2 in the molybdenum concentrate are oxidized to MoO3 and Re2O7, respectively, and large amounts of SO2 are released. Re2O7 is a volatile compound with a melting point of only 297 °C and vapor pressure that increases rapidly with increasing temperature (0.045 kPa at 200 °C, 22.74 kPa at 300 °C, 1795.84 kPa at 400 °C, and 1.58×106 kPa at 650 °C) [26]. Meanwhile, a very small amount of MoO3 will volatilize and lead to molybdenum loss at high temperature [27], because its vapor pressure is much lower than that of Re2O7 (0.0067 kPa at 650 °C, 0.028 kPa at 670 °C, 0.059 kPa at 700 °C) [26]. However, it must be noted that another rhenium oxide with low valance, ReO3, is nonvolatile as its vapor pressure is extremely low (only 0.013 kPa at 400 °C) [26]. Thus, sufficient oxidation of MoS2 and ReS2 is very necessary. In the separation process, MoO3 can remain steady in calcine while Re2O7 evaporates into the flue gas and is enriched in the fume [28]. Moreover, the reaction temperature can be reached by the self-oxidation of molybdenum concentrate, with a massive amount of heat released. Additionally, the method of oxidizing roasting has merits such as being a simple process that requires only basic facilities and has a low cost. These advantages make it a more appropriate method for rhenium-bearing molybdenum concentrate.
Current studies on the oxidation mechanism of MoS2-ReS2 mainly focus on standard-molybdenum concentrate (Mo content >45%). There have been few reports on the oxidation of low-grade molybdenum- rhenium sulfide concentrate as it is considerably more complex than that of standard-molybdenum concentrate due to, for example, the multiple components and impurities and the low-grade of molybdenum. Thus, the phase evolution and roasting characteristics of low-grade concentrate during the oxidizing process were analyzed in this study. The reaction behaviors of molybdenum, rhenium, and sulfur were also revealed, which could provide the basis for effective molybdenum and rhenium extraction.
2 Experimental
2.1 Materials
The low-grade rhenium-bearing molybdenum concentrate was collected from separation in a copper mine. The chemical components of the material are shown in Table 1. The molybdenum content is 39.27%, which is lower than the grade of standard-molybdenum concentrate (Mo content above 45%). The content of valuable and usable rhenium is 0.034% (340 g/t). The gangue in the molybdenum concentrate mainly consists of SiO2, CaO, and MgO, which accounts for 9.77%, 5.23%, and 4.29%, respectively. Moreover, some sulfides such as CuS2 and FeS2 exist in the molybdenum concentrate. The proportion of molybdenum concentrate particles less than 0.074 mm is 65.44%.
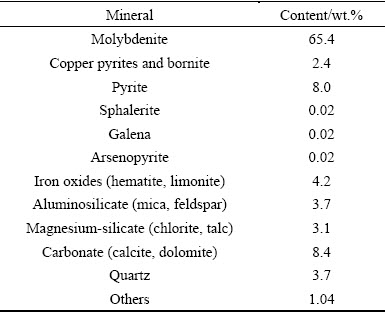
The mineral composition of molybdenum concentrate is given in Table 2. The majority of the molybdenum concentrate is molybdenite; approximately 65.4%. Moreover, the sulfides in the concentrate include copper pyrites, bornite, iron pyrite, arsenopyrite, and sphalerite. The iron-bearing minerals mainly consist of limonite and hematite. Other gangue minerals are composed of aluminosilicate, magnesium silicate, quartz, and carbonate.
Table 1 Chemical composition of molybdenum concentrate (wt.%)
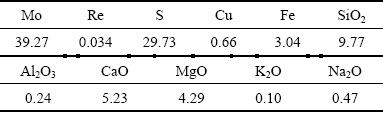
Table 2 Mineral compositions of molybdenum concentrate
2.2 Methods
The equipment schematically shown in Fig. 1 was used to simulate the oxidation process of multiple hearth furnace using a powdery rhenium-bearing, low-grade molybdenum concentrate. During the process, 20 g of dried molybdenum concentrate was put into an open corundum crucible measuring 100 mm × 50 mm × 15 mm and evenly spread onto the crucible. The crucible loaded with molybdenum concentrate was then moved into a muffle furnace and roasted at a setting temperature (400-700 °C) for a certain period (5-90 min) in air. Eventually, the roasted products were removed and ground to -0.074 mm for subsequent analyses.

