
Thermoelectric properties of MxM6o8 (M=Ag, Cu)
SHI Xiao-ya(史啸亚)1, 2, WANG Li(王 丽)1, 2, CHEN Li-dong(陈立东)1, CHEN Xi-hong(陈喜红)1
1. State Key Laboratory of High Performance Ceramics and Superfine Microstructures, Shanghai Institute of Ceramics,
Chinese Academy of Sciences, Shanghai 200050, China;
2. Graduate University of the Chinese Academy of Sciences, Beijing 100080, China
Received 6 October 2008; accepted 8 February 2009
Abstract:
Ag and Cu filled Chevrel phase MxMo6Te8 (x=1.0, 2.0) samples were synthesized by direct solid state reaction and spark plasma sintering. The electrical and thermal properties were investigated in the temperature range of 300-800 K. The results show that both the electrical and thermal properties are affected by filler atoms. Although the electrical conductivity of MxMo6Te8 is slightly higher than that of state-of-the-art thermoelectric material, such as filled skutterudites, the absolute value of Seebeck coefficient is relatively low. Due to the phonon scattering by the filler atoms, the decrease of the thermal conductivity and the lattice thermal conductivity is obvious. As a result, the dimensionless figure of merit(ZT) is improved over the whole temperature region. The highest ZT value is 0.034 at 800 K for the AgMo6Te8 sample.
Key words:
thermoelectric property; Chevrel phase; chalcogenide;
1 Introduction
The ideal thermoelectric(TE) materials should have large Seebeck coefficient(s), and high electrical conductivity?(σ) as well as low thermal conductivity(κ) [1-3]. The TE materials could be evaluated by the figure of merit, ZT, which is defined as ZT=(α2σ/κ)T. Many researches have been focused on materials with special structures. Skutterudites[4-6], for instance, have cavity in their cubic structure that can be filled with atoms to scatter phonons and then reduce the lattice thermal conductivity. Chevrel phase materials have been studied mainly for their superconducting properties since 1971. Similar to the skutterudites, the 3-D framework of Chevrel phase[7] Mo6X8 (X=S, Se, Te) has channels made up of interconnected cavities that can be filled with a wide variety of guest atoms. Therefore, interest in Chevrel phase materials for thermoelectric applications is increased. Generally speaking, the traditional Chevrel phases are molybdenum chalcogenides Mo6X8 (X=S, Se, Te) that can be classified as binaries (Mo6X8), ternaries (MxMo6X8), and pseudo-binaries (Mo6-xMxX8 and Mo6X8-xYx). The unfilled Chevrel phase Mo6X8 is metallic while the ternaries and the pseudo-binaries can be realized acting as semiconductors. Recently, the ternary molybdenum chalcogenides MxMo6X8 (M=Cu, Ag, Fe, rare earth elements, etc; X=S, Se, Te) have been studied for use as TE materials[8-10]. A high ZT value of 0.6 at 1150 K for Cu/FeMo6Se8 was reported in Ref.[9], which shows the potential thermoelectric applications of the Chevrel phase materials. However, little data were provided for the thermoelectric properties of filled telluride Chevrel phase [11-12].
In the present study, both the electrical and thermal properties of Cu/Ag filled Chevrel phases (MxMo6Te8) were studied in the temperature range of 300-800 K and the figure of merit, ZT, was evaluated in the same temperature range.
2 Experimental
Highly pure elements Mo (99.95% pure, powder), Te (99.9999% pure, powder), Cu (99.95% pure, powder), Ag (99.9% pure, powder) were used as the starting materials. The powders were first prepared in the molar ratio of Mo to Te to M of 6:8:x (x=1.0, 2.0) and subsequently, they were synthesized in evacuated silica tubes by the direct solid state reaction method at 800 ℃ for 7 d. In order to get homogeneous samples, the products were ground into fine powders and pressed into bulks for another 7 d annealing at 650 ℃ in the evacuated silica tubes. Finally, the products were prepared using spark plasma sintering (SPS 2040) between 770 K and 800 K for 5 min under 55 MPa uniaxial pressure.
The phase composition of the sample was determined by X-ray diffraction(XRD) analysis (Cu Kα). The electrical conductivity(σ) was measured using a standard four-probe method. The Seebeck coefficient(s) was determined from slope of the thermoelectromotive force (ΔE) vs temperature gradient (0<ΔT<4 K). The thermal conductivity was measured by a laser flash technique (NETZSCH LFA427). All these transport measurements were carried under a flowing Ar atmosphere from 300 K to 800 K. The Archimedes method was adopted to measure the relative densities.
3 Results and discussion
3.1 Electrical properties
The powder XRD pattern of the sample at room temperature shows that single phase filled Chevrel phases MxMo6Te8 is obtained. For Ag filled compounds, we only discuss the samples of the nominal x≤2.0 since the main phase is not obtained when x>2.0. For comparison, Cu filled MxMo6Te8 (x=1.0, 2.0) samples were prepared in this work. The bulk densities of the samples are all over 98% of the theoretical density.
