DOI: 10.11817/j.ysxb.1004.0609.2020-37686
强磁场作用下磁场梯度力对铜电解过程的影响
姚夏妍1,赵芸芸1,鲁兴武1,王军辉2,牛永胜1,程 亮1,李俞良1
(1. 西北矿冶研究院 甘肃省有色金属冶炼新工艺及伴生稀散金属高效综合利用重点实验室,白银 730900;
2. 白银有色集团股份有限公司,白银 730900)
摘 要:
摘 要:Cu2+扩散性能和杂质离子含量是影响阴极铜质量的关键指标,通过在循环管道施加水平取向磁场调控Cu2+扩散性能及强化铜电解自净化过程改善阴极铜质量。从磁分离与磁场影响水分子氢键缔合程度和离子水合作用的角度出发,开展水平取向磁场磁化铜电解液的实验,利用火焰原子吸收分光光度法、黏度计、毛细管、电镜扫描分别研究磁场梯度力对电解液铜酸浓度、杂质离子浓度、黏度、表面张力以及阴极铜表观质量的影响,分析水平取向磁场强化铜电解工艺的机理。结果表明:磁场梯度力一方面可放大铜电解液中的抗磁性离子和水分子的磁能,降低水分子间的氢键缔合程度和电解体系的活化能,加快铜电解反应速率,促进砷锑铋离子形成SbAsO4、BiAsO4以及AsSbO4的沉淀。增大溶解氧量和CaSO4的溶解度,促使镍铁锌离子表面形成氧化性保护膜,以提高电解液的清晰度和阴极铜的产量。同时,磁场梯度力可降低电解液的表面张力,导致电解液气泡增多而引发阴极铜表面生成气孔;另一方面,磁场梯度力可缩小顺磁性离子的磁能,增强离子水合作用,调控阳极溶解和阴极铜析出速度,降低铜损失量,改善阴极铜表观质量。综上可知,水平取向磁场作用于铜电解过程存在最佳磁感应强度。
关键词:
文章编号:1004-0609(2020)-11-2695-11 中图分类号:TF111 文献标志码:A
电解精炼是生产高纯阴极铜不可或缺的关键工序,广泛应用于铜冶炼行业。随着科技发展的需要,为了满足市场对阴极铜日益增长的需求量,铜冶炼企业通常采用提高铜电解的电流密度以提高阴极铜的产量[1],但高电流密度易引发电解液中杂质离子富集、阳极钝化以及阳极泥沉降困难等问题[2-5]。例如锑以三价离子的形式如SbO+或硫酸盐络合物离子通过阳极溶解进入电解液形成[6],然后被电解液中的溶解氧氧化为五价形成阳极泥的状态[7],一旦阳极泥附着在阴极铜上会降低其纯度和表观质量。同时,溶解在电解液中的锑离子易导致阳极钝化[8],引起槽电压升高与电流效率降低。而且随着杂质离子浓度的增加,阳极钝化更频繁,主要原因是杂质离子溶解在电解液中会增加其黏度,加剧硫酸铜结晶,干扰铜离子在阳极表面的传递[9]。生产中一般对应的措施包括提高温度和循环流量,调节添加剂等物理方法[10],因为温度过低会加剧阳极泥团聚,低流速会导致热量补充不足,造成阳极钝化并增加槽电压[11],所以提高流速及温度可减少阴极铜污染的主要来源和漂浮阳极泥的数量[12]。但流速太高又会加大阳极泥沉降难度,温度过高也会影响铜电解工艺,增加能耗,所以实际生产很难调控到最佳状态。常见的除杂化学工艺如萃取法也因易造成电解液的二次污染而很少被使用[13]。另外,NINOMIYA等[14]通过观察Cu-5%Sb(质量分数)阳极在H2SO4-CuSO4电解液中的溶解和钝化过程发现可以通过控制阳极泥颗粒的聚结和硫酸铜的析出防止硫酸盐覆盖整个阳极表面而引起钝化。而姜丽丽等[15]发现磁场可以增大硫酸钙的溶解度,改变硫酸钙的结晶形态,所以可以利用磁处理技术优化铜电解工艺,例如MATSUSHIMA等[16]利用梯度磁场改变了电沉积铜的晶粒尺寸。但OSHIKIRI等[17]发现垂直磁场作用可将铜阳极溶解过程中产生的离子空位转化为微泡,在洛伦兹力诱导的循环溶液中,离子空位相互碰撞形成纳米空泡,纳米气泡与循环流动的溶液进一步碰撞产生微气泡在阴极析出形成气孔。综上所述,有关磁处理的研究还集中在定性研究阶段,仍无统一的结论可解释磁处理机理,对于铜电解过程而言,施加磁场所引起电解液理化性质的变化与阴极铜表观质量之间的关系还未见报道。
基于此,本研究通过在铜电解循环管道上施加水平取向磁场进行磁场协同强化铜电解试验,研究不同磁感应强度下磁场梯度力作用于铜电解液时,离子浓度、黏度、表面张力以及阴极铜表观质量之间的变化规律,推导出磁场梯度力作用下阳极溶解速度、阴极析出速度、铜电解自净化过程、电解体系能量、溶解氧量、离子磁性的差异、离子水合作用以及氢键缔合程度与阴极铜表观质量之间的关系。
1 实验
1.1 试验装置
在原铜电解循环系统的基础上添加可调水平取向磁场进行设备设计与安装,整个试验系统由高位槽、可调水平取向磁场、循环泵、流量计、电解槽、直流电源、实验平台组成,如图 1 所示。其中,电解槽的尺寸为15 cm×15 cm×40 cm,有效体积为1 L,上面架设阳极铜和始极片,阳极铜的尺寸为10 cm×1 cm×15 cm,始极片的尺寸为10 cm×15 cm,阳极铜和始极片通过铜导线与直流电源(上海稳凯电源设备有限公司生产)的正极和负极连接。同时,电解液采用下进上出的循环方式,循环流速通过超声波流量计测量(江苏美安特自动化仪表有限公司生产),其大小通过阀门控制为0.02 m/s,高位槽内置加热装置,可调水平取向磁场装置由深圳怡天磁性材料有限公司制造,其磁感应强度B的可调范围为0~3 T。

图1 试验装置示意图
Fig. 1 Schematic diagram of experiment device
1.2 试验方法
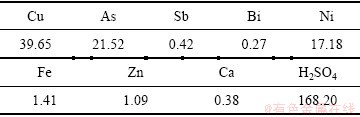
