
Corrosion behavior of Mg and Mg-Zn alloys in simulated body fluid
GAO Jia-cheng(高家诚)1, 2, WU Sha(伍 沙)1, QIAO Li-ying(乔丽英)1, 2, WANG Yong(王 勇)1
1. College of Materials Science and Engineering, Chongqing University, Chongqing 400044, China;
2. National Engineering Research Center for Magnesium Alloy, Chongqing University, Chongqing 400044, China
Received 26 March 2007; accepted 14 November 2007
Abstract:
The corrosion behavior of Mg and Mg-Zn in simulated body fluid was studied. The mass loss of pure Mg, Mg-Zn-Zr and Mg-Zn-Zr-Y in simulated body fluid was measured using photovoltaic scale meter. Corrosion rate was determined through electrochemical tests. Finally, the corrosion mechanism was thermodynamically studied. The results show that the corrosion rate decreases with the lapse of time for both pure Mg and Mg alloys. The purer the alloy, the better the corrosion resistance exhibits. The corrosion behavior of Mg alloy is improved by the addition of trace Y.
Key words:
Mg alloy; bio-compatibility; corrosion resistance;
1 Introduction
Success has been made in the development of various metal-based hard texture implants in the 20th century. Metallic materials continue to play a crucial role as biomaterials[1]. With a density of 1.74 g/cm3, magnesium is much denser than aluminum and steel[2]. The fracture toughness of magnesium is greater than that of ceramics, while the elastic modulus and compressive yield strength are closer to those of natural bone than the case for other commonly used metallic implants. Moreover, magnesium is essential to human metabolism and is naturally found in bone tissue[3-8].
However, the extremely high chemical activity of magnesium would lead to aggravated corrosion in corrosive media containing Cl-[9]. This work aims to study the corrosion resistance of pure magnesium and magnesium alloys in simulated body fluids(SBF), and explores thermo- dynamically their corrosion mechanism.
2 Experimental
99.9% pure magnesium (No.1), ZK60 (Mg-5.4Zn-0.55Zr)(No.2) and Mg-5.6Zn-0.55Zr-0.9Y(No.3) were used in the experiment. Samples were cut into cuboids and polished by abrasive paper. After ultrasonic cleaning in acetone for 10 min, the polished samples were soaked in SBF for 10 d. The corrosion rate was measured by mass loss and electrochemical methods after several soaking periods. The temperature of SBF was maintained at (37±0.5) ℃ and the ion concentrations in SBF are listed in Table 1[10].
Table 1 Ion concentrations in simulated body fluid (mmol/L)\
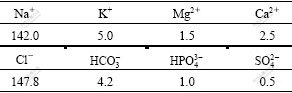
A triple-electrode system was used in the electrochemical measurement. 50 mL SBF with a pH value of 7.4 was used as electrolyte. Saturated calomel, platinum and the sample were set as reference, auxiliary and working electrodes, respectively. After the measurement, the corroded surface of the sample was polished, chemically etched and observed by metallographic microscope. The substance generated on the surface during corrosion was analyzed by XRD.
3 Results and discussion
Fig.1 shows the mass of the samples measured in different soaking periods. Generally, the mass changes were very small for all the three types of samples. After 242 h soaking, pure magnesium exhibited the best corrosion resistance with 0.9% mass loss, followed by Mg-5.6Zn-0.55Zr-0.9Y with 1.7% mass loss and Mg-5.4Zn-0.55Zr(ZK60) with 3.1% mass loss.
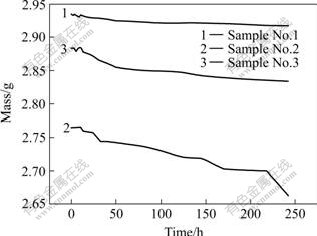
Fig.1 Mass change of samples soaked in simulated body fluid
Black pits began to emerge on the surfaces of samples No.2 and No.3 after soaking for 15 h, which indicated the start of corrosion, while no similar signs were observed for sample No.1. After 24 h of soaking, pits emerged on sample No.1, and the corrosion of samples No.2 and No.3 became aggravated, accompanied by the emergence of a black substance. After 74 h, a white substance was observed on sample No.1, while the corrosion of samples No.2 and No.3 continued to aggravate and there were black precipitates on the surface.
