RGD多肽接枝聚(乳酸-羟基乙酸-L-赖氨酸)的制备与表征
严琼姣,李世普,殷义霞,李 娟
(武汉理工大学 生物材料与工程研究中心,湖北 武汉,430070)
摘 要:
摘 要:以3S-[4-(苄氧羰基氨基)丁基]-吗啉-2,5-二酮和丙交酯为起始原料,制备一种新型的RGD多肽接枝聚(乳酸-羟基乙酸-L-赖氨酸)共聚物(PRGD)。采用核磁共振氢谱、氨基酸分析、接触角测试、MTT实验和环境扫描电镜对其结构和性能进行表征。研究结果表明:RGD接枝量为6.9~14.3 μmol/g;PRGD膜和聚乳酸(PLA)膜的水接触角分别为43.63°和62.45°;PRGD膜表面黏附的嗅鞘细胞较PLA组活性高,细胞密度大,生长状态好。PRGD较PLA具有更好的亲水性和神经细胞亲和性,有望成为一种理想的神经修复材料。
关键词:
中图分类号:TQ316.343 文献标识码:A 文章编号:1672-7207(2008)06-1190-06
Preparation and characterization of RGD peptide modification of poly{(lactic acid)-co-[(glycolic acid)-alt-(L-lysine)]}
YAN Qiong-jiao, LI Shi-pu, YIN Yi-xia, LI Juan
(Biomedical Materials and Engineering Research Center, Wuhan University of Technology, Wuhan 430073, China)
Abstract: A novel RGD peptide modification of poly[LA-co-(GA-alt-Lys)] copolymer(PRGD) was prepared by lactide and 3S-[4-(benzyloxycarbonylamino)butyl]morpholine-2,5-dione(BMD) as starting materials. The structure and properties of poly[LA-co-(GA-alt-Lys)]/RGD were characterized by 1H-NMR, AAA, contact angel measurement, MTT experiments and ESEM. The results show that GRGDY is coupled to poly[LA-co-(GA-alt-Lys)] at a concentration of 6.9-14.3 μmol/g. The water contact angels of PRGD films and PLA films are 43.63° and 62.45°, respectively. Olfactory ensheathing cells(OECs) on the PRGD films exhibit higher viability and the amount of OECs on the PRGD films are much larger than those on PLA films, and moreover, the cells stretch very well. The PRGD has better hydrophilicity and neural cell affinity than PLA, so it is promising in nerve tissue engineering application.
Key words: RGD; poly[LA-co-(GA-alt-Lys)]; cell affinity