Fig. 1 Schematic diagram of oxidizing roasting devices
The concentration of molybdenum was determined via the lead molybdate weight method, and sulfur was titrated with barium chloride. Rhenium was determined via inductively coupled plasma emission spectroscopy (ICP) with a PS-6 PLASMA SPECTROVAC, BAIRD (USA). The X-ray diffraction (XRD) patterns were recorded on a Rigaku Miniflex diffractometer with Cu Kα radiation at 35 kV and 20 mA. TG/DSC curves were obtained on a TG-DSC thermo gravimetric analyzer (Netzsch STA 449 C) in the temperature range of 20-1000 °C at a heating rate of 10 °C/min under air atmosphere. Additionally, all the reagents used in this work, including potassium oxide (K2O), sodium oxide (Na2O), calcium oxide (CaO), and magnesia (MgO), were of analytical grade.
The key aim of oxidizing roasting is to generate as much MoO3 and Re2O7 as possible because MoO3 in calcine can dissolve easily in the ammonia and be recycled by ammonia leaching. Meanwhile, Re2O7 is easy to volatilize, which results in separation from the molybdenum, and can become enriched and recycled from flue gas as well as smoke dust. The entire flow diagram of oxidation roasting-acid leaching is shown in Fig. 2.
The efficiency of the oxidizing roasting process can be evaluated by the following indexes: the rate of soluble molybdenum in the calcine (the rate of molybdenum dissolved in ammonia to total molybdenum) and the volatilization rate of molybdenum, rhenium, and sulfur. Formulas (1)-(3) show the calculation of the volatilization rate. To achieve desirable indexes, the soluble molybdenum content, as well as the volatilization rate of rhenium and sulfur, should be kept at a high level, whereas the volatilization rate of molybdenum should be low.
Volatilization rate of molybdenum:
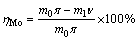 (1)
(1)
Volatilization rate of sulfur:
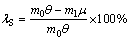 (2)
(2)
Volatilization rate of rhenium:
 (3)
(3)
where ν, μ, and τ stand for the molybdenum, sulfur, and rhenium contents in the calcine, respectively (%); π, θ, and σ stand for the molybdenum, sulfur, and rhenium contents in the molybdenum concentrate, respectively (%); and m0 and m1 stand for the mass of molybdenum concentrate and calcine, respectively (%).

Fig. 2 Flow diagram of oxidation roasting leaching
The rate of soluble molybdenum was detected as follows: 5 g of ground calcine was dissolved in a 100 mL beaker with a liquid/solid ratio of 10:1 and a mass concentration of ammonia of 10%. Calcine was blended with excessive ammonium hydroxide to ensure adequate dissolution. Then, the sealed beaker was placed in a constant temperature oven at 70 °C for 2 h of leaching. Finally, samples were taken out to be filtrated and dried, and the molybdenum content in the leached residue was analyzed.
Rate of soluble molybdenum:
 (4)
(4)
where α and β stand for the molybdenum contents in the calcine and in the ammonia leached residue, respectively (%), and m1 and m2 stand for the mass of calcine and ammonia leaching residue, respectively (%).
3 Results and discussion
3.1 Oxidation behaviors of MoS2
3.1.1 XRD and TG-DSC characterization
The phases of roasted products at different roasting temperatures were analyzed by X-ray diffraction (XRD). The results are given in Fig. 3. MoS2 was not oxidized at 400 °C as molybdenum oxide was not found in the roasted product. When the temperature rose to 450-500 °C, the diffraction peaks of MoS2 decreased gradually while some diffraction peaks of MoO3 started to appear. When the temperature increased to above 600 °C, the diffraction peaks of MoS2 disappeared, and the diffraction peaks of MoO3 became enhanced. Moreover, diffraction peaks of CaMoO4 and MgMoO4 were observed. According to the analyses, the molybdenum phases with sufficient oxidation time were mainly MoO3, MgMoO4, and CaMoO4. These two types of molybdate are considered an obstacle to the leaching of molybdenum because they are difficult to dissolve in the ammonium hydroxide while some types of molybdates easily dissolve in ammonium hydroxide (such as K2MoO4 and Na2MoO4 [18]).

