文章编号:1004-0609(2011)07-1601-06
正极材料LiNi0.8Co0.1Mn0.1O2的合成及电化学性能
岳 鹏, 彭文杰, 王志兴, 李新海, 李灵均, 郭华军, 胡启阳, 张云河
(中南大学 冶金科学与工程学院,长沙 410083)
摘 要:
将液相共沉淀法制备的Ni0.8Co0.1Mn0.1(OH)2与LiOH·H2O混合,固相烧结合成微米级的LiNi0.8Co0.1Mn0.1O2 正极材料。XRD谱表明,合成的LiNi0.8Co0.1Mn0.1O2 正极材料为典型的α-NaFeO2层状结构,无杂质峰;从SEM像可以看出,产物颗粒为类球形,分散性好,由一次粒子紧密堆积而成,平均粒径为3 μm;电化学测试结果表明,在2.8~4.3 V电压范围内,750 ℃焙烧15 h合成的LiNi0.8Co0.1Mn0.1O2材料的电化学性能最优,0.1 C 时,其首次放电容量为186.748 mA·h/g,分别高于700和800 ℃时的首次放电容量172.947和180.235 mA·h/g。材料在0.5和2 C时循环40次后,容量保持率分别为98.32%和88.72%,循环性能良好。
关键词:
LiNi0.8Co0.1Mn0.1O2;正极材料;共沉淀法;电化学性能;
中图分类号:TM912.9 文献标志码:A
Synthesis and electrochemical performance of
micro-size LiNi0.8Co0.1Mn0.1O2 cathode material
YUE Peng, PENG Wen-jie, WANG Zhi-xing, LI Xin-hai, LI Ling-jun, GUO Hua-jun, HU Qi-yang, ZHANG Yun-he
(School of Metallurgical Science and Engineering, Central South University, Changsha 410083, China)
Abstract: The micro-size LiNi0.8Co0.1Mn0.1O2 cathode material was synthesized by mixing Ni0.8Co0.1Mn0.1(OH)2 and LiOH·H2O via solid state reaction followed by heating to elevated temperatures. X-ray diffraction (XRD) patterns show that all samples exhibit typical α-NaFeO2 structure without any other impurities. The scanning electron microscopy (SEM) reveals that the primary particles agglomerate to form secondary particles with an average size of 3 μm, and the particles are near-spherical and well distributed. In the voltage range of 2.8-4.3 V, LiNi0.8Co0.1Mn0.1O2 synthesized at 750 ℃ for 15 h exhibits a higher initial discharge capacity of 186.748 mA·h/g at 0.1 C compared with those of LiNi0.8Co0.1Mn0.1O2 synthesized at 700 and 800 ℃ (172.947 and 180.235 mA·h/g, respectively). The material has excellent cycling performance, in which the capacity retention at the rates of 0.5 and 2 C are 98.32% and 88.72%, respectively, after 40 cycles.