试验所用的电解液、阳极铜和始极片均取自白银有色集团股份有限公司铜业公司。将电解液放置一周以保证添加剂硫脲和骨胶失效,然后装桶密封保存备用,阳极铜和电解液的成分及其含量如表 1和2所列。实验开始时,首先量取5 L的电解液倒入高位槽,电解液由高位槽自流至电解槽,待整个管道充满电解液后开启循环泵,将电解液以0.02 m/s的流速经过可调水平取向磁场输送到高位槽,同时开启加热装置,待电解液温度升高至65 ℃时将打磨和泡洗好的阳极铜和始极片进行干燥称重装槽,同时开启直流电源,计算阴极有效总面积(S)、根据电流密度的公式J=I/S (I为电流强度)确定电流的大小,其中电流密度设定为260 A/m2。
表1 阳极铜的化学成分
Table 1 Chemical composition of anode (mass fraction, %)

表2 电解液的化学成分
Table 2 Chemical composition of copper electrolyte (g/L)
1.2 检测指标
电解4、8、12、16、20、24 h时,测量电解液的黏度、表面张力以及离子浓度。为了保证试验数据的准确性,每组试验重复3 次,取其平均值作为试验结果。电解试验完成后,将残极和阴极铜取出,用稀硫酸煮洗后,进行真空干燥、称取质量、取样。其中,离子浓度采用火焰原子吸收分光光度法测量,阴极铜的表观质量利用电镜扫描观测,电解液的黏度采用NDJ-9S系列黏度计测量。表面张力的测量是利用电解液在一定温度下,不同直径毛细管中的高度差来测量:将毛细管浸没在电解液中,电解液会缓慢上升,这样毛细管可以连续记录电解液上升的高度。因此,电解液的表面张力T可根据式(1)计算[18]。
 (1)
(1)
式中:T 为表面张力(N);D1、D2为两个毛细管的直径(D1=0.3×10-3 m,D2=0.5×10-3 m);h1、h2为毛细管中电解液上升的高度(m);m为电解液质量(g);V为电解液体积(L);g为重力加速度(m/s2)。
2 结果与分析
2.1 磁感应强度对磁化铜电解过程铜/酸的影响
图2(a)所示为在水平取向磁场下磁感应强度B对铜电解过程中Cu2+浓度的影响。由图2可知,当B=0 T时,Cu2+浓度随电解时间的延长呈现出波动性增长趋势,电解时间t为24 h 时,Cu2+浓度由初始值39.65 g/L上升至40.99 g/L。当B=1 T时,Cu2+浓度首先在t=8 h时降低至极小值39.30 g/L,然后在20 h增至极大值41.67 g/L。

图2 磁感应强度对磁化铜电解过程铜/酸的影响
Fig. 2 Effect of magnetic field intensity on copper/acid under different orientation magnetic fields
在B=2 T时,Cu2+浓度在t=4 h时达到极小值39.77 g/L,在t=24 h时达到极大值43.72 g/L。而当B=3 T时,Cu2+浓度的极小值为39.65 g/L,极大值为43.32 g/L。所以水平取向磁场可改变Cu2+浓度的变化趋势,由此可引起酸度和铜电解反应过程发生改变。由图2(b)可知,与未施加磁场相比,随着电解时间的延长,当B=1 T时,硫酸浓度出现降低的趋势,在电解时间为8 h时,硫酸浓度出现极小值,当B为2 T和3 T时,硫酸浓度均出现不同程度地增高,在B=2 T的条件下电解24 h,硫酸浓度出现极大值。由图2(c)可知,阳极质量损失和阴极析出量随着磁感应强度的增大出现先增后减的变化趋势,未施加磁场时,阳极质量损失和阴极析出量分别为86.8 g和73. 2 g,其中,阴极析出量在B=0 T时出现极小值。当B=1 T时,阳极的质量损失和阴极析出量均达到极大值,分别为88.53 g和78.42 g。但阳极质量损失与阴极析出量的差值R在B=0 T时达到极大值,为13.53g,在B=2 T时达到极小值,为8.02 g,在B=3 T时,R值略增至8.88 g,该指标的变化主要取决于阳极质量损失、阴极析出量以及进入阳极泥和电解液中的离子含量。同时,结合表1阳极铜的成分及含量,通过计算可得当B分别为0 T、1 T、2 T和3 T时,铜损失量依次为20.14 g、15.3 g、1.84 g和4.71 g,可见磁场的存在会导致铜电解过程发生一种更为复杂的反应。通常来讲,铜电解过程中会发生如下反应[19]:
Cu-2e=Cu2+ (2)
Cu-e=Cu+ (3)
2Cu+ Cu2++Cu (4)
Cu2++Cu (4)
Cu2SO4+H2SO4+1/2O2=2CuSO4+H2O (5)
Cu2SO4=CuSO4+Cu (6)
Cu2O+2H2SO4+1/2O 2=2CuSO4+2H2O (7)
Cu+H2SO4+1/2O2=CuSO4+H2O (8)
Cu2SO4+H2O=Cu2O+H2SO4 (9)
根据图2(a)和(b)可得,与未加磁场相比,当B=1 T时,Cu2+和H2SO4浓度均有所降低,其中H2SO4浓度在t=12 h时降幅最大,二者浓差达到最大值,为28.82 g/L,根据式(6)可得当B=1 T时,可加剧Cu2O的化学溶解,促使硫酸浓度降低,含铜量增加,导致阴极铜析出量增加。当B=2 T时,电解液中的含铜量和H2SO4浓度随着电解时间的延长而急剧增加,结合式(8),说明B=2 T时可抑制Cu+的歧化反应,可降低铜粉析出量,促进阳极铜和Cu2O溶解以及Cu2SO4的水解,提高H2SO4浓度。而当B=3 T时,Cu2+浓度低于B=2 T时的含铜量,说明磁感应强度B越强,Cu2O的溶解量越少。另外,进入电解液中的亚砷酸盐可与Cu2+发生以下反应:
3Cu2++2HAsO2+3H+→Cu3As+2H2O (10)
所以Cu2+浓度的变化也会受到电解液中砷离子的影响。图2(d)显示As5+浓度在磁场作用下出现降低的趋势,在B=1 T时,As5+浓度达到极小值,可见Cu2+浓度的变化与砷离子存在一定关系。同时,砷离子又可与锑铋离子发生反应,因此,施加水平取向磁场可影响阴极铜的析出量、电解液和阳极泥的成分及其含量。
2.2 磁感应强度对铜电解过程自净化的影响
图3所示为杂质离子含量随磁感应强度的变化。由图3可看出,施加水平取向磁场后,杂质离子浓度均有不同程度地降低。根据图3(a)所示,当B=0 T时,Sb3+浓度随着电解时间t的延长而出现递增趋势,最高可达0.57 g/L。当B=1 T时,Sb3+浓度在t为4~24 h时基本达到平衡状态,为0.31 g/L。而当B=2 T时,Sb3+浓度先减后增,在t=12 h达到极小值,为0.35 g/L,在t=24 h时达到极大值,为0.47 g/L。当B=3 T时,Sb3+浓度出现波动性变化趋势,基本维持在0.38~0.42 g/L的范围内。根据图3(b)可得,Bi3+浓度的变化趋势与Sb3+浓度的变化趋势基本一致,主要是由As5+浓度变化引起,当As5+降低时,Sb3+和Bi3+浓度也会相应地降低,这是因为磁场梯度力可通过调控离子化合价促使电解液中的砷锑铋离子形成沉淀阳极泥,达到铜电解过程自净化的目的,具体反应见式(11)~(14)[20]:
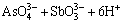 =SbAsO4↓+3H2O (11)
=SbAsO4↓+3H2O (11)
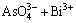 =BiAsO4↓ (12)
=BiAsO4↓ (12)
HSb(OH)6+AsO+→AsSbO4↓+3H2O+H+ (13)
结合图2(d)、图3(a)和(b),根据式(11)~(13)可得,磁场梯度力可促进SbAsO4、BiAsO4以及AsSbO4等沉淀的形成,降低电解液中砷锑铋杂质离子浓度。而通过分析实际生产产生的漂浮阳极泥成分时发现砷锑铋是形成漂浮阳极泥的核心元素(见表3),所以降低电解液中As5+、Sb3+和Bi3+浓度可降低铜电解过程中形成漂浮阳极泥的概率,达到提高电解液清晰度和改善阴极铜表观质量的目的。
表3 漂浮阳极泥的主要化学成分
Table 3 Main chemical composition of floating anode slimes samples (mass fraction, %)

图3(c)所示为不同磁感应强度B对Ni2+浓度的影响。由图3(c)可知,Ni2+浓度随着B的变化出现不同程度的降低,其中在B=1 T时,Ni2+浓度达到极小值。同时Fe2+和Zn2+浓度的变化与Ni2+浓度变化趋势相同,Fe2+浓度在B=3 T时达到极小值,而Zn2+浓度在B=1 T时达到极小值(见图3(d)和(e))。根据铜电解液的黏度η与离子浓度的关系可知(见式(14)),降低Ni2+浓度可降低电解液的黏度[21],从而提高阳极泥的沉降速率和电解液的清晰度。同理,Fe2+和Zn2+浓度越低,电解液清晰度越高,结果将有利于改善阴极铜的质量。图2(f)所示为Ca2+浓度的变化趋势,Ca2+浓度变化比较特殊,其溶解度随着磁感应强度的提高出现递增趋势,当B=3 T时,Ca2+浓度达到极大值。
η=0.07989+0.002868[Cu]+0.0005293[H2SO4]+
0.003349[Ni]+0.004768[As]-0.007038T-
2.175×10-5T [As] (14)
综上所述,结合图3和6,施加水平取向磁场,有助于提高电解液的清晰度和阴极铜的表观质量。

图3 磁感应强度对铜电解过程中杂质离子浓度的影响
Fig. 3 Effect of magnetic field intensity on impurity ion concentration in magnetic field with different orientations
3 基于强磁场环境下磁场梯度力作用于铜电解过程的机理分析
在水平取向磁场作用下,离子会受到磁场梯度力的影响[22],正如式(15)、(16)和(17)所示:
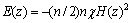 (15)
(15)
式中:E(z)为离子的磁能; 为摩尔磁化率;n为离子的摩尔数;H(z)是位于位置z磁感应强度,而离子所受的磁场梯度力可表示为
为摩尔磁化率;n为离子的摩尔数;H(z)是位于位置z磁感应强度,而离子所受的磁场梯度力可表示为
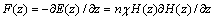 (16)
(16)
由式(16)可知,假设离子是顺磁性的( >0),如铜电解液中的Fe2+、Cu+、Cu2+、H+、Ni2+、以及Ca2+,因磁场梯度力F(z)作用在磁感应强度增加的方向被吸引,其磁场能量E(z)会被缩小并稳定在磁感应强度的最大处。如果离子是反磁性的(