Fig. 3 XRD patterns of products roasted at different temperatures for 90 min
The phases of the roasted products at different roasting time were tested by XRD (Fig. 4). There were several types of Mo-bearing minerals in the roasted product: MoS2, MoO2, and MoO3. The existence of un-oxidized or uncompleted oxidized minerals such as MoS2 and MoO2 was the dominant factor leading to low soluble molybdenum content for short roasting time of 5 min. When the roasting time was extended to 10 min, the diffraction peaks of MoS2 decreased substantially, while the diffraction peaks of MoO3 and MoO2 became somewhat stronger. As the roasting time exceeded 25 min, the diffraction peaks of MoS2 and MoO2 disappeared, while the diffraction peaks of MoO3 became stronger. At the same time, diffraction peaks of MgMoO4 and CaMoO4 were observed. These results indicate that metallic oxide could not react with MoO3 until MoS2 was completely oxidized, and the products were a large number of molybdates, which could not be dissolved by ammonium hydroxide.

Fig. 4 XRD patterns of roasted products for different time at 650 °C
The oxidation process of MoS2 was analyzed by thermal gravity analysis in air under a heating rate of 10 °C/min. From the TG-DSC curves (Fig. 5), it was observed that the entire process involved the evaporation of moisture and the volatilization of volatile-like flotation reagents in the molybdenum concentrate below 400 °C. When the temperature increased to 400-625 °C, two exothermic peaks appeared at approximately 548 °C and 619 °C. Correspondingly, the sample continuously lost mass below 625 °C. All these phenomena represented the rapid generation of MoO2 and MoO3. The main reaction from 625-650 °C was the oxidation of remnant MoO2. Thus, the transformation process of molybdenite was MoS2→MoO2→MoO3, which corresponds to the results shown in Fig. 4. The time interval for the existence of MoO2 was relatively short. When the temperature was over 650 °C, MoO3 started to sublime. According to the TGA results, the mass loss indicates that the sublimation of MoO3 was the major process after the oxidation of remnant MoO2 as the temperature increased above 650 °C.

Fig. 5 TG-DCS curves of molybdenum concentrate in air
3.1.2 Oxidation process of MoS2
During the oxidation of low-grade molybdenum concentrate, metallic sulfide ores were oxidized to metallic oxides and SO2. Possible reactions are as follows:
MoS2+3.5O2(g)=MoO3+2SO2(g) (5)
MeS+1.5O2=MeO+SO2(g) (Me: Fe,Mg,Ca,Cu) (6)
Figure 6 shows the influences of temperature on the oxidation of molybdenum concentrate. Molybdenum could not be oxidized and the rate of soluble molybdenum was below 5% at 400 °C while a portion of SO2 volatilized. Analyzing the reactions in thermodynamics [29] showed that the trend of oxidation of FeS2 is large under normal pressure. Thus, the reason could be that the oxidation process of FeS2 is much easier than that of MoS2. This view is also confirmed in Fig. 3 as the diffraction peaks of Fe2O3 started to appear at 400 °C. Under the trial conditions, the ores contacted fully with the oxygen and the oxidation desulfurization reactions occurred easily. Corresponding to this, the diffraction peaks of sulfate could not be observed (see Figs. 3 and 4), and the sulfur in metallic sulfide mainly volatilized in the forms of SO2. At a temperature from 450 to 500 °C, the oxidation of molybdenum sulfide was reinforced and the volatilization of SO2 increased. The rate of soluble molybdenum increased from 43.14% to 69.09% as the volatilization rate of sulfur increased from 47.60% to 75.57%. The velocity of the oxidizing reaction, especially the oxidation of molybdenum sulfide, increased significantly over 550 °C. Consequently, the rate of soluble molybdenum and the volatilization of sulfur were both above 80%. However, both indexes increased slowly as the temperature increased from 600 to 700 °C. According to the analysis, when the temperature was relatively low at 450-550 °C, MoS2 began to be oxidized and the gas diffused slowly. When the temperature increased to 600-700 °C, MoS2 was almost completely oxidized. The soluble molybdenum approached a peak and the volatilization rate of sulfur was close to 99% at 650 °C. The analyzing results of the reactions in thermodynamics [29] showed that the generated MoO3 could react with some oxides of metallic impurities, forming molybdate and leading to melting and sintering of materials. The main reactions were spontaneous thermodynamics reactions and could be expressed as follows:
MgO+MoO3=MgMoO4(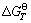 =-54.58-0.02T) (7)
=-54.58-0.02T) (7)
CaO+MoO3=CaMoO4(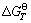 =-163.00-0.01T) (8)
=-163.00-0.01T) (8)
This is why the oxidizing process of molybdenum sulfide was restricted at a temperature of 700 °C or above. The occurrence of the molybdate phase confirms this view (Fig. 3). MoO3 on the surface became drastically volatilized.