神经组织缺损(如周围神经缺损)是临床常见的损伤,传统的治疗方法是采用自体神经移植,但自体神经移植供区有限,难以满足临床的需要,因此,人们希望通过组织工程寻找合适的周围神经再生支架材料替代自体神经[1]。目前,用于周围神经再生的支架材料主要是一些可降解的天然高分子和合成高分子。天然高分子如胶原[2-3],亲水性和细胞亲和性较好,但降解性能和力学性能较差。合成高分子如聚乳酸(PLA)[4-7],降解性能和力学性能较好,但其亲水性差,细胞亲和性不好。
理想的周围神经再生支架材料不仅具有良好的力学性能,而且还要具有良好的细胞亲和性,在植入时能诱导神经细胞快速地黏附与生长[8]。因此,胶原和聚乳酸作为周围神经再生支架材料都存在着一定的局限性。聚乳酸与胶原相比,缺乏细胞识别信号(如精氨酸-甘氨酸-天冬氨酸, RGD),不利于神经细胞黏附、增殖、分化[9-10],并且聚乳酸缺乏反应活性官能团, 不能与RGD多肽直接结合[11-16]。
L-赖氨酸含有亲水性侧氨基,且其侧氨基可偶联RGD多肽。在此,本文作者将L-赖氨酸与α-羟基酸(羟基乙酸,乳酸)共聚,通过L-赖氨酸的侧氨基引入短肽RGD,制备一种新型的聚合物-RGD多肽接枝聚(乳酸-羟基乙酸-L-赖氨酸)。此聚合物含有亲水性氨基和促神经细胞黏附的RGD短肽,能有效改善聚乳酸的亲水性和神经细胞亲和性,兼具聚乳酸和胶原的优点,是一种更理想的神经修复材料。
1 实 验
1.1 主要试剂和仪器
主要试剂:甘氨酸-精氨酸-甘氨酸-天冬氨酸-酪氨酸(GRGDY),购自吉尔生化(上海)有限公司;3S-[4-(苄氧羰基氨基)丁基]-吗啉-2,5-二酮、丙交酯、聚乳酸(PLA),为实验室自制;其他试剂均为上海国药集团AR级。
主要仪器:采用Varian Inova 300核磁共振仪测试1H-NMR,采用日立835-50型氨基酸分析仪进行氨基酸分析(AAA),采用ZAP显微镜测试水接触角,采用Quanta 200环境扫描电子显微镜观察环境扫描电镜。
1.2 材料的合成
1.2.1 RGD多肽接枝聚(乳酸-羟基乙酸-L-赖氨酸)的合成路线
RGD多肽接枝聚(乳酸-羟基乙酸-L-赖氨酸)的合成路线如图1所示。
1.2.2 聚(乳酸-羟基乙酸-Nε-苄氧羰基-L-赖氨酸)(3)的制备
按摩尔比1?9分别称取单体3S-[4-(苄氧羰基氨基) 丁基]-吗啉-2,5-二酮(BMD) (1)4 g和丙交酯(2)16.2 g,

图1 RGD多肽接枝聚(乳酸-羟基乙酸-L-赖氨酸)的合成
Fig.1 Synthesis of poly{(lactic acid)–co-[(glycolic acid)-alt-(L-lysine)]}
置于干净的安培管中,然后,注入浓度为30 mg/mL的辛酸亚锡氯仿溶液2 mL,抽真空使氯仿挥发,在酒精喷灯上将安培管熔断封口,置于温度为140 ℃的油浴中聚合48 h。将产物溶于氯仿中,甲醇沉淀,真空干燥,得14.3 g聚(乳酸-羟基乙酸-Nε-苄氧羰基-L-赖氨酸)。
1.2.3 聚(乳酸-羟基乙酸-L-赖氨酸)(4)的制备
将10 g聚(乳酸-羟基乙酸-Nε-苄氧羰基-L-赖氨酸)溶于200 mL乙酸乙酯/甲醇(体积比为2?1)溶液中,加入0.5 g 10%的钯/碳催化剂,通氢气反应48 h,过滤,真空回收溶剂,将残留液倒入100 mL无水乙醚中,立即生成大量沉淀,真空干燥,得8 g聚(乳酸-羟基乙酸-L-赖氨酸)。
1.2.4 RGD多肽接枝聚(乳酸-羟基乙酸-L-赖氨酸)(5)的制备
将5 g聚(乳酸-羟基乙酸-L-赖氨酸)溶于100 mL二氯甲烷,再依次向其中加入50 mL二甲亚砜,100 mg GRGDY,85 mg N, N′-羰基二咪唑,于室温反应4 h,真空回收二氯甲烷,残留液变浑浊,加入水,析出沉淀,经过滤和真空干燥,得到4.5 g RGD多肽接枝聚(乳酸-羟基乙酸-L-赖氨酸)(简称为PRGD)。
1.3 PRGD膜和PLA膜的制备