Table 1 illustrates the sample characterization of MxMo6Te8. The lattice thermal conductivity(κL), which can be estimated by subtracting the carrier contribution κE via the Wiedemann-Franz law (κE=LσT, where L is Lorentz number) from the total thermal conductivity, decreases with increasing filler atoms in both Ag filled and Cu filled Chevrel phases at room temperature. Different from the state-of-the-art “PGEC”[1,13] materials, such as filled skutterudites, the Chevrel phase shows the opposite trend. The larger and heavier the filler elements are, the more the phonons scattered from rattling of the filler atoms in skutterudites would be[5]. However, as shown in Table 1, the lattice thermal conductivities of the Cu filled samples are much smaller than those of Ag filled compounds when compared at the same temperature and the same filling fraction. This is due to the distinct effect of the filling atoms in the two series. The atoms in filled skutterudites act as the scattering center while in Chevrel phase the filling atoms bond with tellurium (M—Te). Fig.1(a) shows the 3-D framework of Mo6Te8 units and Fig.1(b) shows the 3-D framework of Mo6Te8 units connected by intercluster Mo—Te bonds. Cu/Ag atoms separate the Mo6Te8 units in adjacent sheets. It is found that the filler atoms are not in the center of the units but at the channel between the Mo6Te8 units, which coordinates Ag/Cu by Te. Furthermore, M—Te bonding by S—P hybrid in Ag filled Chevrel phase is much stronger than that in Cu filled Chevrel phase. As a result, Cu atoms are relatively active in the structure, which reduces the stability of the structure and causes the atoms “rattling”, like filled skutterudites. In this case, the mass effect is not so obvious in the Chevrel phase materials.
Table 1 Chemical composition and physical parameters for MxMo6Te8 at room temperature
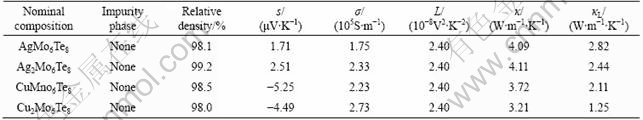

Fig.1 Crystal structures of Mo6Te8 (a) and Ag/Cu filled crystal structure of MxMo6Te8 (b)
Fig.2 shows the temperature dependence of electrical conductivity for all the samples as well as the data of Mo3Te4, reported by KUROSAKI et al[14]. The electrical conductivities of the composites exhibit metallic property, decreasing gradually with the rising temperature from room temperature to 800 K. The filler atoms increase the electrical conductivities of the matrix in this temperature range for both Ag filled and Cu filled series. For Ag filled samples, when the filling fraction is small, the electrical conductivity changes slightly with the temperature just like the trend of the matrix. However, the electrical conductivity decreases obviously when

Fig.2 Temperature dependence of electrical conductivity for Ag and Cu filled MxMo6Te8
adding two Ag atoms into Mo6Te8. The electrical conductivity of Cu filled compound decreases much faster compared with the matrix Mo3Te4 below 600 K, while it changes to a nearly flat line when the temperature goes higher. We consider it may be caused by the balance of the filler atoms. The fully filled Chevrel phase is supposed to have four atom channels. However, we cannot get pure compounds when x>2.0 and the fully filled Chevrel phase should be thermodynamically unstable one. So, at the relatively low temperature (around room temperature), the filler atoms are stable. The filler atoms get excited with the rising temperature, and reach the best position and the balance in the structure. Finally, at the high temperature (above 600 K), the property trace of the filled Chevrel phase and the matrix are alike.
Fig.3 shows the temperature dependence of Seebeck coefficient for all the samples. The matrix and Ag filled compounds perform p-type properties and the values increase with increasing temperature. The Seebeck coefficient of AgxMo6Te8 samples has larger value than that of Mo3Te4 in almost all temperature range. Meanwhile, AgMo6Te8 has larger Seebeck coefficient than Ag2Mo6Te8. This is because Ag2Mo6Te8 possesses higher carrier concentration than AgMo6Te8 as we mentioned above in Fig.2. Unexpectedly, the Seebeck coefficient values of Cu filled samples go from negative to positive that could be found in some intrinsic semiconductors. It is suggested that the weak S—P hybrid of Cu—Te makes it sensitive by adding electron from Cu into the structure. As a result, the Seebeck coefficient has a change of p-type to n-type.

Fig.3 Temperature dependence of Seebeck coefficient for MxMo6Te8
3.2 Thermal properties and figure of merit ZT
The thermal conductivities of all the samples studied increase as the temperature increases (Fig.4). It is obvious that the filler elements indeed reduce the thermal conductivity. The more the amount of the filler atoms are, the more the thermal conductivity will be reduced. Cu filled system has much lower thermal conductivity than Ag filled sample. It can be explained by the influence of Cu atoms on the weak bonding with the structure of the Chevrel phases, in which phonons are greatly scattered by the filler atoms. As a result, the thermal conductivity is lowered greatly (as listed in Table 1). Around 600 K, there is an inflexion of thermal conductivity for CuxMo6Te8, which agrees well with the results shown in the electrical conductivities above.