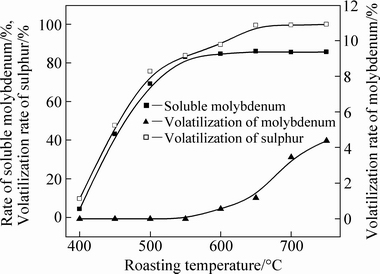
Fig. 6 Effect of roasting temperature on oxidation of molybdenum
The effects of roasting time on the oxidization of molybdenum concentrate are shown in Fig. 7. The molybdenum concentrate was oxidized quickly at 650 °C. When the roasting lasted for 5 min, the rate of soluble molybdenum reached 25.70% and the volatilization rate of sulfur was 40.98%. As the roasting continued, sulfur became greatly oxidized and its volatilization rate reached 96%. The rate of soluble molybdenum was only 73.76% and the increase was lower than that of sulfur volatilization. In the first 15 min, sulfide minerals were oxidized, and the MoO3 product had a disproportionation reaction with MoS2, forming a low valence state of molybdenum such as MoO2, which led to a low content of soluble molybdenum in the early roasted product. After 20 min of roasting, the rate of soluble molybdenum

Fig. 7 Effect of roasting time on oxidation of molybdenum at 650 °C
changed slightly, which reflected the oxidation of low valence state molybdenum, and the volatilization rate of sulfur increased slightly. As the roasting proceeded, the retention rate of molybdenum decreased due to volatilization of MoO3. When the roasting time exceeded 25 min, the volatilization rate of sulfur increased to 99.70% and the volatilization rate of molybdenum continued to increase, whereas the rate of soluble molybdenum was approximately 80%.
3.2 Oxidation behavior of ReS2
3.2.1 Oxidation process of ReS2
The oxidizing roasting process of rhenium-bearing molybdenum concentrate is a complicated physic- chemical process. Based on the calculation of thermodynamic data [29], the relationships between 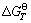 and T in the reactions were analyzed, and the results are shown in Fig. 8. The main reactions of rhenium include ReS2 oxidation, the disproportionation reaction of Mo-Re-S, and the formation of rhenate. Under the standard state, ReS2 can be directly oxidized into Re2O7 (formula (9)). At the beginning of oxidation, ReS2 can be easily oxidized. However, Re2O7 is also easily deoxidized by MoS2 that has not been oxidized, forming low valence states of the rhenium oxides like ReO2 and ReO3 (formulas (14) and (15)). Re2O7 can also be deoxidized into ReO3 by SO2 but not ReO2. According to the thermodynamic analysis, ReS2 was oxidized along with the molybdenite, and the products were ReO2, ReO3 and Re2O7. In the later stage of oxidation, ReO2 and ReO3 were oxidized into Re2O7, which became volatilized, MoS2 became completely oxidized, and the concentration of SO2 decreased.
and T in the reactions were analyzed, and the results are shown in Fig. 8. The main reactions of rhenium include ReS2 oxidation, the disproportionation reaction of Mo-Re-S, and the formation of rhenate. Under the standard state, ReS2 can be directly oxidized into Re2O7 (formula (9)). At the beginning of oxidation, ReS2 can be easily oxidized. However, Re2O7 is also easily deoxidized by MoS2 that has not been oxidized, forming low valence states of the rhenium oxides like ReO2 and ReO3 (formulas (14) and (15)). Re2O7 can also be deoxidized into ReO3 by SO2 but not ReO2. According to the thermodynamic analysis, ReS2 was oxidized along with the molybdenite, and the products were ReO2, ReO3 and Re2O7. In the later stage of oxidation, ReO2 and ReO3 were oxidized into Re2O7, which became volatilized, MoS2 became completely oxidized, and the concentration of SO2 decreased.