>0),如铜电解液中的Fe2+、Cu+、Cu2+、H+、Ni2+、以及Ca2+,因磁场梯度力F(z)作用在磁感应强度增加的方向被吸引,其磁场能量E(z)会被缩小并稳定在磁感应强度的最大处。如果离子是反磁性的( <0),如砷锑铋离子,它们在磁场之外是稳定的,即它们对磁场是排斥的,所以金属离子在磁场中的敏感性和迁移距离均不同,且迁移距离通常会随着磁化率的增加而增大。根据离子水合作用以及离子溶剂化理论得到,Cu2+、Fe2+、Zn2+、Ni2+、Ca2+等离子会与若干个水分子相互作用形成离子水合作用,进而影响离子的水解程度[23-24],所以当顺磁性离子的磁能被缩小时,体系能量随之降低,离子水合作用和水解反应得到加强,化学水化层厚度增加。同时,在F(z)作用下,Cu+因磁能被减小其歧化反应受到抑制,所以在B为1 T和2 T时,铜粉析出量以及铜损失量急剧减少,Cu2SO4的水解反应得到加强。当B=3 T时,水分子在F(z)作用下其磁能被放大,并转化为体系的能量,降低氢键缔合程度,减弱磁场对Cu+歧化反应的抑制作用而引发铜损失量回升。另一方面电解液经磁化后表面张力降低(见图5(b)),根据表面张力与体系能量关系(见式(17))。
<0),如砷锑铋离子,它们在磁场之外是稳定的,即它们对磁场是排斥的,所以金属离子在磁场中的敏感性和迁移距离均不同,且迁移距离通常会随着磁化率的增加而增大。根据离子水合作用以及离子溶剂化理论得到,Cu2+、Fe2+、Zn2+、Ni2+、Ca2+等离子会与若干个水分子相互作用形成离子水合作用,进而影响离子的水解程度[23-24],所以当顺磁性离子的磁能被缩小时,体系能量随之降低,离子水合作用和水解反应得到加强,化学水化层厚度增加。同时,在F(z)作用下,Cu+因磁能被减小其歧化反应受到抑制,所以在B为1 T和2 T时,铜粉析出量以及铜损失量急剧减少,Cu2SO4的水解反应得到加强。当B=3 T时,水分子在F(z)作用下其磁能被放大,并转化为体系的能量,降低氢键缔合程度,减弱磁场对Cu+歧化反应的抑制作用而引发铜损失量回升。另一方面电解液经磁化后表面张力降低(见图5(b)),根据表面张力与体系能量关系(见式(17))。
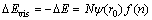 (17)
(17)
式中: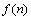 为密度;
为密度; 是两个分子相互作用的平均势能;N为分子数量;
是两个分子相互作用的平均势能;N为分子数量;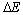 为活化能;
为活化能; 是距离为r0两分子的平均势能,其中
是距离为r0两分子的平均势能,其中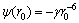 ,
,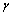 是表面张力[25]。由此可得:密度和分子数量一定的条件下,表面张力和活化能越小,两个分子间的距离r0以及分子相互作用的平均势能越大。如图4所示,在F(z)的作用下模型中顺磁性离子P的磁能被缩小,其离子水合作用得到加强,化学水化层厚度因此而增加,表现为离子P与水分子形成的球簇结构增大,抗磁性离子D则相反。水分子在F(z)作用下氢键作用被削弱,在图中表现为水分子链断裂。而水分子氢键缔合程度的减弱一方面可引起电解液表面张力的降低,且磁感应强度越强,电解液的表面张力和活化能越小(见图5(b)),Cu2+扩散速度以及阴极铜的析出速度也越快。同时,磁处理存在“记忆效应”,实验发现:铜电解液在磁场强度为3 T、温度为65 ℃、流速0.02 m/s的条件下单独磁化铜电解液后表面张力降低约20%~ 35%,并且这种状态能够维持48 h以上,表明磁化铜电解过程弛豫时间长。另一方面,Cu2+的扩散速度因Cu2+和H+的化学水化层厚度增加而受到影响,因此,阴极析出量随B的提高出现先增后减的趋势(见图2(c))。同时,水分子间的氢键作用的减弱可引起气体和难溶性盐的溶解度增加[26],因此,磁场梯度力可增加铜电解液中溶解氧量和CaSO4的溶解度(见图3(f))。另外,Ca2+由于受到磁能的影响引发化学水化层厚度增加及离子水合作用加强,导致 Ca2+与SO42-之间不易形成盐桥,结果将进一步降低硫酸钙析出量。而水分子间的氢键减弱导致水分子间距增大,减小对氧气分子的压缩,电解液中溶解氧因此而增加,主要会引发下列反应[27]:
是表面张力[25]。由此可得:密度和分子数量一定的条件下,表面张力和活化能越小,两个分子间的距离r0以及分子相互作用的平均势能越大。如图4所示,在F(z)的作用下模型中顺磁性离子P的磁能被缩小,其离子水合作用得到加强,化学水化层厚度因此而增加,表现为离子P与水分子形成的球簇结构增大,抗磁性离子D则相反。水分子在F(z)作用下氢键作用被削弱,在图中表现为水分子链断裂。而水分子氢键缔合程度的减弱一方面可引起电解液表面张力的降低,且磁感应强度越强,电解液的表面张力和活化能越小(见图5(b)),Cu2+扩散速度以及阴极铜的析出速度也越快。同时,磁处理存在“记忆效应”,实验发现:铜电解液在磁场强度为3 T、温度为65 ℃、流速0.02 m/s的条件下单独磁化铜电解液后表面张力降低约20%~ 35%,并且这种状态能够维持48 h以上,表明磁化铜电解过程弛豫时间长。另一方面,Cu2+的扩散速度因Cu2+和H+的化学水化层厚度增加而受到影响,因此,阴极析出量随B的提高出现先增后减的趋势(见图2(c))。同时,水分子间的氢键作用的减弱可引起气体和难溶性盐的溶解度增加[26],因此,磁场梯度力可增加铜电解液中溶解氧量和CaSO4的溶解度(见图3(f))。另外,Ca2+由于受到磁能的影响引发化学水化层厚度增加及离子水合作用加强,导致 Ca2+与SO42-之间不易形成盐桥,结果将进一步降低硫酸钙析出量。而水分子间的氢键减弱导致水分子间距增大,减小对氧气分子的压缩,电解液中溶解氧因此而增加,主要会引发下列反应[27]:
Cu++O2=Cu2++ (18)
(18)
As(Ⅲ)+ →As(Ⅳ)+H2O2 (19)
→As(Ⅳ)+H2O2 (19)
As(Ⅳ)+O2→As(Ⅴ)+  (20)
(20)
2H++2e+O2→H2O2 (21)
2Cu++2H++O2→ 2Cu2++H2O2 (22)
根据式(21)~(22)可得:提高溶解氧量可降低Cu+形成Cu2+和H2O2的活性,促进阳极溶解速率。因此,电解液中存在氧气时可加快阳极溶解速率是由于溶解氧可降低Cu+的活性[28]。而磁化电解液后溶解氧量的提高将进一步降低Cu+的活性,降低铜粉析出量。
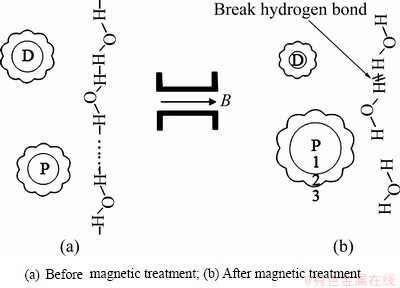
图4 磁场梯度力影响氢键缔合程度和离子水合作用的理论模型
Fig. 4 Theoretical model of influence of magnetic field gradient force on hydrogen bond association degree and ion hydration (B—Magnetic flux density; D—Diamagnetic ion; P—Paramagnetic ion; 1—Chemical hydration shell; 2—Physical hydration shell; 3—Free hydration shell)
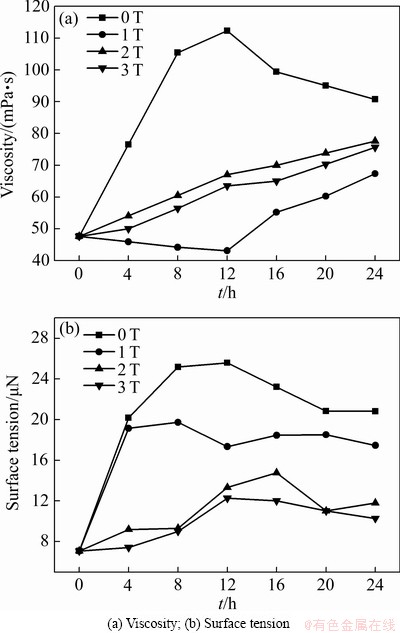
图5 磁感应强度对黏度和表面张力的影响
Fig. 5 Effect of magnetic field intensity on viscosity and surface tension
同时,铜与氧因磁性差异在F(z)作用下导致他们之间的距离增大。另外,溶液的黏度也会影响迁移距离,对于在黏性介质η中运动的流体动力半径为R的离子,漂移速度v(z)与磁场梯度力F(z)的关系式为
 (23)
(23)
因而水平取向磁场可根据离子磁性的差异达到分离离子效果,随着磁感应强度的增强,虽然溶解氧量得到提升,但Cu+的磁能却不断被降低,加之磁分离作用加剧,导致Cu2O的生成量先增加后降低,引起Cu2SO4的水解量和H2SO4浓度增加。因此,当B为2 T和3 T时,H2SO4浓度达到极大值。
另外,阳极铜中的砷锑铋在电解过程中首先以三价离子形式进入电解液,然后在Cu+和溶解氧的作用下,As3+逐渐被氧化为As5+,少量Sb3+被氧化为Sb5+,Bi离子则主要以 Bi3+存在,所以未施加水平取向磁场时,电解液中As5+浓度随电解时间的延长而不断增加,其主要原因是As3+不断被氧化导致电解过程中砷锑铋离子浓度不断上升。当B=1 T时,砷锑铋的磁能得到提升,并且由于砷锑铋同属抗磁性元素,因此,根据式(11)~(13)可得,水平取向磁场可促进SbAsO4、BiAsO4以及AsSbO4等沉淀的形成,导致As5+、Sb3+以及Bi3+达到最小值。当B为2 T和3 T时,根据式(18)~(20)表明:活性氧量和As5+浓度受溶解氧影响而不断提高,降低SbAsO4、BiAsO4以及AsSbO4等沉淀的形成量,导致As5+、Bi3+和Sb3+浓度有所提升。对于Ni2+而言,由于NiO在铜电解精炼过程中基本上都没有溶解,大部分集中在阳极泥中[29]。因此,在F(z)的作用下,溶解氧量增加促使镍表面形成氧化膜,但随着磁感应强度不断增加,溶解氧的磁能不断被缩小,导致Ni2+浓度又出现回升趋势[30]。同理,Fe2+和Zn2+的表面也会形成保护膜,其变化趋势同Ni2+浓度一致。
磁场梯度力通过作用于铜电解液而影响阴极铜的质量,如图6所示,当B=0 T时,电解液中杂质离子超标,易引起与铜电位接近的杂质离子直接在阴极析出,如砷锑铋等离子。另一方面,结合式(15)可知,杂质离子浓度增加会引起电解液黏度增大。结果如图5(a)所示,未施加磁场时,阳极泥沉降性能受电解液黏度的影响而降低,形成漂浮阳极泥黏附在阴极铜表面而影响阴极铜的表观质量(见图6(a))。当B=1 T时,与未加磁场相比,电解液的表面张力降低,引起电解液中气泡增多,Cu2+扩散速度和阴极铜析出速度加快,结果导致阴极铜表面形成气孔[31],如图6(b)所示。当B=2 T时,与B=1 T时相比,由于表面张力更小,电解液气泡量更多,阴极铜表面形成的气孔数量更多(见图6(c))。而当B=3 T时,由于F(z)的作用对Cu2+磁能的影响,Cu2+化学水化层厚度增加,其扩散性能受到影响,阴极铜的析出速度减慢,降低气泡对阴极铜质量的影响。同时,Cu2+离子水合作用加强,水化层厚度增加,强化对电解液的压缩,减弱微气泡的聚合,引起黏度适量增加,导致电解液中的气泡数量降低[32],阴极铜表面不易形成气孔,并且由于杂质离子浓度降低,阴极铜的表观质量得到极大地改善,如图6(d)所示,具体作用原理如图7所示:经过磁场梯度力作用后,一方面水分子的氢键缔合程度减弱,水分子间距增加导致微气泡聚合形成气泡而影响阴极铜的表观质量,另外,更多的氧气也会溶解在电解液中形成溶解氧影响铜电解反应;另一方面受磁场梯度力的影响,电解液中Cu2+和H+与水分子形成的球簇结构增大,在一定程度上可调控Cu2+的扩散速度。同时,Cu2+和H+球簇结构的增大也会通过碰撞和压缩降低电解液中的气泡数量以减少阴极铜表面的气孔数量。

