B2O3替代KBF4用氟盐法制备Al-Ti-B晶粒细化剂的研究
宜宾学院计算物理重点实验室
兰州理工大学甘肃省有色金属新材料省部共建国家重点实验室
摘 要:
晶粒细化是提高铝合金强度和塑性,改善铸件质量的重要途径。目前,铝合金的晶粒细化主要基于Al-Ti-B三元合金体系采用氟盐法制备,通过在熔融铝合金中添加Al-Ti-B细化剂棒使α-Al晶粒细化,从而获得均匀、细小、等轴晶粒组织。然而,该方法的KBF4和K2Ti F6混合卤盐,尤其是KBF4,增加了生产成本,引起更多的氟化物排放和难处理的残渣,且反应过程造成的热量损失很难由熔体自身的放热反应来弥补。因此,寻找一种更经济、更高效的化合物替代KBF4,为制备新型、质优的Al-Ti-B晶粒细化剂提供了方向。实验表明:用B2O3完全替代氟盐法中的KBF4盐,降低了晶粒细化性能,产生大量的残渣,且降低了熔体的流动性,使浇铸过程更加困难;当B2O3与除渣助熔剂预先混合,再与K2Ti F6混合加入到熔体中时,在不考虑添加物损失的情况下,铝熔体的微观组织结构和细化效率均会得到明显的改善;当B2O3和KBF4各添加50%时,钛的回收率和晶粒的细化效率,几乎与单独添加KBF4时一致。由此得出结论:在不损失晶粒细化效率的前提下,可以用B2O3部分替代KBF4,但绝非全部。该方法的优势在于:具有更低的氟化物排放量和颗粒物添加量,在相同质量下,B2O3提供的硼含量达到4倍,价格也更便宜。
关键词:
中图分类号: TG292;TG223
作者简介:任峻(1978-),男,四川巴中人,硕士,副教授,研究方向:金属基复合材料、轻质合金;电话:13778963061;E-mail:lilyrj@163.com;
收稿日期:2014-05-29
基金:四川省教育厅重点项目(12ZA202)资助;
Manufacture of Al-Ti-B Grain Refiner by Reaction of Complex Halide Salts with Molten Aluminum in B2O3 Alternative KBF4
Ren Jun Tao Qingui Ma Ying
Key Laboratory of Computational Physics,Yibin University
State Key Laboratory of Gansu Advanced Nonferrous Metal Materials,Lanzhou University of Technology
Abstract:
Grain refinement was one of the means of increasing strength and plasticity of aluminum alloy and improving quality of castings. Currently,the grain refinement of aluminum alloys mainly depended on the Al-Ti-B alloy ternary system,which added Al-TiB grain refiner robs into molten aluminum in the reaction of complex halide salts to refine α-Al and obtain a uniform,fine and equiaxed structure. However,the mixed malts of the method,KBF4 and K2Ti F6,particularly KBF4,greatly increased the cost of production,caused more fluoride emissions and needed troublesome residue treatment process. Besides,it was difficult to compensate the heat loss of the reaction-self from the exothermic reaction of the molten aluminum. Therefore,it was a direction to find a more economical and efficient compound which could replace KBF4 salt for manufacturing high quality Al-Ti-B grain refiner. The experiment showed that KBF4 was completely replaced by B2O3 during the complex halide salts with molten aluminum to prepare Al-Ti-B grain refiner,which could not only reduce the refiner performance and produce a large amount of residue,but also decrease the fluidity of the molten aluminum and lead to more difficulty to the casting process. With premixing B2O3 and deslagging agent and then adding K2 Ti F6to the molten aluminum,the microstructure and refine efficiency of aluminum alloy would be significantly improved without considering the loss of additives. When each of B2O3 and KBF4was added by 50%,the recovery rate of Ti and the grain refiner efficiency of aluminum alloy were almost consistent with the case that only KBF4 was added. Therefore,we concluded that B2O3 could be used to replace KBF4 only partially but not completely on the premise that grain refining efficiency was not taken into consideration. This method could produce lower fluoride emissions and particulates additive. Moreover,B content that provided by B2O3 was nearly 4 times higher,and the additive of this method was cheaper than other additives in the same mass.