Fig. 8 Relationship between  and T for rhenium-bearing mineral oxidation reactions
and T for rhenium-bearing mineral oxidation reactions
3.2.2 Volatilization of rhenium
The influence of roasting temperature and roasting time on the volatilization of rhenium was also studied (Fig. 9). The volatilization rate of rhenium was very low at 400 °C when ReS2 began to be oxidized. After heating to 450-500 °C, the volatilization rate of rhenium increased but was still below 40%. As the temperature increased to 600-650 °C, rhenium was significantly oxidized and the volatilization rate approached 70%. However, the volatilization rate did not markedly change when the temperature continued to increase. Research on the volatilization of rhenium for different roasting time at 650 °C showed that the volatilization rate of rhenium increased as roasting time proceeded in the early oxidation phase (0-20 min). The volatilization rate rapidly reached 44.38% as the oxidation time increased to 20 min and increased more slowly when the time continued to increase from 20 to 90 min. As the roasting time exceeded 90 min, rhenium could no longer oxidize; the volatilization rate was almost 70% for a roasting time of 90 min.

Fig. 9 Effects of roasting temperature on volatilization rate of rhenium with roasting for 90 min (a) and roasting time on volatilization rate at 650 °C (b)
Into the pure substance of MoS2, 0.034% of analytically pure ReS2 was added to simulate the Re-bearing molybdenum concentrate. The effects of adding 1% alkali oxides or alkaline earth oxides on the volatilization of rhenium were tested at 650 °C for 90 min, and the results are shown in Fig. 10. Without the addition of alkali oxides or alkaline earth oxides, the volatilization rate of rhenium was close to 100% during the roasting process, which indicates that almost all the rhenium sulfide can be oxidized to volatile material of Re2O7. However, because alkali oxides or alkaline earth oxides exist in sulfide ore, the volatilization of rhenium was very low due to the generation of non-volatile rhenate. K2O had the greatest effect on the volatilization of rhenium of all the tested metal oxides, followed by Na2O, CaO, and MgO. It was verified that metal oxides in molybdenum concentrate inhibit the volatilization of rhenium.

Fig. 10 Effect of metal oxides on rhenium volatilization rate tested at 650 °C
3.3 Discussion of MoS2 and ReS2 oxidation
The relationships among the oxidation of molybdenum, the volatilization of rhenium, and the volatilization of sulfur are shown in Fig. 11. The oxidation of rhenium always lagged behind that of molybdenum and sulfur. The process of rhenium oxidation can be divided into two stages. From 0–20 min, both MoS2 and ReS2 are oxidized simultaneously. But only a high valence state of rhenium, such as Re2O7, can easily volatilize, which reduced the speed of rhenium volatilization compared to that of MoS2 oxidation. As the roasting time exceeded 20 min, MoS2 became completely oxidized and the removal rate of sulfur approached 100%. Rhenium continued to oxidize at a relatively low speed between 20 and 90 min. Therefore, the volatilization of rhenium requires its thorough oxidization after complete MoS2 oxidation and SO2 removal, which explains why the volatilization of rhenium lagged behind the oxidation of molybdenum. Due to the influence of impurities, such as Na, K, Ca, and other alkaline-earth metals in the reaction system, a mass of rhenate formed; the volatilization rate of rhenate only reached 70%. Note that the chemical formulas of the rhenate were MⅠReO4 or MⅡ(ReO4)2 because MⅠ and MⅡ were monovalent and divalent cations, respectively.

Fig. 11 Effect of roasting time on roasting process of molybdenum concentrate
As stated above, in contrast to standard molybdenum concentrate, more complex physico- chemical reactions occur during the roasting process of low-grade molybdenum-rhenium sulfide concentrate, such as the disproportionation reaction between oxidation products and sulfide, and the formation of molybdate and rhenate. These reactions inhibited the oxidation and separation of molybdenum and rhenium. In the roasting process, ReS2 was oxidized along with the molybdenite. The whole oxidation process is shown in Fig. 12.