图6 磁感应强度对阴极铜表观质量的影响
Fig. 6 Effect of magnetic field intensity on surface quality of cathode copper
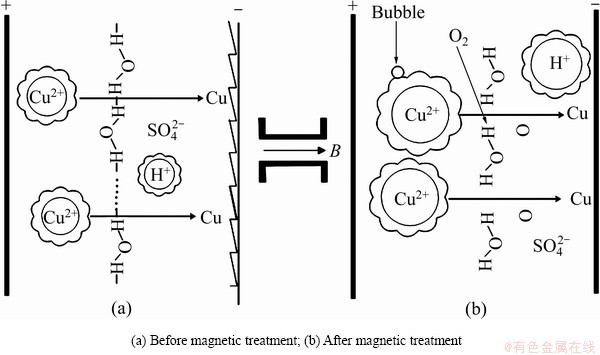
图7 磁场梯度力影响铜电解过程的理论模型
Fig. 7 Theoretical model of influence of magnetic field gradient force on copper electrolysis process
4 结论
1) 在水平取向磁场条件下,离子由于磁性的差异受到磁场梯度力的作用,铜电解液中的抗磁性离子和水分子磁能被放大,顺磁性元素的磁能被缩小,促使电解液的表面张力降低,且磁感应强度越强,表面张力越低,结果降低电解液活化能,加快铜电解反应速度,加强电解液的自净化过程,增大溶解氧量和CaSO4的溶解度。
2) 铜电解过程中,电解液由于磁场梯度力的作用,水分子的磁能转化为体系能量,导致氢键缔合程度降低,Cu2+扩散速度加快,电解液中的气泡增多,阴极铜表面易形成气孔;但铜离子活性降低,Cu2+离子水合作用加强,减弱微气泡聚合,阴极铜的表观质量得到改善,表面无气孔。同时,Cu+的歧化反应被抑制,铜损失量降低,阳极溶解速度和阴极铜析出速度降低,因此,水平取向磁场作用于铜电解过程存在最佳磁感应强度。
3) 水平取向磁场作用于铜电解过程时,恰当的磁场梯度力可促进砷锑铋离子的相遇机会,磁场梯度力达到一定数值后又会阻碍砷锑铋离子相遇,另外,磁场梯度力可扩大砷锑铋的磁能并将其转化为自身能量,因此,恰当的磁场梯度力可促进SbAsO4、BiAsO4以及AsSbO4等沉淀的形成,降低电解液中砷锑铋杂质离子浓度。同时,恰当的磁场梯度力可促进镍铁锌离子表面形成氧化性保护膜,降低电解液中Ni2+、Fe2+、Zn2+离子浓度。所以,恰当的水平磁场可强化铜电解自净化过程,降低电解液中杂质离子浓度,提高电解液清晰度,改善阴极铜质量,综上可知,水平取向磁场作用于铜电解过程存在最佳磁感应强度,为3 T。
4) 水平取向磁场作用于铜电解过程时,Ca2+因其磁性的影响自身磁能降低导致离子水合作用加强,阻止Ca2+与 之间形成盐桥。同时,电解液表面张力降低,水分子间氢键断裂引起Ca2+溶解度增加,所以Ca2+浓度随着磁感应强度的增加而增加,在磁感应强度为3 T时,达到极大值。
之间形成盐桥。同时,电解液表面张力降低,水分子间氢键断裂引起Ca2+溶解度增加,所以Ca2+浓度随着磁感应强度的增加而增加,在磁感应强度为3 T时,达到极大值。
REFERENCES
[1] 姚夏妍, 王军辉, 余江鸿, 鲁兴武, 李彦龙, 焦晓斌. 磁场协同作用改善阴极铜质量的机理探讨与验证[J]. 中国有色冶金, 2019, 48(3): 14-18.
YAO Xia-yan, WANG Jun-hui, YU Jiang-hong, LU Xing-wu, LI Yan-long, JIAO Xiao-bin. Mechanism discussion and verification of improving cathode copper quality by magnetic field synergy[J]. China Nonferrous Metallurgy, 2019, 48(3): 14-18.
[2] MITRA A, MALLIK M, SENGUPTA S, BANTHIA S, DAS K, DAS S. Effect of anodic passivation at high applied potential difference on the crystal shape and morphology of copper electrodeposits: thermodynamics and kinetics of electrocrystallization[J]. Crystal Growth & Design, 2017, 17(4): 1539-1549.
[3] KIVILUOMA M, AALTONEN M, AROMAA J, LUNDSTROM M, FORSEN O. Development of characterization methods for adherent anode slimes in copper electrorefining[J]. Physicochemical Problems of Mineral Processing, 2016, 52(1): 295-302.
[4] KALLIOMAKI T, WILSON B P, AROMAA J, LUNDSTROM M. Diffusion coefficient of cupric ion in a copper electrorefining electrolyte containing nickel and arsenic[J]. Minerals Engineering, 2019, 134: 381-389.