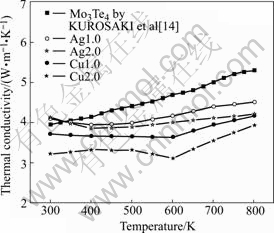
Fig.4 Temperature dependence of thermal conductivity for MxMo6Te8
The thermoelectric figure of merit is calculated from the measured σ, s, and κ. Fig.5 shows ZT values for all the samples. Ag filled Chevrel phases reach much higher ZT in the temperature range while Cu filled samples start with low ZT value and rise to about 0.025 at 800 K for Cu2Mo6Te8. The largest figure of merit reaches 0.034 at 800 K for AgMo6Te8.

Fig.5 Temperature dependence of ZT value for MxMo6Te8
4 Conclusions
1) Chevrel phases MxMo6Te8 (M=Ag, Cu) were synthesized and the thermoelectric properties were evaluated in the temperature range from room temperature (about 300 K) to about 800 K. The filling of Ag/Cu increases the electrical conductivity and Seebeck coefficient. By scattering the phonons, the lattice thermal conductivity decreases. Thus, the figure of merit, ZT value, rises to 0.034 at 800 K.
2) Different S—P hybrids between filler atom and Te influence a lot on the physical properties. The weak bonding (Cu—Te) results in a larger electrical conduc- tivity while lower Seebeck coefficient and thermal conductivity. The strong bonding (Ag—Te) causes relatively lower electrical conductivity but higher Seebeck coefficient and thermal conductivity.
3) Finally, the ZT values of MxMo6Te8 (M=Ag, Cu) are extremely lower than those of the state-of-the-art thermoelectric materials. In order to further optimize the performance of the Chevrel phase materials, more emphasis should be put on the electrical properties through changing the carrier concentration.
References
[1] ROWE D M. CRC handbook of thermoelectrics [M]. Boca Roton, FL: CRC Press, 1995.
[2] TRITT T M. Thermoelectric materials—holey and unholy semiconductors [J]. Science, 1999, 283: 804-805.
[3] DISALVO F J. Thermoelectric cooling and power generation [J]. Science, 1999, 285: 703-706.
[4] SALSE B C, MANDRUS D, CHARKOUMAKOS B C, KEPPENS V, THOMPSON J R. Filled skutterudite antimonides: Electron crystals and phonon glasses [J]. Physical Review B, 1997, 56: 15081-15089.
[5] ZHAO X Y, SHI X, CHEN L D, ZHANG W Q, ZHANG W B, PEI Y Z. Synthesis and thermoelectric properties of Sr-filled skutterudite SryCo4Sb12 [J].Journal of Applied Physics, 2006, 99: 053711.
[6] CHEN L D, KAWAHARA T, TANG X F, GOTO T, HIRAI T, DYCK J S, CHEN W, UHER C. Anomalous barium filling fraction and n-type thermoelectric performance of BayCo4Sb12 [J]. Journal of Applied Physics, 2001, 90: 1864-1868.
[7] CHEVREL R, SERGENT M, PRIGENT J. New phases of ternary sulfureted molybdenum [J]. Journal of Solid State Chemistry, 1971, 3: 515-519. (in French)
[8] CAILLAT T, FLEURIAL J P. Thermoelectric properties of the semiconducting Chevrel phase Mo2Re4Se8 [J]. Journal of Physics and Chemistry of Solids, 1998, 59: 1139-1144.
[9] NUNES R W, MAZIN I I, SINGH D J. Theoretical search for Chevrel-phase-based thermoelectric materials [J]. Physical Review B, 1999, 59: 7969-7972.
[10] TSUBOTA T, OHTAKI M, EGUCHI K. New superconducting ternary molybdenum chalcogenides InMo6Se8, T1Mo6S8, and T1Mo6Se8 [J]. Journal of the Ceramic Society of Japan, 1997, 107: 697-701.
[11] MILLER G J, SMITH M. Hexamolybdenum octatelluride Mo6Te8 [J]. Acta Crystallogr C, 1998, 54: 709-710.
[12] BERRY F J, FORGAN E M, GIBBS C D. Metal molybdenum tellurides: Synthesis characterization and superconducting properties of compounds of the type Mo6-xMxTe8 (M=Ru or Rh) [J]. Solid state Communications, 1988, 66(6): 667-670.
[13] SALES B C. Electron crystals and phonon glasses: A new path to improved thermoelectric materials [J]. MRS Bulletin, 1995, 23: 15-21.
[14] KUROSAKI K, KOSUGA A, UNO M, YAMANAKA S. Thermoelectric properties of Mo3Te4 [J]. Journal of Alloys and Compounds, 2002, 334: 317-323.
Foundation item: Projects(2007CB607502, 2007CB607503) supported by the National Basic Research Program of China
Corresponding author: CHEN Li-dong; Tel: +86-21-52414804; E-mail: chenlidong@mail.sic.ac.cn
DOI: 10.1016/S1003-6326(08)60326-X
(Edited by YANG Hua)