Key words: LiNi0.8Co0.1Mn0.1O2; cathode material; co-precipitation; electrochemical performance
随着便携电子设备、混合电动汽车和电动汽车等的发展,体积小、能量密度高的锂离子电池具有良好的发展前景。其中,层状正极材料LiCoO2已经在商业上广泛应用,但其对环境的非友好型和高成本限制了其进一步应用,因此,其他正极材料的探索、开发与应用成为研究的热点[1-4]。
与LiCoO2相比,层状结构的LiNi1-x-yCoxMnyO2 (0≤x≤0.5,0≤y≤0.3) 镍钴锰正极材料具有价廉和容量高等优点,被认为是可替代LiCoO2的正极材料 之一[5-6]。OHZUKU和MAKIMURA[7]合成了具有电化学活性的LiNi1/3Co1/3Mn1/3O2材料,在2.5~4.3 V电压区间内放电容量达到160 mA·h/g [8],在2.8~4.6 V电压区间内可达200 mA·h/g[9]。在文献[10-13]中,研究者合成了性能优良LiNi0.8Co0.1Mn0.1O2材料,此材料受到了广泛的关注。
在材料制备过程中,常用的方法有固相法和液相共沉淀法。固相法操作简单,但合成产物不易达到原子级的混合,合成温度高,时间长,能耗高;液相共沉淀法克服了固相法的缺点,但前驱体制备的反应时间较长(一般大于12 h),合成样品的粒径较大(10~40 ?m)[11, 14-16]。锂离子电池材料的电化学性能不仅与制备方法有关,也与材料的粒径有关。SCLAR等[17]和DENG等[18]指出,微尺寸或纳米尺寸的材料有利于缩短锂扩散路径,从而改善材料的电化学性能。
本文作者将液相共沉淀法制备的Ni0.8Co0.1Mn0.1(OH)2与LiOH·H2O混合,固相烧结合成微米级LiNi0.8Co0.1Mn0.1O2正极材料。研究pH值、c(NH3)/c(M)(M为Ni、Co和Mn)、陈化时间th对前驱体振实密度的影响,将具有高振实密度的Ni0.8Co0.1Mn0.1(OH)2前驱体与适量LiOH·H2O混合,制备LiNi0.8Co0.1Mn0.1O2材料,研究合成温度对产物LiNi0.8Co0.1Mn0.1O2性能的影响。
1 实验
以NiCl2·6H2O(AR)、CoCl2·6H2O(AR)、MnCl2·4H2O (AR)和NaOH(AR)为合成原料,按比例配制浓度为2 mol/L的混合溶液(n(Ni):n(Co):n(Mn)= 8?1?1),同时配制2 mol/L的NaOH溶液。在50 ℃水浴中,将配好的溶液与适量氨水同时加入反应容器中,控制pH值在一定范围,在搅拌速度为800 r/min条件下反应5 min,得到沉淀物。将生成的沉淀物抽滤、洗涤,在真空干燥箱内干燥,得到Ni0.8Co0.1Mn0.1(OH)2前驱体。将前驱体与适量的LiOH·H2O混合均匀,送入程式控温管式炉内,通入O2,在700、750和800 ℃焙烧15 h,随炉冷却至室温后取出,得到LiNi0.8Co0.1Mn0.1O2材料。
采用X射线衍射仪(XRD)和扫描电镜(SEM)分别分析样品的晶体结构和形貌。XRD测试采用日本Rigaku公司生产的D/max 2550VB+18kW转靶X射线衍射仪(X射线源Cu Kα,电流300 mA,管电压50 kV,步宽0.02°,扫描速率8 (°)/min,衍射角扫描范围10°~80°);SEM测试采用JEOL公司生产的JSM-5600LV扫描电镜,在20 kV下观察样品的表面形貌。采用Thermo ICAP6300型ICP-AES测定产物中元素含量。振实密度的测定过程如下:将20 g样品置于50 mL的量筒中,振实至体积不再变化,则其振实密度为质量与体积的比。
