Trans. Nonferrous Met. Soc. China 24(2014) 1571-1577
Effect of biological pretreatment on flotation recovery of pyrolusite
Zhi-chao YANG1, Ya-li FENG1, Hao-ran LI2, Wei-da WANG1, Qing TENG1
1. Civil and Environment Engineering School, University of Science and Technology Beijing, Beijing 100083, China;
2. State Key Laboratory of Biochemical Engineering, Institute of Process Engineering, Chinese Academy of Sciences, Beijing 100190, China
Received 19 June 2013; accepted 6 September 2013
Abstract:
Bacillus mucilaginosus was used in pretreatment of pyrolusite to facilitate the flotation removal of quartz from pyrolusite minerals. Quartz was activated by B. mucilaginosus, whereas pyrolusite was unaffected at pH 7 with laurylamine as collector. Flotation recovery of pyrolusite with B. mucilaginosus pretreatment is 73.62%, slightly lower than that of the process without biopretreament, namely 74.70%. The grade of concentrate of recovered pyrolusite is 19.44%, 2.18% higher than that of the recovered pyrolusite without B. mucilaginosus pretreatment (17.26%). The results of FTIR and SEM showed that no bacteria were adsorbed on the surface of quartz or pyrolusite, indicating that the better selectivity and collectability of flotation resulted from bacterial byproducts. And interaction of bacterial byproducts such as extracellular bacterial polysaccharide, extracellular bacterial protein and acetic acid, on minerals were studied by FTIR and adsorption.
Key words:
flotation; pyrolusite; quartz; laurylamine;
1 Introduction
Pyrolusite is an important mineral consisting essentially of manganese dioxide (MnO2). Quartz, as its primary associated mineral, is difficult to be separated from pyrolusite. Pyrolusite ore flotation practice is well described in the technical literature and typically involves flotation of manganese dioxide using octyl hydroxamate as collector [1]. Hydroxamate forms strong chelate bonds with manganese ions and consequently strongly chemisorbs on manganese-containing minerals. What’s more, FUERSTENAU and SHIBATA [2] used electrokinetics to interpret the flotation and interfacial behavior of manganese dioxide. Part of the studies also involved the flotation of manganese dioxide with oleic acid or oleate soap, the most commonly used collector for the flotation of oxide minerals. There is almost no research using cationic reverse flotation for the removal of silicate gangues from pyrolusite ore.
Cationic reverse flotation of quartz is the most important technique for the concentration of ores for the silicate minerals [3]. The mechanism of amine-silicate interaction has been studied extensively by indirect methods and ex-situ measurements such as flotation recovery response, adsorption experiments, contact angle, zeta-potential and FTIR spectroscopy in the last several decades.
Biobeneficiation is a process that utilizes microorganisms as surface modifiers to enhance the separation of one mineral from another by flotation or flocculation [4-9]. Most biological activities, such as bioleaching and biobeneficiation, depend on attachment or adsorption of microorganisms to the mineral surfaces. Although the use of microorganisms in ore leaching is well established, the mechanism of biobeneficiation is not fully explained. The development of biotechnology is promising in solving some problems generated by mineral processing [10]. Separation of quartz from hematite/corundum mixture was successfully achieved with the aid of E. coli strain Sip [11]. As well known, some of microorganisms have a selective activating or depressing effect on mineral froth flotation [12]. The effect of using microorganisms can be realized by the adhesion of microorganism cells onto the mineral surface, the oxidation of the mineral surface by chemolithotrophic bacteria and attack of metabolic reagents produced by the microorganism cells.
Bacillus mucilaginosus is a common soil bacterium, and usually used as a model bacterium in studying microbe-mineral interactions. Several reaction mechanisms of B. mucilaginosus weathering silicate minerals were proposed [13-15]. These studies regarded B. mucilaginosus weathering silicate minerals, variations in the concentrations of various ions composing the silicates, but variations in the concentrations could not reflect weathering rates. Actually the silicate minerals, were dissolved, further formed secondary silicate minerals [16]. Therefore, it is interesting to study the flotation of the silicate minerals weathered by B. mucilaginosus.