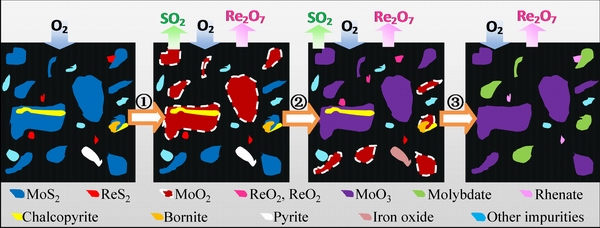
Fig. 12 Roasting process of Mo-Re-S system in molybdenum concentrate
Step 1: In the early stage of oxidation, MoS2 was oxidized to MoO2, and ReS2 was simultaneously oxidized to Re2O7.
Step 2: Re2O7 was deoxidized into low valence states of rhenium oxides such as ReO2 and ReO3 by MoS2 and SO2 because of the disproportionation reaction between oxidation products and sulfide. At the same time, MoO2 was continuously oxidized to MoO3 with increasing roasting temperature or roasting time.
Step 3: The oxidation of rhenium lagged behind that of molybdenum and sulfur. In the late stage of oxidation, MoS2 was completely oxidized and rhenium was continuously volatilized during the formation of Re2O7. Finally, due to the influence of impurities, MoO3 and Re2O7 reacted with the metallic oxide, forming a large amount of molybdate and rhenate, and the oxidation process was restricted. Thus, the maximum rate of soluble molybdenum was approximately 80% and the final volatilization rate of rhenium was below 70%. Further research should focus on the inhibition or extraction of molybdate and rhenate.
4 Conclusions
(1) MoS2 began to be oxidized at 450 °C and achieved complete oxidation over 600 °C. MoO3 started to volatilize when the temperature exceeded 650 °C and violently volatilized at 700 °C. The oxidative products of MoS2 were mainly MoO2, MoO3, and molybdate. The maximum rate of soluble molybdenum was approximately 80% due to insoluble molybdate caused by the high impurity content in molybdenum concentrate.
(2) With increasing roasting temperature and roasting time, the volatilization rate of rhenium increased but still lagged behind the oxidation efficiency of molybdenum. When molybdenum was completely oxidized, the volatilization rate of rhenium improved significantly. The formation of rhenate and the difficulty in oxidizing the encapsulated low valence state of rhenium resulted in a final volatilization rate below 70%.
(3) The oxidizing roasting process of rhenium- bearing molybdenum concentrate involved oxidization of the low valence states of molybdenum and rhenium, disproportionation of the Mo-Re-S system, and the formation of molybdate and rhenate. The main products of rhenium sulfide were ReO2, ReO3, and Re2O7. Re2O7 can react with MoS2 and SO2 spontaneously, and rhenium can volatilize adequately after the thorough oxidation of MoS2 and the complete release of SO2.
References
[1] GUPTA C K. Extractive metallurgy of molybdenum [M]. Boca Raton: CRC Press, 1992.
[2] SCHEINER B, LINDSTORM R. Extraction of molybdenum from ores by electro-oxidation [J]. Febs Letters, 1971, 444: 217-221.
[3] USGS, U.S. Geological Survey Mineral Commodity Summaries 2017 [EB/OL]. https://minerals.usgs.gov/minerals/pubs/commodity/molybdenum/ mcs-2017-molyb.pdf.
[4] GERHARDT N, PALANT A, DUNGAN S. Extraction of tungsten (VI), molybdenum (VI) and rhenium (VII) by diisododecylamine [J]. Hydrometallurgy, 2000, 55: 1-15.
[5] XIANG Si-yun, YAO Ying, WAN Yu-nan. Comparative study on trace element excretions between nonanuric and anuric patients undergoing continuous ambulatory peritoneal dialysis [J]. Nutrients, 2016, 8: 826-15.
[6] KUMAR M, MANKHAND T, MURTHY D. Refining of a low-grade molybdenite concentrate [J]. Hydrometallurgy, 2007, 86: 56-62.