[5] PENG Ying-lin, ZHENG Ya-jie, CHEN Wen-mi. The oxidation of arsenic from As(Ⅲ) to As(Ⅴ) during copper electrorefining[J]. Hydrometallurgy, 2012, 129/130: 156-160.
[6] ZHANG Wang-bing, YANG Xin-an, DONG Yong-ping, CHU Xiang-feng. Application of alkaline mode electrochemical hydride generation for the detection of As and Sb using atomic fluorescence spectrometry[J]. Spectrochimica Acta Part B: Atomic Spectroscopy, 2010, 65(7): 571-578.
[7] HISKEY J B. Mechanism and thermodynamics of floating slimes formation[C]// Proceedings of T.T. Chen Honorary Symposium on Hydrometallurgy, Electrometallurgy and Materials Characterization. Orlando: TMS Annual Meeting & Exhibition, 2012: 101-112.
[8] SAFIZADEH F, GHALI E. Monitoring passivation of Cu-Sb and Cu-Pb anodes during electrorefining employing electrochemical noise analyses[J]. Electrochimica Acta, 2011, 56(1): 93-101.
[9] ZENG Wei-zhi, WANG Shi-jie, MICHAEL L F. Two-phase flow modeling of copper electrorefining involving impurity particles[J]. Journal of The Electrochemical Society, 2017, 164(9): 233-241.
[10] YANG Jian-guang, LIU Shan, ZENG Wei-zhi, LI Bo, LI Ken, HU Hui, DING Wen-jie. Experimental analysis of the performance of innovative circulation configurations for cleaner copper electrolysis[J]. Hydrometallurgy, 2019, 189: 105-145.
[11] ZENG Wei-zhi, WANG Shi-jie, MICHAEL L F. Experimental studies of the effects of anode composition and process parameters on anode slime adhesion and cathode copper purity by performing copper electrorefining in a pilot-scale cell[J]. Metallurgical and Materials Transactions B, 2016, 47(5): 3178-3191.
[12] ZENG Wei-zhi, MICHAEL L F, WANG Shi-jie. Studies of anode slime sintering/coalescence and its effects on anode slime adhesion and cathode purity in copper electrorefining[J]. Journal of The Electrochemical Society, 2015, 163(2): 14-31.
[13] ARTZER A, MOATS M, BENDER J. Removal of antimony and bismuth from copper electrorefining electrolyte: Part Ⅱ—An investigation of two proprietary solvent extraction extractants[J]. JOM, 2018, 70(10): 2033-2040.
[14] NINOMIYA Y, SASAKI H, KAMIKO M, YOSHIKAWA T, MAEDA M. Passivation of Cu-Sb anodes in H2SO4-CuSO4 aqueous solution observed by the channel flow double electrode method and optical microscopy[J]. Electrochimica Acta, 2019, 309: 300-310.
[15] JIANG Li-li, YAO Xia-yan, YU Hai-tao, HOU Xin-gang, ZOU Zong-shu, SHEN Feng-man, LI Chuan-tong. Effect of permanent magnetic field on water association in circulating water[J]. Desalination and water treatment, 2017, 79(10): 152-160.
[16] MATSUSHIMA H, ISPAS A, BUND A, PLIETH W, FUKUNAKA Y. Magnetic field effects on microstructural variation of electrodeposited cobalt films[J]. Journal of solid state electrochemistry, 2007, 11(6): 737-743.
[17] OSHIKIRI Y, AOGAKI R, MIURA M, SUGIYAMA A, MORIMOTO R, MIURA M, MOGI I, YAMAUCHI Y. Microbubble formation from ionic vacancies in copper anodic dissolution under a high magnetic field[J]. Electrochemistry, 2015, 83(7): 549-553.
[18] JIANG Li-li, YAO Xia-yan, YU Hai-tao, HOU Xin-gang, ZOU Zong-shu, SHEN Feng-man, LI Chuan-tong. Effect of permanent magnetic field on scale inhibition property of circulating water[J]. Water Science & Technology, 2017, 76(8): 1981-1991.
[19] 李晓静. 旋流电积技术进行铜电解液净化脱铜除杂的研究[D]. 长沙: 中南大学, 2012.
LI Xiao-jing. Study on the purification and impurity removal of copper electrolyte by cyclone electrowinning technology[D]. Changsha: Central South University, 2012.
[20] 彭映林, 郑雅杰, 陈文汨. 铜电解过程中As(Ⅲ)净化作用及其氧化动力学[J]. 中国有色金属学报, 2012, 22(6): 1798-1803.
PENG Ying-lin, ZHENG Ya-jie, CHEN Wen-mi. Purification and oxidation kinetics of As(Ⅲ) in copper electrolysis[J]. The Chinese Journal of Nonferrous Metals, 2012, 22(6): 1798-1803
[21] TAINA K, AJI A T, RINTALA L, AROMAA J, LUNDSTROM M. Models for viscosity and density of copper electrorefining electrolytes[J]. Physicochemical Problems of Mineral Processing, 2017, 53(2): 1023-1037.
[22] FUJIWARA M, KODOI D, DUAN W, TANIMOTO Y. Separation of transition metal ions in an inhomogeneous magnetic field[J]. Cheminform, 2001, 32(39): 3344-3345.
[23] O’BRIEN J T, WILLIAMS E R. Coordination numbers of hydrated divalent transition metal ions investigated with IRPD spectroscopy[J]. The Journal of Physical Chemistry A, 2011, 115(51): 14612-14619.
[24] 胡殿俭. 金属离子在水溶液中的溶剂化研究[D]. 北京: 中国石油大学, 2007.
HU Dian-jian. Solvation of metal ions in aqueous solution[D]. Beijing: China University of Petroleum, 2007.
[25] BRANDT W. Calculation of intermolecular force constants from polarizabilities[J]. Journal of Chemical Physics, 1956, 24(3): 501-508.
[26] 姚夏妍, 汪友元, 鲁兴武, 程 亮, 李彦龙, 焦晓斌. 磁场效应强化湿法冶金的现状及前景[J]. 中国有色冶金, 2019, 48(1): 8-13.