In this work, pyrolusite was pretreated by B. mucilaginosus and then cationic reverse flotation silicate gangues from pyrolusite was conducted with laurylamine as collector. The objective of the present investigation is to understand the underlying probability of laurylamine reverse flotation silicate minerals from pyrolusite and the role of B. mucilaginosus pretreatment.
2 Experimental
2.1 Materials preparation
The sample of pyrolusite was obtained from a mine in the southern part of China, and dry ground using a porcelain ball and then sieved through 74 μm standard specification sieve. The chemical analysis of ore samples indicated that the sample contained mainly: 59.08% SiO2, 15.24% Mn, and 7.19% Fe. By combining the information obtained from XRD and ore microscopy, an approximate mineralogical composition of the ore sample was pyrolusite (15%-16%), quartz (58%-60%), hematite (10%-12%), and mica (3%-4%).
2.2 Bacterial strain, media and growth
A pure strain of B. mucilaginosus, which was stored at the Environmental Biological Science and Technology Research Center, Institute of Geochemistry, Chinese Academy of Sciences, was used in this study. B. mucilaginosus was cultured and maintained in the liquid medium with 5.0 g/L C12H22O11 (sucrose), 2.0 g/L Na2HPO4, 0.5 g/L MgSO4·7H2O, 0.005 g/L FeCl3, 0.1 g/L CaCO3, 0.5 g/L Al2O3·2SiO2·2H2O (kaolin) and pH of 7.0-7.2. A 10% active cell culture was added to the medium and incubated in a rotary shaker at 100 r/min at 30 °C. The cells were harvested from the culture at the beginning of the stationary phase of their growth. After 48 h of incubation, the liquid suspensions containing the cells were used in pyrolusite biotreatment.
2.3 Biological pretreatment
50 g pyrolusite powder, 25 mL B. mucilaginosus suspension, and a desired amount of medium were added to a 500 mL flask. The total volume of the pulp was 250 mL and all tests were conducted at pH 7. Afterwards, the pulp was incubated at 30 °C on a rotary shaker at 150 r/min so that the microbe and mineral interaction occurred. Biological pretreatment time varied in different tests. In the control experiment, deionized water was added to the system instead of B. mucilaginosus suspension and the medium.
2.4 Flotation tests
The flotation tests were carried out in a 0.5 L laboratory flotation cell, using 50 g mineral sample. Laurylamine was used as the collector, while sodium hexametaphosphate was used as modifier. All the reagents used in this study were of analytical grade. The biotreatment samples were first transferred to flotation cell and diluted with deionized water to 0.5 L and then the modifier and collector were added and conditioned for 3 min and floated for 6 min at pH 7. The effects of biotreatment time and different dosages of collector and modifier on recovery rate were examined. Tests were also performed in the absence of biotreatment. Once the flotation product was obtained, the concentrates and tailings were washed, filtered, dried and finally weighed to obtain the percentage of flotation. The floatability was calculated as the grade and recovery of the concentrate.
The average values of the results of two paralleled tests were shown in this paper. Furthermore, to check the reliability of the results obtained, several pyrolusite recovery tests were carried out.
2.5 Fourier transformed infrared spectroscopy
The BRUKER ALPHA-T instrument was used for recording the infrared absorption spectra; a KBr matrix was used as background. The spectra of pyrolusite, before and after the B. mucilaginosus pretreatment, were evaluated. The B. mucilaginosus cell suspension and the minerals samples were filtered and dried at 75 °C. The dried powder was properly mixed in a KBr matrix. All the spectra were recorded between 4000 and 700 cm-1.