[7] YIN Zhi-gang, SUN Wei, HU Yue-hua, GUAN Qing-jun, ZHANG Chen-hu. Depressing behaviors and mechanism of disodium bis (carboxymethyl) trithiocarbonate on separation of chalcopyrite and molybdenite [J]. Transactions of Nonferrous Metals Society of China, 2017, 27: 883-890.
[8] XIAN Peng-fei, ZHOU Sheng-fan, WANG Ming-yu, WANG Xue-wen, CHEN Bian-fang. Extraction of molybdenum and nickel from roasted Ni-Mo ore by hydrochloric acid leaching, sulphation roasting and water leaching [J]. Transactions of Nonferrous Metals Society of China, 2017, 27: 220-226.
[9] LASHEEN T, EI-AHMADY M, HASSIB H. Molybdenum metallurgy review: Hydrometallurgical routes to recovery of molybdenum from ores and mineral raw materials [J]. Mineral Processing & Extractive Metallurgy Review, 2015, 36: 145-173.
[10] WU Zhi-hong, ZHOU Wan-cheng, LUO Fa. Effect of MoSi2content on dielectric and mechanical properties ofMoSi2/Al2O3composite coatings [J]. Transactions of Nonferrous Metals Society of China, 2012, 22: 111-115.
[11] FENG Bao-qi, GUO Jin-liang, MA Gao-feng. Research on separation and extraction of rhenium [J]. Molybdenum Industry, 2013, 37: 12-15. (in Chinese)
[12] USGS, U.S. Geological Survey Mineral Commodity Summaries 2017 [EB/OL]. https://minerals.usgs.gov/minerals/pubs/commodity/rhenium/ mcs-2016-rheni.pdf.
[13] HUANG Hui, CHEN Fu-liang, JIANG Yan. The current situation of domestic molybdenum resources and molybdenum smelting analysis [J]. Yunnan Metallurgy, 2014, 43: 66-70. (in Chinese)
[14] KETCHAM V, COLTRINATI E, HAZEN W. Pressure oxidation process for the production of molybdenum trioxide from molybdenite: US Patent, 08327980 [P]. 2000-11-21.
[15] AMELUNXEN P, WILMOT J, HAZEN W. System and method for conversion of molybdenite to one or more molybdenum oxides: EP Patent, 11182640 [P]. 2007-11-13.
[16] HOSSEIN S, JALIL V K, ABOLFAZL B, IDA D M, FRANCESCO V. An enhanced dissolution rate of molybdenite and variable activation energy [J]. Hydrometallurgy, 2018, 17: 52-63.
[17] FU Yun-feng, XIAO Qing-gui, GAO Yi-ying, NING Peng-ge, XU Hong-bin. Direct extraction of Mo(VI) from acidic leach solution of molybdenite ore by ion exchange resin: Batch and column adsorption studies [J]. Transactions of Nonferrous Metals Society of China, 2018, 28: 1660-1669.
[18] ZHANG Qi-xiu, ZHAO Qin-sheng. Tungsten-molybdenum metallurgy [M]. Beijing: Metallurgical Industry Press, 2005. (in Chinese)
[19] CAO Zhan-fang, ZHONG Hong, LIU Guang-yi. Electric-oxidation kinetics of molybdenite concentrate in acidic NaCl solution [J]. Canadian Journal of Chemical Engineering, 2010, 87: 939-944.
[20] ROMANO P, ALGUACIL F, MUNOZ J. Comparative study on the chalcopyrite bioleaching of a molybdenite concentrate with mesophilic and thermophilic bacteria [J]. Fems Microbiology Letters, 2001, 196: 71-75.
[21] CHEN Jia-wu, GAO Cong-jie, ZHANG Qi-xiu. Leaching of nickel-molybdenum sulfide ore in membrane biological reactor [J]. Transactions of Nonferrous Metals Society of China, 2011, 21: 1395-1401.
[22] SHI Li-hua, WANG Xue-wen, WANG Ming-yu. Extraction of molybdenum from high-impurity ferromolybdenum by roasting with Na2CO3 and CaO and leaching with water [J]. Hydrometallurgy, 2011, 108: 214-219.