实验电池的正极片由合成的活性物质、乙炔黑与粘结剂PVDF 按质量比8?1?1混合,加入N-甲基吡咯烷酮(NMP),均匀涂覆在铝箔上,并在真空干燥箱中干燥。在充满氩气的手套箱中组装成CR2025型扣式电池,负极为金属锂片,电解液为1 mol/L LiPF6/EC+DMC+EMC(体积比1?1?1)混合溶液,隔膜为Celgard 2400聚丙稀微孔膜。实验电池在Neware公司生产的充放电测试仪上完成,测试温度为常温,充放电电压为2.8~4.3 V。
2 结果与讨论
氨水浓度与pH值均是制备Ni0.8Co0.1Mn0.1(OH)2的重要影响因素。苏继桃等[19]从理论上分析了M2+-NH3-OH--H2O体系,指出pH值过低使沉淀不完全,pH过高则易引入杂质相。氨水在前驱体制备过程中充当络合剂,它可以与Ni、Co和Mn离子优先结合形成络合物,延缓了氢氧化物直接沉淀,对Ni0.8Co0.1Mn0.1(OH)2晶核的形成速度起到控制作 用[20]。
表1所列为不同合成条件对前驱体振实密度的影响。从表1可以看出,当pH<11时,随着pH值的增加,振实密度增加;当pH>11时,随着pH值的增 加振实密度降低。在较低pH值下,可能有部分氨水络合物没有完全转变为氢氧化物沉淀,从而造成沉淀不完全。在较高pH值下,晶核的过饱和度高,形成速率快,而晶体生长速度慢,因此,形成的微晶结构粒度较小,造成振实密度降低[21]。
表1 合成条件对Ni0.8Co0.1Mn0.1(OH)2振实密度的影响
Table 1 Tap density of Ni0.8Co0.1Mn0.1(OH)2 depending on different synthetic conditions
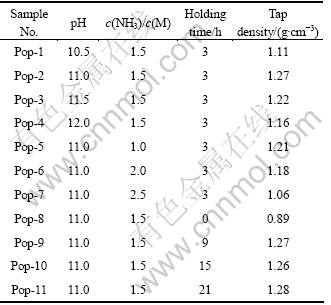
在一定的pH值下,随着c(NH3)/c(M)的增加,振实密度逐渐增加,当c(NH3)/c(M)=1.5时,振实密度达到1.27 g/cm3;但当c(NH3)/c(M)>1.5后,随着c(NH3)/c(M)的增加,振实密度呈减小趋势。当c(NH3)/c(M)较低时,部分金属离子未能与氨水发生络合反应,导致产生细碎的晶粒,并使其振实密度降低;当c(NH3)/c(M)较高时,将阻碍沉淀反应,从而降低振实密度[22]。
随着th的增长,振实密度逐渐增加,当th 超过3 h时,振实密度无明显变化。因此,保持一定的th有利于晶体的进一步长大,修饰晶体形貌,使晶体粒径均一。另外,也有利于Na+和Cl-等离子从晶体中脱出,避免杂质离子进入前驱体中。
前驱体的振实密度与pH、c(NH3)/c(M)和th 紧密相关。以高振实密度为目标,在pH=11、c(NH3)/c(M)=1.5及th =3 h时,可以制备出高振实密度的Ni0.8Co0.1Mn0.1(OH)2前驱体。
图1所示为pH=11、c(NH3)/c(M)=1.5及th =3 h时制备的Ni0.8Co0.1Mn0.1(OH)2 (Pop-2)的XRD谱。从图1可以看出,前驱体的衍射特征峰与β-Ni(OH)2的相符,无杂质峰,说明制备的前驱体为单一相的Ni0.8Co0.1Mn0.1(OH)2前驱体[23]。通过ICP检测,前驱体中n(Ni):n(Co):n(Mn)为7.867:0.977:1.052,与理论比例近似,摩尔比满足n(Ni):n(Co):n(Mn)=8:1:1。因此,通过液相共沉淀法可以制备出满足化学计量比、无杂相的Ni0.8Co0.1Mn0.1(OH)2前驱体。
将Ni0.8Co0.1Mn0.1(OH)2前驱体(Pop-2)与LiOH·H2O按摩尔比n(Li)/n(Ni+Co+Mn)为1.05?1混合,将混后料置于管式炉内分别在700、750和800 ℃合成LiNi0.8Co0.1Mn0.1O2 材料。