2.6 Scanning electron microscopy
Scanning electron microscopy (SEM) was used to check the surface images of the mineral particles before and after biological pretreatment. After washing and drying, the minerals of the adsorption tests were gold coated under vacuum in a BAL-TEC sputter coater. Secondary electron images were acquired in a Carl Zeiss-DSM 630 scanning electron microscope.
2.7 Adsorption experiment
Adsorption experiments were carried out on two samples (quartz and manganese dioxide). In each case, 5.0 g sample was added to 20 mL metabolic product suspension of B. mucilaginosus. The suspension was then shaken for some time by a vortex shaker. The optical density (OD330) of the supernatant was then measured to determine the adsorption rate. The suspension was obtained by centrifuging cell supernatant at 7000 r/min, then the supernatant was filtered through 0.45 μm filtering membrane.
3 Results and discussion
3.1 Flotation results
3.1.1 Effect of collector concentration
Figure 1 shows the flotation recoveries of quartz and pyrolusite as a function of concentrations of collector at pH 7, using 500 g/t sodium hexameta- phosphate as modifier.
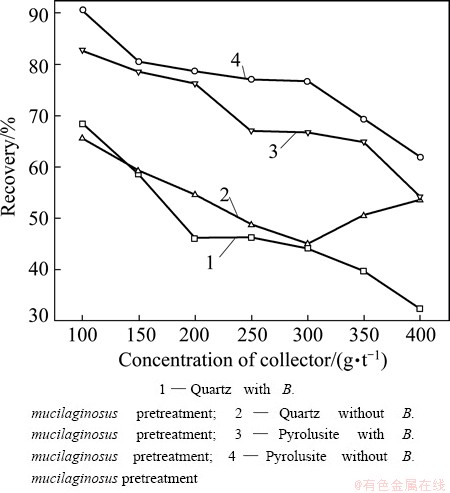
Fig. 1 Flotation recoveries of quartz and pyrolusite as function of concentration of collector (with 500 g/t sodium hexametaphosphate) at natural pH
Pyrolusite can be floated with laurylamine, while quartz is the tailings as reported in the literature. As the laurylamine concentration increases, the recovery of pyrolusite decreases. As shown by curve 4 in Fig. 1, the recovery of the untreated pyrolusite mineral decreased from about 90% to 62% while laurylamine concentration increased from 100 to 400 g/t. And the quartz recovery decreased from 65% to 45% while laurylamine concentration increased from 100 to 300 g/t and then an obvious increment of quartz recovery was observed as laurylamine concentration went up to 400 g/t. This increment could be attributed to the preferential adsorption of laurylamine onto the pyrolusite surface due to the high concentration of the collector. Surprisingly, laurylamine which is a strong collector for quartz does not float pyrolusite after B. mucilaginosus pretreatment. According to the results, after the mineral samples were pretreated by B. mucilaginosus, the recovery of quartz decreases from 68% to 32% as the collector concentration increased from 100 to 400 g/t, which is quite different from the results of the untreated samples (curve 2 in Fig. 1). The results showed that B. mucilaginosus pretreatment improved the quartz floatability. It was noted that the lower recovery of pyrolusite was obtained when the mineral samples were pretreated by B. mucilaginosus, but the distinction was not obvious. As seen from curve 3 in Fig. 1, the maximum difference between the recoveries of pyrolusite and quartz was 10% when the concentration of collector was 250 g/t. Therefore, the pretreatment with B. mucilaginosus is feasible to separate quartz from pyrolusite.
3.1.2 Modifier concentration
The effects of sodium hexametaphosphate concentrations on quartz and pyrolusite flotation were studied with 200 g/t laurylamine at pH 7 and the results are shown in Fig. 2.