[23] ALEKSANDROV P V, MEDVEDEV A S, MILOVANOV M F, IMIDEEV V A, KOTOVA S A. Molybdenum recovery from molybdenite concentrates by low-temperature roasting with sodium chloride [J]. International Journal of Mineral Processing, 2017, 161: 13-20.
[24] ZOU Zhen-qiu, ZHOU Qin-jian. Recovery of molybdenum and rhenium from molybdenite concentrate in Dexing copper ore by lime roasting-N235 extraction method [J]. Mining and Metallurgical Engineering, 2002, 22: 79-84. (in Chinese)
[25] ZHOU Qiu-sheng, YUN Wei-tao, XI Jun-tao, LI Xiao-bin, QI Tian-gui. Molybdenite-limestone oxidizing roasting followed by calcine leaching with ammonium carbonate solution [J]. Transactions of Nonferrous Metals Society of China, 2017, 27: 1618-1626.
[26] CHEN Jia-yong. Handbook of hydrometallurgy [M]. Beijing: Metallurgical Industry Press, 2008. (in Chinese)
[27] GOLMAKANI M H, VAHDATI J, BABAKHANI A. A novel method for direct fabrication of ferromolybdenum using molybdenite via self-propagation high temperature synthesis [J]. Materials Chemistry and Physics, 2017, 194: 9-16.
[28] HEVIA R, SOTO-KREBS L. Rhenium extraction process: US Patent 193459 [P]. 1971-10-28.
[29] YE Da-lun. Thermodynamics data book of practical inorganic substance [M]. Beijing: Metallurgical Industry Press, 2002. (in Chinese).
粉状含铼低品位钼精矿焙烧过程中ReS2和MoS2的氧化行为
范晓慧,邓 琼,甘 敏,陈许玲
中南大学 资源加工与生物工程学院,长沙 410083
摘 要:钼精矿氧化焙烧工艺在工业应用中具有显著的优势。然而,低品位钼精矿因其比标准钼精矿复杂得多而在应用过程中存在许多问题。通过热力学计算、焙烧实验、热重分析和物相分析等,研究粉状含铼低品位钼精矿的氧化行为。结果表明,MoS2从450 °C氧化,当温度达到600 °C时,MoO3与金属氧化物反应并形成钼酸盐。最终,由于不可溶的钼酸盐生成,约80%的MoO3溶解氨水中。由于Re2O7、MoS2和SO2之间互相反应生成低价铼氧化物ReO2和ReO3,铼的挥发速度远落后于钼的氧化速度。当MoS2氧化完全后,铼的挥发加强,最终铼挥发率接近70%。查明了低品位钼精矿在焙烧过程中钼、铼氧化效率低的主要原因,为后续强化钼、铼的提取奠定了基础。
关键词:低品位钼精矿;铼;氧化焙烧;热力学;物相演变
(Edited by Xiang-qun LI)
Foundation item: Projects (U1760107, U1660206) supported by the National Natural Science Foundation of China; Project (2013zzts064) supported by the Innovation Foundation for Postgraduate of Central South University, China
Corresponding author: Min GAN; Tel: +86-13467517674; E-mail: ganminhao@126.com
DOI: 10.1016/S1003-6326(19)64994-0
Abstract: The oxidation roasting process of molybdenum concentrate has significant advantages in industrial applications. However, utilization of low-grade mineral has many problems because it is more complex than the standard concentrate. In this study, the oxidation behaviors of powdery rhenium-bearing low-grade molybdenum concentrate were investigated through thermodynamic calculation, roasting experiments, thermogravimetric analysis, and phase analysis. The results obtained show that oxidation of MoS2 begins at 450 °C, and MoO3 reacts with metal-oxide forming molybdate at 600 °C. Finally, MoO3 can be dissolved in ammonia with a maximum content of approximately 80%. The volatile speed of Re was considerably slower than the oxidation speed of MoS2 because the nonvolatile products ReO2 and ReO3 were generated in reactions among MoS2, SO2, and Re2O7. The final volatilization rate of Re was almost 70%. This study determined the problems related to the roasting of low-grade molybdenum concentrate, which lays the scientific foundations for subsequent enhancement of molybdenum and rhenium extraction.