图2所示为不同温度下合成材料在0.1 C时的首次充放电曲线。从图2可以看出,750 ℃合成的LiNi0.8Co0.1Mn0.1O2 在0.1 C时的初始放电容量为186.748 mA·h/g,高于700和800 ℃时的172.947和180.235 mA·h/g。
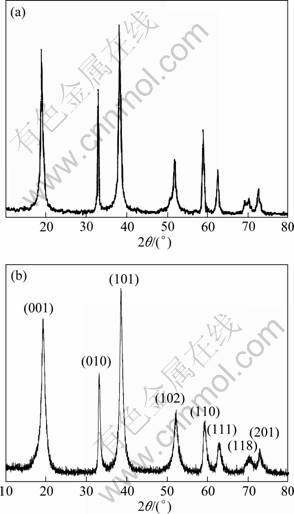
图1 β-Ni(OH)2及pH=11、c(NH3)/c(M)=1.5及th =3 h时制备的Ni0.8Co0.1Mn0.1(OH)2的XRD谱
Fig.1 XRD patterns of β-Ni(OH)2 (a) and Ni0.8Co0.1Mn0.1(OH)2 particles synthesized at pH=11, c(NH3)/c(M)=1.5 and th=3 h (b)
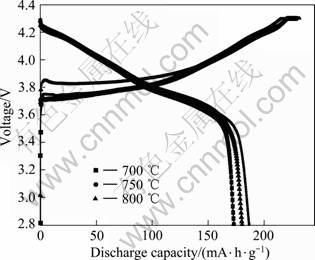
图2 不同温度下合成的LiNi0.8Co0.1Mn0.1O2在0.1 C时的首次充放电曲线
Fig.2 Initial charge-discharge (0.1 C rate) curves of LiNi0.8Co0.1Mn0.1O2 synthesized at different temperatures
图3所示为不同温度下合成的LiNi0.8Co0.1Mn0.1O2在1 C时的循环性能曲线。从图3可以看出,750 ℃合成的LiNi0.8Co0.1Mn0.1O2在1 C时的放电容量为165.74 mA·h/g,40次循环后放电容量为145.76 mA·h/g,容量保持率为87.95%。700和800 ℃合成的LiNi0.8Co0.1Mn0.1O2在1 C时的放电容量分别为151.433和165.55 mA·h/g,40次循环后容量保持率分别为82.19%和77.72%。
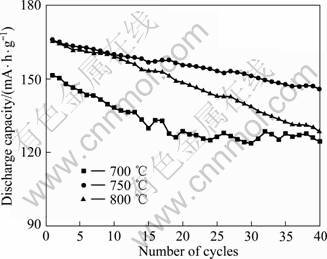
图3 不同温度下合成的LiNi0.8Co0.1Mn0.1O2在1 C时的循环性能
Fig.3 Cycling performance (0.1 C rate) of LiNi0.8Co0.1Mn0.1O2 synthesized at different temperatures
综合图2与3可知,750 ℃合成的LiNi0.8Co0.1Mn0.1O2的性能最优。为了分析其原因,从产物的XRD谱与SEM像进行分析。
在XRD谱中,(006/102)与(108/110)衍射峰的明显分裂表示材料层状结构的完整性[24]。I(003)/I(104)表示阳离子混排程度[25],一般认为,当I(003)/I(104)>1.2时,产物的阳离子混排度较小,材料层状结构良好,电化学性能优异。
图4所示为不同温度下焙烧15 h合成的LiNi0.8Co0.1Mn0.1O2的XRD谱。从图4可以看出,各温度合成的样品衍射谱相近,衍射峰明显,均具有α-NaFeO2结构,其中,Li和过渡金属分别占据3a和3b位;XRD谱中不存在杂质峰,说明产物相单一。700 ℃合成样品的(006/102)与(108/110)两对衍射峰分裂不明显,初步判断该样品的层状结构不完整;根据衍射数据计算出I(003)/I(104)=1.13,阳离子混排的情况较严重,从而导致材料的电化学性能变差。750和800 ℃合成样品的(006/102)与(108/110)两对衍射峰均明显分裂,层状结构完整,I(003)/I(104)分别为1.49和1.21,阳离子混排程度小。750 ℃合成样品的I(003)/I(104)最大,电化学性能最佳。