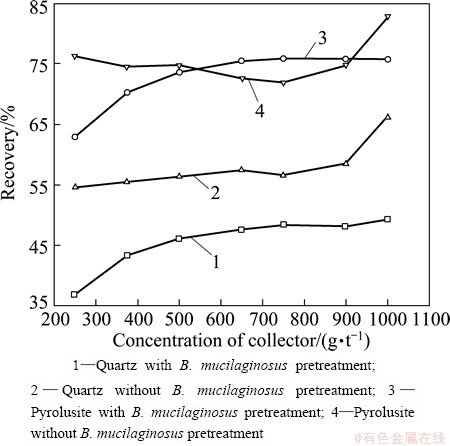
Fig. 2 Flotation recoveries of quartz and pyrolusite as function of concentrations of modifier (with 200 g/t laurylamine) at natural pH
The quartz recovery of the B.mucilaginosus pretreated sample increased from 36% to 49% as the concentration of the modifier increased from 250 to 1000 g/t. With increasing sodium hexametaphosphate concentration, the quartz recovery remained almost constant at 57% without B. mucilaginosus pretreatment. However, when the concentration of sodium hexametaphosphate was more than 900 g/t, the quartz recovery increased rapidly.
The pyrolusite recovery remained at about 74% followed by a rapid increase to 82% when the concentration of sodium hexametaphosphate increased from 900 to 1000 g/t without B. mucilaginosus pretreatment. With the increased use of sodium hexametaphosphate, the pyrolusite recovery increased from 62% to 75% after B. mucilaginosus pretreatment.
When the mineral samples were not pretreated by B. mucilaginosus, the recovery of quartz increased and pyrolusite recovery also increased, showing that sodium hexametaphosphate played an insignificant role. The appropriate amount of sodium hexametaphosphate depressed the recovery of pyrolusite, but the quartz recovery was also depressed at the same time after B. mucilaginosus pretreatment. Sodium hexametaphosphate lacks of selectivity as a modifier.
3.1.3 Biological pretreatment time
The relationship between the biological pretreatment time by B. mucilaginosus and the flotation activity of the mineral samples was also investigated. Flotation results as a function of B. mucilaginosus pretreatment time were analyzed at a concentration of laurylamine of 200 g/t. In Fig. 3 it is shown that the recovery of pyrolusite decreased when the pretreatment time increased from 0 to 144 h. No significant activation of flotation was obtained after 144 h of biopretreatment with B. mucilaginosus. When biopretreatment time was extended, foam product yield increased and improved the grade of concentrate effectively. At the same time,the selectivity of collector became bad. This deterioration of selectivity resulted from more adsorption of a number of bacteria and the bacterial byproduct on the surface pyrolusite ore as the pretreatment time increased.
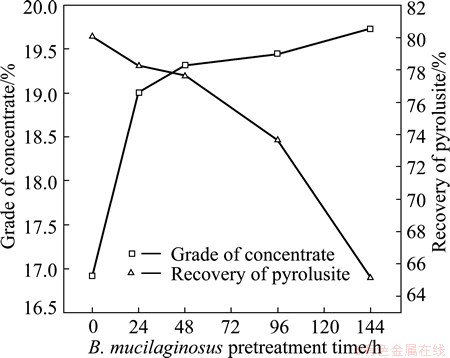
Fig. 3 Grade of concentrate and flotation recovery of pyrolusite as function of B. mucilaginosus pretreatment time at pH 7
3.1.4 Influence of pulp composition
In order to investigate the influence of B. mucilaginosus on flotation efficiency, the flotation tests were carried out using deionised water, liquid medium and inoculated medium to form flotation pulp. The recovery and grade of concentrate are presented in Fig. 4.