Keyword:
aluminum alloys; Al-Ti-B; grain refinement;
Received: 2014-05-29
铝合金因其质轻、易加工、耐久性高和良好的物理力学性能等特点,在高新电子领域得到越来越广泛的应用,但后续深加工对铝合金的冶金组织、成分和性能提出了更高的要求。晶粒细化是提高铝合金强度和塑性,改善铸件质量的重要途径之一[1,2]。目前,铝合金很大程度上依赖于Al-Ti-B中间合金的三元合金体系,典型的做法是在铝合金熔融过程中添加Al-Ti-B晶粒细化剂棒,借助机械振动、电磁振动等外来能量使 α-Al晶粒细化,从而控制铸造晶粒尺寸,获得均匀、细小、等轴晶粒组织的目的[3,4,5,6]。但是,为铝合金的生产提供Al-Ti-B晶粒细化剂的诸多方法中[7,8,9,10],采用在熔融铝合金中加入KBF4和K2Ti F6混合卤盐的氟盐法是最传统的方法之一[11,12,13,14,15]。实验研究证明,该方法也存在一些不足,混合卤盐所含的B和Ti相对较少,昂贵的原料大大增加了生产成本,并引起更多的氟化物排放量和难处理的残渣,尤其是KBF4,且大量颗粒物质所造成的熔体冷却的热量损失很难由放热反应产生的热量弥补[15]。因此,用B和Ti含量更高的化合物替代KBF4和K2Ti F6的混合卤盐,成为研究的热点[16,17,18,19,20,21,22]。本文在Al-5Ti-B晶粒细化剂制取过程中,用B2O3代替KBF4,在不同的实验条件下,通过细化参数的分析以确定最佳的工艺,旨在为今后更进一步的研究提供可能的方法。
1 实验
据Birol的研究[12],拟分别采用4 次实验制备Al-5Ti-B晶粒细化剂的不同合金,每次制备1000g,原料添加量如表1 所示。
制备合金1 时,先预混含有KBF4和K2Ti F6的商业纯度盐,再加入到液态铝熔体中,以制备标准的Al-5Ti-B晶粒细化剂[15]。根据实验的安排,制备其他3 种合金时,KBF4将完全或部分被B2O3所取代。制备合金2 时,其工艺流程与制备合金1 相一致,但B2O3作为B元素唯一的载体化合物加入到铝熔体中。原料添加量表明,每制备1000 g的合金2 能减少约84 g的添加物。制备合金3 的过程与制备合金2 相同,但B2O3加入到铝熔体之前,须预先进行除渣处理,最后再与K2Ti F6混合加入到铝熔体中,该过程相对于合金2 的制备减少约70 g的添加物。制备合金4 时,化合物KBF4和B2O3同时提供B元素,B2O3添加量减少50% ,其余由KBF4提供,为避免KBF4和K2Ti F6的混合盐与B2O3在铝熔体内混合反应,须将合金4 熔体提前30 min放置于800 ℃ 的电阻炉内充分搅拌,待熔融的KAl F4盐完全反应后再加入反应物B2O3。
表1 制备1000 g的Al-5Ti-B晶粒细化剂的原料添加量Table 1Total of raw material for preparation of 1000 g Al-5Ti-B grain refiners / g 下载原图
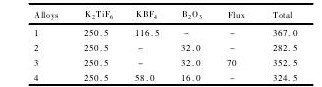
表1 制备1000 g的Al-5Ti-B晶粒细化剂的原料添加量Table 1Total of raw material for preparation of 1000 g Al-5Ti-B grain refiners / g
最后,将样品合金1 ~ 4,按标准金相制样方法,用0. 5% 的HF试剂腐蚀并用光学显微镜( OM) 观察组织特征,通过X射线衍射( XRD) 实验确定铝基中不溶性硼化物颗粒含量,分别进行细化效果评估。