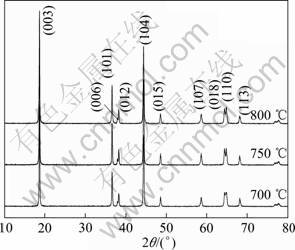
图4 不同温度下焙烧15 h合成的LiNi0.8Co0.1Mn0.1O2的XRD谱
Fig.4 XRD patterns of LiNi0.8Co0.1Mn0.1O2 synthesized at different temperatures for 15 h
图5所示为不同温度下合成样品LiNi0.8Co0.1Mn0.1O2的SEM像。从图5可以看出,700 ℃合成样品颗粒粒径均一性差,分布不均匀;750 ℃合成的样品颗粒粒径均一,分布均匀;800 ℃合成样品出现大块团聚,可能是晶粒熔化粘结造成的,这种现象不利于电解液的充分浸润,造成锂离子脱嵌困 难[26],从形貌方面解释了800 ℃合成样品性能较750 ℃样品差的原因。
从图5(c)可以看出,750 ℃合成样品为类球形,平均粒径为3 μm,每个颗粒均由粒径为0.1~0.2 μm的一次粒子紧密堆积而成。
对750 ℃下合成性能优良的LiNi0.8Co0.1Mn0.1O2进行不同倍率的充放电实验。图6和7所示分别为不同倍率下LiNi0.8Co0.1Mn0.1O2的放电容量曲线和循环性能。从图6和7可以看出,0.5、1和2 C 时的放电容量高,充放电循环可逆性好。LiNi0.8Co0.1Mn0.1O2 在0.5和2 C时的放电容量分别为173.714 和155.83 mA·h/g,40次循环后,容量保持率分别为98.32%和88.72%,循环性能良好。
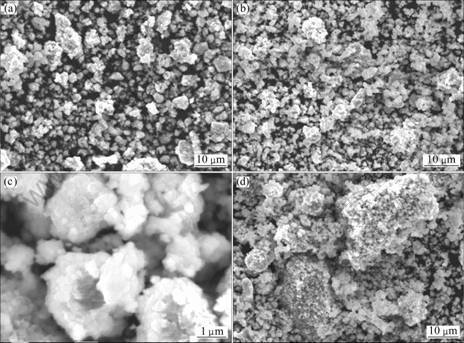
图5 不同温度下合成样品LiNi0.8Co0.1Mn0.1O2的SEM像
Fig.5 SEM images of LiNi0.8Co0.1Mn0.1O2 synthesized at different temperatures: (a) 700 ℃; (b) 750 ℃; (c) 750 ℃; (d) 800 ℃
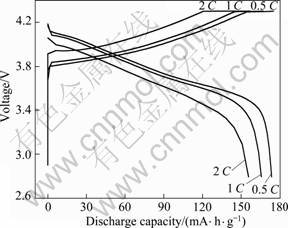
图6 LiNi0.8Co0.1Mn0.1O2在0.5、1和2C时的充放电曲线
Fig.6 Charge-discharge curves of LiNi0.8Co0.1Mn0.1O2 at 0.5, 1 and 2 C
3 结论
1) 采用液相共沉淀法制备Ni0.8Co0.1Mn0.1(OH)2前驱体,最优条件为pH=11,c(NH3)/c(M)=1.5,陈化时间th=3 h。Ni0.8Co0.1Mn0.1(OH)2的特征峰与β-Ni(OH)2的相符合,无杂质峰;ICP测试表明,前驱体中Ni、Co和Mn的摩尔比为8?1?1。
2) 合成的微米级LiNi0.8Co0.1Mn0.1O2材料具有优良的电化学性能、完整的层状结构,阳离子混排度低,颗粒粒径约为3 μm。
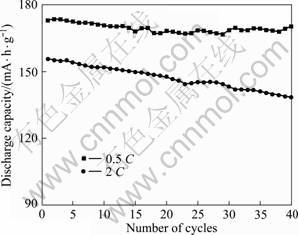
图7 LiNi0.8Co0.1Mn0.1O2在0.5 and 2 C时的循环性能
Fig.7 Cycling performances of LiNi0.8Co0.1Mn0.1O2 at 0.5 and 2 C
3) LiNi0.8Co0.1Mn0.1O2在2.8~4.3 V充放电范围及0.5 C的充放电倍率下,放电容量为173.714 mA·h/g,40次循环后容量保持率为98.32%。
REFERENCES
[1] GOODENOUGH J B, KIM Y G. Challenges for rechargeable Li batteries[J]. ChemistryofMaterials, 2010, 22(3): 587-603.