分别称取适量的PRGD和PLA溶于乙酸乙酯中,配制成10%的溶液,倒入玻璃模板中,室温挥发溶剂成膜后再真空干燥,除去膜中残余乙酸乙酯。
1.4 性能测试
1.4.1 接触角的测定
常温状态下,采用ZAP显微镜(德国莱茨Leitz)观测PLA和PRGD膜的静态水接触角。
先将PLA和PRGD膜,经紫外照射灭菌后放入96孔板(每组5个样),再将嗅鞘细胞以合适的密度接种于96孔板中,培养3 d,用酶标仪测其吸光度(OD值),检测波长为570 nm。
1.4.3 环境扫描电镜观察
将嗅鞘细胞与PLA和PRGD膜分别共培养3 d后,将膜取出,经0.01 mol/L磷酸缓冲液清洗2次,2.5%戊二醛固定后,直接进行环境扫描电镜观察。
2 结果与讨论
2.1 核磁共振氢谱分析(1H-NMR)
图2所示为聚(乳酸-羟基乙酸-Nε-苄氧羰基-L-赖氨酸)的核磁共振氢谱。图2中化学位移7.345(g)处是单体BMD中苯环的质子峰,化学位移1.58(h)和5.16(b)处分别是丙交酯中甲基和次甲基的质子峰,表明BMD与丙交酯共聚生成了聚(乳酸-羟基乙酸-Nε-苄氧羰基- L-赖氨酸)。根据质子峰的积分面积之比即为各基团物质的量之比的原理,可计算共聚物中各单体的摩尔分数。共聚物中BMD的摩尔分数可根据下式计算:

![]()
BMD与丙交酯按摩尔比1?9参与共聚反应, 共聚产物中BMD的理论摩尔分数应为0.10,而实际仅为0.077,其原因是BMD与丙交酯相比,反应活性较低。
图3所示为是聚(乳酸-羟基乙酸-L-赖氨酸)的核磁共振氢谱。图3与图2相比,化学位移7.345处苯环的质子峰消失,化学位移5.297(f)处新增L-赖氨酸的侧氨基峰,表明Pd/C催化氢解可完全脱除聚(乳酸-羟基乙酸-Nε-苄氧羰基-L-赖氨酸)中的苄氧羰基,生成聚(乳酸-羟基乙酸-L-赖氨酸)。

图2 聚(乳酸-羟基乙酸-Nε-苄氧羰基-L-赖氨酸)的核磁共振氢谱
Fig.2 1H-NMR spectrum of poly[LA-co-(GA-alt-Nε-benzyloxycarbonyl-L-lys)]

图3 聚(乳酸-羟基乙酸-L-赖氨酸)的核磁共振氢谱
Fig.3 1H-NMR spectrum of poly[LA-co-(GA-alt-Lys)]
2.2 氨基酸分析(AAA)
PRGD的氨基酸测试结果如表1所示。可见,每克PRGD主链含赖氨酸277 μmol,赖氨酸侧氨基接枝GRGDY 6.9~14.3 μmol,接枝率仅为2.5%~5.2%。Barrera等[16]研究表明材料只需含有极少量(如3.1 μmol/g )GRGDY肽链就能有效促进细胞对材料表面的黏附,故本实验中选择聚(乳酸-羟基乙酸-L-赖氨酸)与GRGDY的投料比是50?1,通过改变两者投料比,可在很大范围内调节PRGD中GRGDY的含量。
表1 PRGD氨基酸分析
Table 1 Amino acid analysis of PRGD μmol/g

2.3 接触角的测定
静态水接触角常被用于评价材料的亲/疏水性,水接触角越小,说明材料亲水性越好。图4所示为PLA膜和PRGD膜水接触角的显微镜图像。可见,PLA膜水接触角为62.45°,PRGD膜水接触角为 43.63°,PRGD膜比PLA膜亲水性有明显的提高。这是由于PLA缺乏亲水性基团,而PRGD中含有大量L-赖氨酸的亲水性基团(—NH2),能有效增强材料的亲水性。按照细胞的液滴模型,亲水性材料的表面有利于神经细胞黏 附[17],因此,PRGD膜较PLA膜更有利于细胞黏附。

(a) PLA膜;(b) PRGD膜
图4 2种膜水接触角的显微镜图像
Fig.4 Microscopic images of water contact angel of PLA and PRGD films
2.4 MTT实验
材料与嗅鞘细胞共培养3 d后,通过MTT比色法测各组材料表面黏附细胞的吸光度,结果如下。