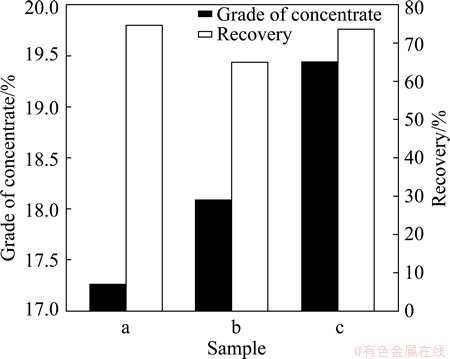
Fig. 4 Comparison of efficiency of using deionised water (a), liquid medium (b) and inoculated medium (c) to form flotation pulp
The results showed that the highest grade of concentrate (19.44%) was obtained at pH 7, using the inoculated medium to form flotation pulp compared with liquid medium (18.09%) and deionised water(17.26%). While the recovery of pyrolusite is 73.62%, which is only slightly lower than that of the deionised water and 8.57% higher than that of the liquid medium. This means that the inoculated medium is more efficient in pyrolusite flotation than either liquid medium or deionised water when operating at optimal conditions. This further evidenced bacterial preconditioning.
The study indicated that the bacteria or bacterial byproduct adsorbed to the mineral surface can change the surface properties of the minerals. The micro-organism cell surface consists of polymers, peptides, proteins and mycolic acids [17]. These materials must adhere to the mineral surface directly and utilize cell surface associated or extracellular biopolymers to catalyze chemical reactions on the mineral surface [18]. The biosurfactants produced by B. mucilaginosus possess the ability to decrease the surface tension of water from 72 to 28.6 mN/m [19]. The goal of the following experiments is to investigate whether the bacteria or bacterial byproducts were adsorbed to the surface of minerals.
3.2 FTIR analysis
As shown in Fig. 5(a), FTIR spectra of the pyrolusite sample, obtained before the B. mucilaginosus pretreatment, showed the characteristics bands for pyrolusite (absorbance bands of 3445 cm-1assigned to hydroxyl groups, 1091 cm-1assigned to Si—O). The FTIR spectra of pyrolusite, after B. mucilaginosus pretreatment, showed similar characteristics bands as the untreated one. This indicated that absorbance bands of 3422 cm-1and 1091 cm-1 might not associate with functional groups presented on the B. mucilaginosus cell wall which could interact with the sample surfaces. However, some modifications could be observed compared with the initial results. The absorbance band of hydroxyl group was broadened and the wave number 3445 cm-1 move lower, 3422 cm-1, due to the role of hydrogen bonding. It may be caused by not enough dry samples in infrared analysis test. The absorbance band of 1091 cm-1was widened, which may be subject to interference from other bands.
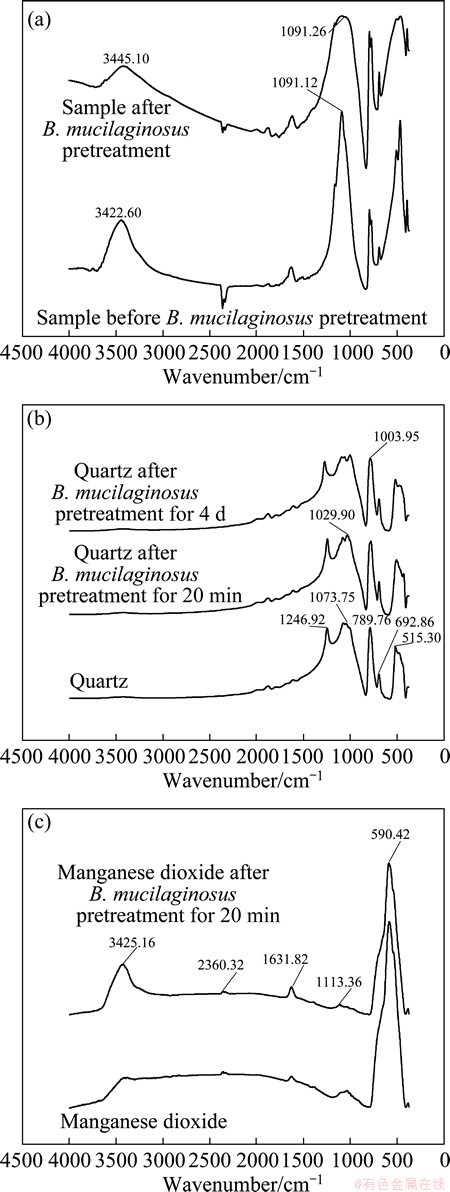
Fig. 5 FTIR spectra of samples of pyrolusite(a), quartz(b) and manganese dioxide(c) before and after B. mucilaginosus pretreatment
Figure 5(b) shows the FTIR spectra of quartz before and after B. mucilaginosus pretreatment. Characteristic absorbance bands were observed. Minor modifications were seen after the sample was biologically pretreated. Furthermore, it could be observed the absorbance bands of 1029 and 1003 cm-1 associated with C—OH groups, indicating the interaction between the microbes and the quartz surface. In the microbe-mineral interaction processes, B. mucilaginosus contacted with mineral grains and formed bacterium-mineral complexes by its extracellular polysaccharide, extracellular bacterial protein and acetic acid.