[2] ZHENG Jun-chao, LI Xin-hai. LiFePO4 with enhanced performance synthesized by a novel synthetic route[J]. Journal of Power Sources, 2008, 184(2): 574-577.
[3] DELMAS C, MENETRIER M, CROGUENNEC L, SAADOUNE I. An overview of the Li(Ni, M)O2 systems: Syntheses, structures and properties[J]. Electrochimica Acta, 1999, 45(1/2): 243-253.
[4] YUE Hong-jun, HUANG Xing-kang, L? Dong-ping, YANG Yong. Hydrothermal synthesis of LiMn2O4/C composite as a cathode for rechargeable lithium-ion battery with excellent rate capacity[J]. Electrochimica Acta, 2009, 54(23): 5363-5367.
[5] LIU Z L, YU A S, LEE J Y. Synthesis and characterization of LiNi1-x-yCoxMnyO2 as the cathode materials of secondary lithium batteries[J]. Journal of Power Sources, 1999, 81/82: 416-419.
[6] LIAO P Y, DUH J G, SHEEN S R. Effect of Mn content on the microstructure and electrochemical performance of LiNi0.75-xCo0.25MnxO2 cathode materials[J]. Journal of the Electrochemical Society A, 2005, 152(9): 1695-1700.
[7] OHZUKU T, MAKIMURA Y. Layered lithium insertion material of LiCo1/3Ni1/3Mn1/3O2 for lithium-ion batteries[J]. Chemistry Letters, 2001, 30(7): 642-643.
[8] SHAJU K M, SUBBA RAO G V, CHOWDARI B V R. Performance of layered Li(Ni1/3Co1/3Mn1/3)O2 as cathode for Li-ion batteries[J]. Electrochimica Acta, 2002, 48(2): 145-151.
[9] YABUUCHI N, OHZUKU T. Novel lithium insertion material of LiCo1/3Ni1/3Mn1/3O2 for advanced lithium-ion batteries[J]. Journal of Power Sources, 2003, 119/121: 171-174.
[10] KIM M H, SHIN H S, SHIN D, SUN Y K. Synthesis and electrochemical properties of LiNi0.8Co0.1Mn0.1O2 and LiNi0.8Co0.2O2 via co-precipitation[J]. Journal of Power Sources, 2006, 159: 1328-1333.
[11] 王希敏, 王先友, 易四勇, 曹俊琦. 层状锂离子电池正极材料LiNi0.8Co0.1Mn0.1O2 的制备及性能[J]. 过程工程学报, 2007, 7(4): 817-821.
WANG Xi-min, WANG Xian-you, YI Si-yong, CAO Jun-qi. Synthesis and characteristics of layered LiNi0.8Co0.1Mn0.1O2 cathode material for lithium rechargeable batteries[J]. The Chinese Journal of Process Engineering, 2007, 7(4): 817-821.
[12] WOO S W, MYUNG S T, BANG H, KIM D W, SUN Y K. Improvement of electrochemical and thermal properties of LiNi0.8Co0.1Mn0.1O2 positive electrode materials by multiple metal (Al, Mg) substitution[J]. Electrochimica Acta, 2009, 54(15): 3851-3856.
[13] SUN Y K, MYUNG S T, PARK B C, PRAKASH J. High-energy cathode material for long-life and safe lithium batteries[J]. Nature Materials, 2009, 8: 320-324.
[14] CHERALATHAN K K, KANG N Y, PARK S H, LEE Y J, CHOI W C. Preparation of spherical LiNi0.8Co0.15Mn0.05O2 lithium-ion cathode material by continuous co-precipitation[J]. Journal of Power Sources, 2010, 195: 1486-1494.
[15] WANG Zhao-xiang, SUN Yu-cheng, CHEN Li-quan, HUANG Xue-jie. Electrochemical characterization of positive electrode material LiNi1/3Mn1/3Co1/3O2 and compatibility with electrolyte for lithium-ion batteries[J]. Journal of the Electrochemical Society A, 2004, 151(6): 914-921.