材料表面细胞的吸光度如图5所示。可见,PRGD材料表面细胞的吸光度(OD值)明显比PLA组的高。
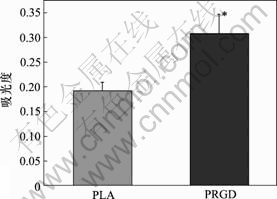
图5 材料表面细胞的吸光度
Fig.5 OD value of cells seeded on films’ surface
(*与PLA组比,P<0.01)
由于MTT法测得的吸光度与细胞的生长活性成正 比,说明PRGD膜表面黏附的嗅鞘细胞活性比PLA组的强。
材料与细胞共培养3 d后,通过环境扫描电镜观察(见图6)显示,两组材料均有细胞生长,PLA膜表面生长的嗅鞘细胞少,呈线性排列。PRGD膜表面嗅鞘细胞成簇生长,细胞密度大,且细胞的胞体较大,铺展良好,与原代细胞形态接近,表明PRGD膜比PLA膜更有利于嗅鞘细胞黏附和生长。
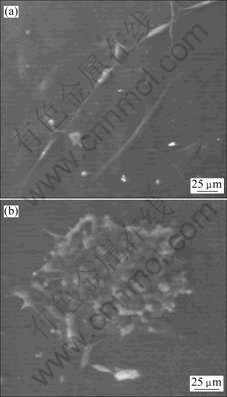
(a) PLA组;(b) PRGD组
图6 环境扫描电镜观察嗅鞘细胞在PLA和PRGD膜上的生长情况
Fig.6 ESEM micrograph of OECS cultured on PLA and PRGD films
3 结 论
a. 以3S-[4-(苄氧羰基氨基)丁基]-吗啉-2,5-二酮与丙交酯为起始原料经共聚、催化氢解和偶联三步反应,制备了一种RGD多肽接枝聚(乳酸-羟基乙酸-L-赖氨酸)共聚物。共聚物中GRGDY含量为6.9~ 14.3 μmol/g,RGD接枝率为2.5%~5.2%。
b. RGD多肽接枝聚(乳酸-羟基乙酸-L-赖氨酸)与聚乳酸相比亲水性明显提高。RGD多肽接枝聚(乳酸-羟基乙酸-L-赖氨酸)膜的水接触角为43.63°,聚乳酸膜的水接触角为62.45°。
c. RGD多肽接枝聚(乳酸-羟基乙酸-L-赖氨酸)与聚乳酸相比具有更好的神经细胞亲和性,是一种更理想的周围神经再生支架材料。将嗅鞘细胞与2种材料体外共培养,经MTT实验和环境扫描电镜观察显示PRGD膜表面黏附的嗅鞘细胞较PLA组活性高,细胞密度大,生长状态好。
参考文献:
[1] Belkas J S, Shoichett M S, Midha R. Peripheral nerve regeneration through guidance tubes[J]. Neurological Research, 2004, 26(2): 151-160.
[2] Itoh S, Takakuda K, Kawabata S, et al. Evaluation of cross-linking procedures of collagen tubes used in peripheral nerve repair[J]. Biomaterials, 2002, 23(23): 4475-4481.
[3] Kitahara A K, Suzuki Y, Qi P, et al. Facial nerve repair using a collagen conduit in cats[J]. Scandinavian Journal of Plastic and Reconstructive Surgery and Hand Surgery, 1999, 33(2): 187-193.
[4] Evans G R, Brandt K, Widmer M S, et al. In vivo evaluation of poly(L-lactic acid) porous conduits for peripheral nerve regeneration[J]. Biomaterials, 1999, 20(12): 1109-1115.
[5] 王身国, 侯建伟, 贝建中, 等. 聚d,l-乳酸及其对20毫米断缺神经诱导修复的研究[J]. 高技术通讯, 2000(8): 15-18.