From Fig. 5(c), it was found that manganese dioxide, after 20 min of B. mucilaginosus pretreatment, the absorbance band at about 3425 cm-1 corresponding to O—H of the hygroscopic sample remained unchanged in position and shape. It suggested that no B. mucilaginosus was adsorbed on the manganese dioxide surface because no new bands were observed.
From all the FTIR spectra, no characteristic peak associated with amide functional groups present on the bacteria cell wall were observed after bacteria pretreatment, indicating that no bacteria were adsorbed on the mineral surface.
3.3 SEM analysis
Figure 6 shows the SEM results of the pyrolusite sample after 4 d of B. mucilaginosus pretreatment. The selected areas of the SEM photomicrographs showed that no B. mucilaginosus cell attached onto the mineral surfaces. The results obtained in the SEM study are in good agreement with the mineral FTIR spectra. It is confirmed that there is no amide maybe associated with functional groups present on the bacteria cell wall on the sample surface after bacteria pretreatment. This showed that the improvement of flotation performance was not attributed to adsorption of bacteria.
3.4 Adsorption
The adsorption behavior of metabolic products as a function of time was studied in neutral environment. The initial metabolic products suspension optical density at 330 nm (OD330) was 0.4236. The adsorption of metabolic products on the surface of quartz and manganese was measured by determining the optical density of residual suspension in the pulp. Figure 7 shows that the adsorption rates of metabolic products onto quartz were 27.90% in 5 min, 16.57% in 10 min onto manganese dioxide and 17.80% in 30 min onto manganese dioxide. The adsorption of metabolic products on the surface of two minerals increased with increasing time. The adsorption of metabolic products on the surface of quartz increased quickly with increasing time, but the adsorption on the surface of manganese dioxide increases slowly with increasing time. This suggests that quartz has a very high affinity for the metabolic products compared with manganese dioxide. These results are in agreement with the results of FTIR analysis.

Fig. 6 SEM images of pyrolusite sample before (a, b) and after (c, d) 4 d of B. mucilaginosus pretreatment
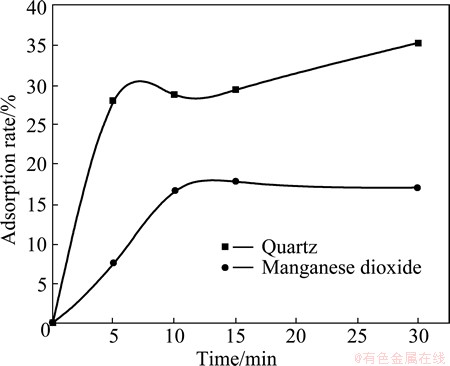
Fig. 7 Adsorption rate of metabolic products of B. mucilaginosus onto quartz and manganese dioxide as function of time
4 Conclusions
1) Flotation separation quartz from pyrolusite is possible using laurylamine as collector.
2) Flotation behavior of quartz from pyrolusite minerals after B. mucilaginosus pretreatment exhibited an obvious advantage compared with pyrolusite minerals before pretreatment. The flotation of pyrolusite minerals after B. mucilaginosus pretreatment can obtain higher grade concentrates and ensure that the recovery is not reduced.