2 结果与讨论
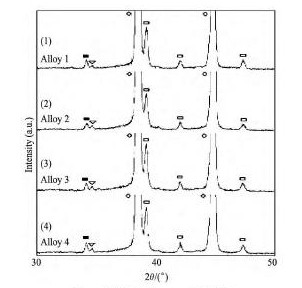
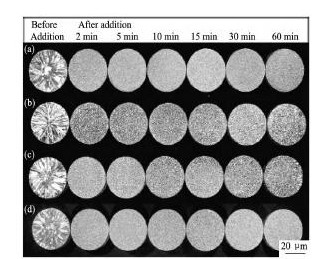
采用氟盐法制备的Al-5Ti-B晶粒细化剂,α-Al相中分散着大量的可溶性铝化物和不溶性硼化物,前者呈块状且尺寸小于20 μm,而硼化物颗粒很多,往往聚集成块、尺寸也较小( 图1( a) ) ,通过XRD分析得知其大部分为Ti B2( 图2( 1) ) 。当加入平均尺寸为100 μm的Al-5Ti-B晶粒2 min后,合金的细化效果非常明显( 图3( a) ) ,因此它作为评定实验中制备的4 种合金细化剂细化效果的标准。
氟盐法中主要的化学反应:
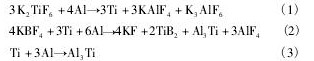
液态铝减少了K2Ti F6的量,只要达到溶解饱和度,Ti就能以Al3Ti颗粒形式析出,Ti的回收率可达到99. 4%[12,13]。合金1 较理想化的微观组织结构,主要是因为尺寸为亚微米的Ti B2粒子数量占优势,且Al3Ti粒子呈块状形态、尺寸范围较宽,同时大量的Al3Ti和Ti B2颗粒具有较高的晶粒细化效率和较少的残渣量。
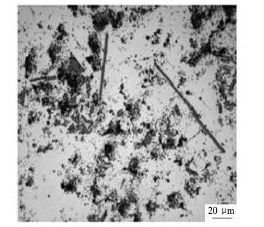
采用与制备合金1 相同的工艺,仅用B2O3作为B元素的添加物制备合金2 时,钛的回收率下降到70%( 表2) ,较低的钛回收率亦表现在合金相图中。合金2 中,虽然铝化物较少但显示的结构与合金1 很类似( 图1( b) ) ,偶尔还会看到花瓣状( 图4) 。
图1制备合金的微观组织Fig.1 OM images of prepared alloys
(a)Alloy 1;(b)Alloy 2;(c)Alloy 3;(d)Alloy 4
图2 制备合金的XRD衍射光谱Fig. 2 XRD patterns of prepared alloys
可见,随着B2O3化合物的添加,伴随Ti B2粒子的形成,熔融铝可能和随后生成的Al B2与Al3Ti发生了一系列的反应:

当然,也可能通过溶质Ti与B2O3直接发生了如下还原反应:
表2 制备合金1 ~ 4 的成分含量及Ti回收率Table 2 Constituents and Ti recovery of prepared Alloys 1~ 4 ( %,mass fraction) 下载原图
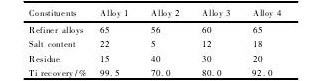
表2 制备合金1 ~ 4 的成分含量及Ti回收率Table 2 Constituents and Ti recovery of prepared Alloys 1~ 4 ( %,mass fraction)
图3制备合金的细化性能测试Fig.3 Refining performance testing results of prepared alloys
(a)Alloy 1;(b)Alloy 2;(c)Alloy 3;(d)Alloy 4
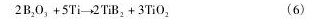
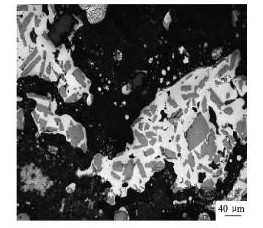
由于熔体中存在过饱和的Al,即便Al B2和Al3Ti两种反应物在熔融液中含量很多,但反应( 5) 都进行得极为迟缓[22]。另一方面,反应( 6) 具有很大的反应动力,Ti直接被B2O3氧化为Ti B2,这也是合金2 的Ti回收率下降的一种合理解释[23]。同时,X射线衍射谱中存在大量的Al B2,表明反应( 4) 也正常发生了。随着熔体温度升高,尤其高于900 ℃ 时,反应( 5) 将会使Al3Ti颗粒快速凝固成非等轴的结晶块状铝,其部分滞留在铝熔体残渣中而降低了合金的流动性( 图5) 。