WANG Sheng-guo, HOU Jian-wei, BEI Jian-zhong, et al. Study on 20 mm of nerve gap recovered by poly- d,l-lactide guide[J]. High Technology Communication, 2000(8): 15-18.
[6] Wan Y, Wen D J. Preparation and characterization of porous conducting poly(D,L-lactide) composite membranes[J]. Journal of Membrane Science, 2005, 246(2): 193-201.
[7] 董红让, 徐永年, 黄继锋, 等. 聚乳酸/神经生长因子缓释导管修复周围神经缺损实验研究[J]. 中国临床解剖学杂志, 2003, 21(5): 482-485.
DONG Hong-rang, XU Yong-nian, HUANG Ji-feng, et al. Experimental study of bridging peripheral nerve defect with biodegradable PDLLA/NGF controlled release conduit[J]. Chinese Journal of Clinical Anatomy, 2003, 21(5): 482-485.
[8] 徐海星, 闫玉华, 万 涛, 等. 壳聚糖-L-乳酸复合不对称膜的制备与表征[J]. 中南大学学报: 自然科学版, 2007, 38(3): 433-438.
XU Hai-xing, YAN Yu-hua, WAN Tao, et al. Preparation and characterization of chitosan-L-lactic acid composite asymmetric membrane[J]. Journal of Central South University: Science and Technology, 2007, 38(3): 433-438.
[9] Lee D Y, Choi B H, Park J H, et al. Nerve regeneration with the use of a poly(L-lactic acid-co-glycolic acid)-coated collagen tube filled with collagen gel[J]. Journal of Cranio-maxillofacial Surgery, 2006, 34(1): 50-56.
[10] Dai W, Belt J, Saltzman W M. Cell-binding peptides conjugated to poly(ethylene glycol) promote neural cell aggregation[J]. Bio/Technology, 1994, 12: 797-801.
[11] Alvarez-Barreto J F, Shreve M C, Deangelis P L, et al. Preparation of a functionally flexible, three-dimensional, biomimetic poly(L-lactic acid) scaffold with improved cell adhesion[J]. Tissue Engineering, 2007, 13(6): 1205-1217.
[12] Lieb E, Hacker M, Tessmar J, et al. Mediating specific cell adhesion to low-adhesive diblock copolymers by instant modification with cyclic RGD peptides[J]. Biomaterials, 2005, 26(15): 2333-2341.
[13] Ho M H, Hou L T, Tu C Y, et al. Promotion of cell affinity of porous PLLA scaffolds by immobilization of RGD peptides via plasma treatment[J]. Macromol Biosci, 2006, 6(1): 90-98.
[14] Deng C, Tian H Y, Zhang P B, et al. Synthesis and characterization of RGD peptide grafted poly(ethylene glycol)-b-poly(L-lactide)-b-poly(L-glutamic acid) triblock copolymer[J]. Biomacromolecules, 2006, 7(2): 590-596.
[15] Deng C, Chen X S, Yu H J, et al. A biodegradable triblock copolymer poly(ethylene glycol)-b-poly(L-lactide)-b-poly (L-lysine): synthesis, self-assembly and RGD peptide modification[J]. Polymer, 2007, 48(1): 139-149.
[16] Barrera D A, Zylstra E, Lansbury P T, et al. Synthesis and RGD peptide modification of a new biodegradable copolymer: poly(lactic acid-co-lysine)[J]. J Am Chem Soc, 1993, 115: 11010-11011.
[17] Fan Y W, Cui F Z, Chen L N, et al. Improvement of neural cell adherence to silicon surface by hydroxyl ion implantation[J]. Surf Coat Tech, 2000, 131(1/3): 355-359.
收稿日期:2008-01-10;修回日期:2008-04-09
基金项目:国家重点基础研究发展计划资助项目(2005CB623905)
通信作者:李世普(1946-),男,辽宁新民人,教授,博士生导师,从事生物医用材料研究;电话:027-87216470;E-mail: Lishipu46@126.com