[16] LUO Xu-fang, WANG Xian-you, LIAO Li, GAMBOA S, SEBASTIAN P J. Synthesis and characterization of high tap-density layered LiNi1/3Mn1/3Co1/3O2 cathode material via hydroxide co-precipitation[J]. Journal of Power Sources, 2006, 158: 654-658.
[17] SCLAR H, KOVACHEVA D, ZHECHEVA E, STOYANOVA R, LAVI R, KIMMEL G. On the performance of LiNi1/3Mn1/3Co1/3O2 nanoparticles as a cathode material for lithium-ion batteries[J]. Journal of the Electrochemical Society A, 2009, 156(11): 938-948.
[18] DENG C, ZHANG S, FU B L, YANG S Y, MA L. Synthetic optimization of nanostructured LiNi1/3Mn1/3Co1/3O2 cathode material prepared by hydroxide co-precipitation at 273 K[J]. Journal of Alloys and Compound, 2010, 496: 521-527.
[19] 苏继桃, 苏玉长, 赖智广. 制备镍, 钴, 锰复合氢氧化物的热力学分析[J]. 电池工业, 2008, 13(1): 18-22.
SU Ji-tao, SU Yu-chang, LAI Zhi-guang. Thermodynamic analysis of preparation of multiple hydroxide of Ni, Co and Mn[J]. Chinese Battery Industry, 2008, 13(1): 18-22.
[20] 胡国荣, 刘艳君, 彭忠东, 杜 柯. 控制结晶法合成球形正极材料LiNi0.8Co0.2O2及其电化学性能[J]. 中国有色金属学报, 2007, 17(1): 59-67.
HU Guo-rong, LIU Yan-jun, PENG Zhong-dong, DU Ke. Synthesis and properties of spherical cathode materials LiNi0.8Co0.2O2 by controlled crystallization method[J]. The Chinese Journal of Nonferrous Metals, 2007, 17(1): 59-67.
[21] 冷拥军, 张鉴清, 成少安, 曹楚南. 高堆积密度球形氢氧化镍制备及其理论分析[J]. 化学学报, 1998, 56: 557-563.
LENG Yong-jun, ZHANG Jian-qing, CHENG Shao-an, CAO Chun-nan. Preparation and theoretical analysis of spherical nickel hydroxide with high tapping density[J]. Acta Chimica Sinica, 1998, 56: 557-563.
[22] 邓新荣. 锂离子电池镍系正极材料的制备及室温固相表面包覆技术研究[D]. 长沙: 中南大学, 2008: 31-33.
DENG Xin-rong. Study on the preparation of nickel-based cathode materials for lithium-ion batteries and the surface coating technology by solid state reaction at room temperature[D]. Changsha: Central South University, 2008: 31-33.
[23] CHANG Z R, LI G G, ZHAO Y J, DING Y C. Influence of preparation conditions of spherical nickel hydroxide on its electrochemical properties[J]. Journal of Power Sources, 1998, 74: 252-254.
[24] LIAO P Y, DUH J G, SHEU S R. Structural and thermal properties of LiNi0.6-xMgxCo0.25Mn0.15O2 cathode materials[J]. Journal of Power Sources, 2008, 183: 766-770.
[25] CHOI Y M, PYUN S I, MOON S I. Effects of cation mixing on the electrochemical lithium intercalation reaction into porous Li1-δNi1-yCoyO2 electrodes[J]. Solid State Ionics, 1996, 89(1/2): 43-52.
[26] WU She-huang, YANG Chi-wei. Preparation of LiNi1-yCoyO2 based cathode materials for lithium batteries by a co-precipitation method[J]. Journal of Power Sources, 2005, 146: 270-274.
(编辑 陈卫萍)
基金项目:国家重点基础研究发展计划资助项目(2007CB613607)
收稿日期:2010-06-04;修订日期:2010-09-19
通信作者:彭文杰,副教授,博士;电话:0731-88836633;E-mail: pwj.csu@163.com