通过分析得知,无论加入何种添加物到铝熔体中,都有一定的放热效应,使熔体温度升高,但升高的幅度存在一定的区别。实验发现,添加B2O3-K2Ti F6混合物的温升效应更明显,放热量更大,但这不能作为解释合金2 出现花瓣状颗粒的原因,因为从合金1 观察到的典型硼化物形态数量较合金2 略有增加,但Al B2的小部分增加( 图2( 2) )预计不会对生成Al3Ti的一系列反应有影响。可见,合金2 完全依靠B2O3提供B元素,仅制备了一个低效的晶粒细化剂( 图3( b) ) ,低的Ti回收率和花瓣状的铝相,以及Al B2粒子含量较高是细化低效的原因[24,25]。
图4 合金2 中花瓣状Al3Ti相Fig. 4 Petal Al3Ti phase in Alloy 2
图5 制备合金2 时滞留在废渣中的Al3Ti相Fig. 5 Remaining Al3Ti phase in slag prepared Alloy 2
用B2O3代替KBF4,不但降低了晶粒细化性能,同时也产生了大量的残渣( 表2) ,其中部分悬浮在熔体中,降低了熔体合金的流动性,使浇铸更加困难。因此,在制备合金3 和4 的过程中,为克服制备合金2 时遇到的浇铸问题,采取了除渣工艺和调整反应物添加顺序的考量,使商业除渣与B2O3-K2Ti F6共同作用以推动氧化物分离和改善熔体流动性的目的。
实验过程得知,除渣工艺不但有助于提高Ti的回收率,且能减少渣质约25% ( 表2) ,使合金3的Ti回收率得到大幅提高,且硼化物和铝含量较多,具有类似合金1 的特征( 图1( c) 和图2( 3) ) 。
因此,合金3 的晶粒细化效率显然比合金2 高( 图3( c) ) ,但添加物加入30 min后晶粒细化效果有轻微的减退迹象。可见,使用除渣去杂工艺几乎完全抵消了由B2O3带来的颗粒剩余量( 表2) ,有效回收渣质中的铝含量并提高合金的细化效果,但仍有改进的余地,使细化效率达到合金1 的水平,这也是当前工作的一个重要目标。
制备合金4 时,B元素一半来源于B2O3,另一半以KBF4的形式提供。其目的,一是减少B2O3氧化剂的数量,通过KBF4促进生成KAl F4; 二是提供两种不同的除渣助熔剂,以更好地改善氧化物的润湿性,提高Ti的回收率( 从合金3 的80. 0% 提高到合金4 的92. 0% ) ,以达到较高的细化效果。
3结论
当B2O3完全取代时,制备的细化剂具有较低的钛回收率,细化性能降低,产生大量的残渣,微观组织Al3Ti相呈花瓣状,Al B2相含量较高。同时,熔体的流动性亦降低,浇铸更加困难。当B2O3与除渣助熔剂预先混合,再与K2Ti F6混合加入到铝熔体中,在不考虑添加物损失时,铝熔体的微观组织结构和细化效率均会得到明显的改善。当B2O3和KBF4各添加50% 时,钛的回收率和晶粒的细化效率,几乎与单独添加KBF4时一致,微观组织均匀细小,未发生明显偏聚。因此。在不损失晶粒细化效率的前提下,可以用B2O3替代KBF4,但只是部分而非完全。该方法的优势在于: 具有更低的氟化物排放量和颗粒物添加量,在相同质量下,B2O3提供的硼含量高出近4 倍,价格也更便宜。
参考文献
[8] Jackson M J,Graham I D,Mater J.Mechanical stirring of Al-B alloys[J].Sci.Lett.,1994,13(10):754.