3) The results of FTIR and SEM show that the effect of flotation is not due to the adsorption of bacteria because there are no bacteria on the surface of quartz and pyrolusite.
4) The selective flotation of quartz from pyrolusite minerals can be enhanced by the metabolic products of B. mucilaginosus, such as extracellular bacterial polysaccharide, extracellular bacterial protein and acetic acid.
5) The products of B. mucilaginosus are more easily adsorbed on the surface of quartz than manganese dioxide.
References
[1] NATARAJAN R, FUERSTENAU D W. Adsorption and flotation behavior of manganese dioxide in the presence of octyl hydroxamate [J]. International Journal of Mineral Processing, 1983, 11: 139-153.
[2] FUERSTENAU D W, SHIBATA J. On using electrokinetics to interpret the flotation and interfacial behavior of manganese dioxide [J]. International Journal of Mineral Processing, 1999, 57: 205-217.
[3] KOU J, TAO D, XU G. A study of adsorption of dodecylamine on quartz surface using quartz crystal microbalance with dissipation [J]. Colloids and Surfaces A: Physicochemical and Engineering Aspects, 2010, 368: 75-83.
[4] SHARMA P K, DAS A, HANUMANTHA RAO K, FORSSBERG K S E. Surface characterization of Acidithiobacillus ferrooxidans cells grown under different conditions [J]. Hydrometallurgy, 2001, 71: 285-292.
[5] NATARAJAN K A, DEO N. Role of bacterial interaction and bioreagents in iron ore flotation [J]. International Journal of Mineral Processing, 2001, 62(1-4): 143-157.
[6] PATRA P, NATARAJAN K A. Role of mineral specific bacterial proteins in selective flocculation and flotation [J]. International Journal of Mineral Processing, 2008, 88 (1-2): 53-58.
[7] SANTHIYA D, SUBRAMANIAN S, NATARAJAN K A, HANUMANTHA RAO K, FORSSBERG K S E. Bio-modulation of galena and sphalerite surfaces using Thiobacillus thiooxidans [J]. International Journal of Mineral Processing, 2001, 62(1-4): 121-141.
[8] SHARMA P K, HANUMANTHA RAO K. Adhesion of Paenibacillus polymyxa on chalcopyrite and pyrite: Surface thermodynamics and extended DLVO theory [J]. Colloids and Surfaces B: Biointerfaces, 2003, 29: 21-38.
[9] MOHAMMAD H F, HAMID K, MOHAMMAD R. Coal flotation using a biosurfactant from Pseudomonas aeruginosa as a frother [J]. Korean J Chem Eng, 2010, 27(5): 1527-1531.
[10] SMITH R W, MISRA M, DUBEL J. Mineral bioprocesing and the future [J]. Minerals Engineering, 1991, 4: 1127-1141.
[11] FARAHAT M, HIRAJIMA T, SASAKI K, DOI K. Adhesion of Escherichia coli onto quartz, hematite and corundum: Extended DLVO theory and flotation behavior [J]. Colloids and Surfaces B: Biointerfaces, 2009, 74: 140-149.
[12] YELLOJI RAO M K, NATARAJAN K A, SOMASUNDARAN P. Effect of biotreatment with Thiobacillus ferrooxidans on the flotability of sphalerite and galena [J]. Minerals & Metallurgical Processing, 1992, 7(5): 95-100.
[13] LIU Wu-xing, XU Xu-shi, WU Xiang-hua, YANG Qi-yin, LUO Yong-ming, CHRISTE Peter. Decomposition of silicate minerals by Bacillus mucilaginosus in liquid culture [J]. Environmental Geochemistry and Health, 2006, 28: 133-140.
[14] BASAK B B, BISWAS D R. Influence of potassium solubilizing microorganism (Bacillus mucilaginosus) and waste mica on potassium uptake dynamics by Sudan grass (Sorghum vulgare Pers.) grown under two alfisols [J]. Plant and Soil, 2009, 317: 235-255.