YAO Xia-yan, WANG You-yuan, LU Xing-wu, CHENG Liang, LI Yan-long, JIAO Xiao-bin. Current situation and Prospect of magnetic field effect enhanced hydrometallurgy[J]. China Nonferrous Metallurgy, 2019, 48(1): 8-13
[27] HUG S J, CANONICA L, WEGELIN M, GECHTER D, VOV GUNTEN U. Solar oxidation and removal of arsenic at circumneutral pH in iron containing waters[J]. Environmental Science & Technology, 2001, 35(10): 2114-2121.
[28] KANZAKI Y, BRUCKENSTEIN S. Anodic dissolution of copper in oxygenated sulfuric acid solution as examined by rotating ring-disk electrode[J]. Electrochemistry, 2013, 81(7): 547-552.
[29] CHEN T T, DUTRIZAC J E. A mineralogical overview of the behavior of nickel during copper electrorefining[J]. Metallurgical transactions B, 2007, 21(2): 229-238.
[30] ZHANG Ming. Study of the effects of the magnetic field on the anodic dissolution of nickel with In-line digital holography[J]. International Journal of Electrochemical Science, 2018, 13(1): 739-751.
[31] CHANG Shih-chieh, WANG Ying-lang, HUNG Chi-cheng, LEE Wen-his, HWANG Gwo-jen. Role of surface tension in copper electroplating[J]. Journal of Vacuum Science & Technology A Vacuum Surfaces and Films, 2007, 25(3): 566-569.
[32] WEISSENBORN P K, PUGH R J. Surface tension and bubble coalescence phenomena of aqueous solutions of electrolytes[J]. Langmuir, 1995, 11(5): 1422-1426.
Effect of magnetic field gradients force on copper electrorefining under intense magnetic field
YAO Xia-yan1, ZHAO Yun-yun1, LU Xing-wu1, WANG Jun-hui2, NIU Yong-sheng1, CHEN Liang1, LI Yu-liang1
(1. Key Laboratory of New Process for Non-ferrous Metal Smelting and Rare Metal High Utilization Efficiency in Gansu Province, Northwest Research Institute of Mining and Metallurgy, Baiyin 730900, China;
2. Baiyin Nonferrous Group Co., Ltd., Baiyin 730900, China)
Abstract: The diffusion property of Cu2+ and the concentration of impurity ions are the key factors affecting the quality of cathode copper. The horizontal oriented magnetic field is installed on the circulating water pipeline in this paper to improve the quality of cathode copper by regulating the diffusion property of Cu2+ and strengthen the self purification process of copper electrolysis. From the perspective of magnetic separation and the influence of magnetic field on water clusters and ion hydration, the experiment of magnetizing copper electrolyte with horizontal orientation magnetic field was carried out. The effects of magnetic field gradient force on the concentration of cupric acid, impurity ion, viscosity, surface tension and the apparent quality of cathode copper were studied by flame atomic absorption spectrophotometry, viscometer, capillary tube and scanning electron microscope, and the mechanism of strengthening copper electrolysis process by horizontal orientation magnetic field was analyzed. The results show that the magnetic field gradient force can enlarge the magnetic energy of diamagnetic ions and water molecules in copper electrolyte, reduce the degree of hydrogen bond association of water molecules and the activation energy of electrolysis system, accelerate the reaction of copper electrolysis and the precipitation of SbAsO4, BiAsO4 and AsSbO4, increase the solubility of dissolved oxygen and CaSO4, so it can promote the formation of oxide protective film on the surface of Ni, Fe and Zn, improve the clarity of electrolyte and increase cathode production. On the other hand, the magnetic field gradient force can reduce the magnetic energy of paramagnetic ions, regulate the dissolution of anode and the precipitation of cathode copper, reduce copper loss and improve the apparent quality of cathode copper. At the same time, the magnetic field gradient force can reduce the surface tension of the electrolyte. As a result, the number of bubbles in the electrolyte increases and the pores form on the surface of copper cathode. To sum up, there is an optimum magnetic field intensity when the horizontal orientation magnetic field acts on the copper electrolysis process.
Key words: metal materials; copper electrolysis; magnetic field gradient force; energy, self purification
Foundation item: Project(GGLD-2019-28) supported by Research Project on Industrial Green Low Carbon Transition and Upgrading in Gansu Province, China; Project(2019-1-12G) supported by Baiyin Science and Technology Plan, China
Received date: 2019-12-02; Accepted date: 2020-04-28
Corresponding author: YAO Xia-yan; Tel: +86-18393862375; E-mail: 1141557523@qq.com
(编辑 李艳红)
基金项目:甘肃省工业绿色低碳转型升级研究课题(GGLD-2019-28);白银市2019年科技计划项目(2019-1-12G)
收稿日期:2019-12-02;修订日期:2020-04-28
通信作者:姚夏妍,研究员,硕士;电话:18393862375;E-mail:1141557523@qq.com
[1] 姚夏妍, 王军辉, 余江鸿, 鲁兴武, 李彦龙, 焦晓斌. 磁场协同作用改善阴极铜质量的机理探讨与验证[J]. 中国有色冶金, 2019, 48(3): 14-18.
[19] 李晓静. 旋流电积技术进行铜电解液净化脱铜除杂的研究[D]. 长沙: 中南大学, 2012.
[20] 彭映林, 郑雅杰, 陈文汨. 铜电解过程中As(Ⅲ)净化作用及其氧化动力学[J]. 中国有色金属学报, 2012, 22(6): 1798-1803.
[24] 胡殿俭. 金属离子在水溶液中的溶剂化研究[D]. 北京: 中国石油大学, 2007.
[26] 姚夏妍, 汪友元, 鲁兴武, 程 亮, 李彦龙, 焦晓斌. 磁场效应强化湿法冶金的现状及前景[J]. 中国有色冶金, 2019, 48(1): 8-13.