[15] KUPRIYANVA-ASHINA F G, KRINARI G A, KOLPAKOV A L, LESCHINLKAYA I B. Degradation of silicate minerals by Bacillus mucilaginosus using Bacillus intermedius RNase [M]//Advances in Geoecology. Towards Sustainable Land Use, Vols I & II: Furthering Cooperation Between People and Institutions. 1998: 813-818.
[16] MO Bin-bin, LIAN bin. Interactions between bacillus mucilaginosus and silicate minerals (weathered adamellite and feldspar): Weathering rate, products, and reaction mechanisms [J]. Chin J Geochem, 2011, 30: 187-192.
[17] van der WAL A, NORDE A, ZEHNDER A J B, LYKLEMA J. A determination of the total charge in the cell walls of gram-positive bacteria [J]. Colloids and Surfaces B: Biointerfaces, 1997, 9: 81-100.
[18] CHANDAPHARA M N, NATARAJAN K A, SOMASUNDARAN P. Surface chemical characterization of Acidithiobacillus ferroxidans grown in presence of different metal ions [C]//Proceedings of the XXIII International Mineral Processing Congress IMPC. Istanbul, 2006: 442-447.
[19] DIDYK A M, SADOWSKI Z. Flotation of serpentinite and quartz using biosurfactants [J]. Physicochem Probl Miner Process, 2012, 48(2): 607-618.
生物预处理对软锰矿浮选的影响
杨志超1,冯雅丽1,李浩然2, 王维大1,滕 青1
1. 北京科技大学 土木与环境工程学院,北京 100083;
2. 中国科学院 过程工程研究所 生化工程国家重点实验室,北京 100190
摘 要:研究了B. mucilaginosus 菌从软锰矿浮选脱除石英的作用,B. mucilaginosus菌对软锰矿表现出良好的选择性和回收能力。当采用十二胺为捕收剂,在pH为7时进行浮选,经 B. mucilaginosus 菌处理的石英被活化,而软锰矿则不受影响。结果表明,经 B. mucilaginosus 菌预先处理的软锰矿锰回收率较不经细菌处理的锰回收率略低,从 74.70% 降至 73.62%。但精矿品位从 17.26% 提高到19.44%。红外分析和扫描电镜结果表明,浮选效果的改善不是细菌吸附在石英或者软锰矿表面造成的。通过红外分析研究了细菌代谢物如胞外多糖、蛋白和乙酸在矿物表面的吸附。
关键词:浮选;软锰矿;石英;十二胺
(Edited by Hua YANG)
Foundation item: Projects (21176026, 21176242) supported by the National Natural Science Foundation of China; Project (2012AA062401) supported by the Hi-tech Research and Development Program of China
Corresponding author: Ya-li FENG; Tel: +86-10-62311181; E-mail: ylfeng126@126.com
DOI: 10.1016/S1003-6326(14)63227-1
Abstract: Bacillus mucilaginosus was used in pretreatment of pyrolusite to facilitate the flotation removal of quartz from pyrolusite minerals. Quartz was activated by B. mucilaginosus, whereas pyrolusite was unaffected at pH 7 with laurylamine as collector. Flotation recovery of pyrolusite with B. mucilaginosus pretreatment is 73.62%, slightly lower than that of the process without biopretreament, namely 74.70%. The grade of concentrate of recovered pyrolusite is 19.44%, 2.18% higher than that of the recovered pyrolusite without B. mucilaginosus pretreatment (17.26%). The results of FTIR and SEM showed that no bacteria were adsorbed on the surface of quartz or pyrolusite, indicating that the better selectivity and collectability of flotation resulted from bacterial byproducts. And interaction of bacterial byproducts such as extracellular bacterial polysaccharide, extracellular bacterial protein and acetic acid, on minerals were studied by FTIR and adsorption.