Fig.2 shows the Tafel curves obtained in the measurement. Generally, with higher corrosion potential and lower corrosion current density, the corrosion rate will be smaller, which means better corrosion resistance of the material. Therefore, the current density, which corresponds to the value at the crossing point of the tangent lines of the two curves in each figure, can indicate the electrochemical corrosion rate of the measured sample.
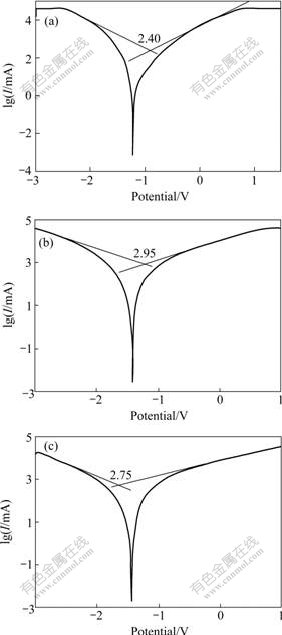
Fig.2 Tafel curves of samples: (a) Pure Mg; (b) ZK60; (c) Mg- 5.6Zn-0.55Zr-0.9Y
Table 2 Electrochemical corrosion rates of samples
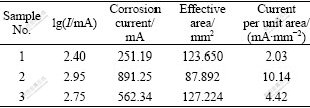
Based on the curves shown in Fig.2, the measured results can be converted into the corrosion currents and current densities, as listed in Table 2, from which it is obvious that sample No.1 has the best corrosion resistance, while sample No.2 performs poorly. However, the corrosion resistance of sample No.2 increases by 56.4% with the addition of 0.9% Y.
Fig.3 shows the microstructures and morphologies of the corroded surfaces of magnesium alloys after 170 h soaking in SBF, under optical microscope.
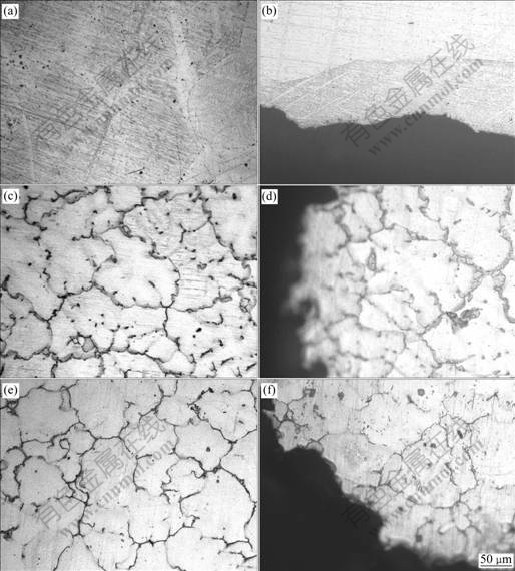
Fig.3 Microstructures and morphologies after 170 h soaking in SBF: (a, b) Pure magnesium; (c, d) EK60; (e, f) Mg-5.6In-0.55Zr- 0.9Y
As shown in the metallographic images, the micro- structure of ZK60 was made up of homogeneous equiaxed grains with fishbone structures on the boundaries,which could be inferred as eutectics. Around the boundaries were dark areas, which were generally considered to be caused mainly by segregation of Zr on the boundaries.
Grain boundaries were hardly detected on Mg alloys with the addition of Zn and Y. The microstructure exhibited a network of a black compound, which was a Zn-enriched phase. From Fig.3, it was observed that sample No.1 had a very thin corrosion layer with a flat boundary, which implied that corrosion centralized on the surface, while sample No.2 and No.3 were seriously corroded and corrosion mostly occurred in the boundary area.
Fig.4 shows the variation of pH value during 170 h of corrosion process. The pH value of pure Mg changed slowly in the whole process, while that of ZK60 changed quite fast with the pH value maintaining at 11.2 after 170 h.
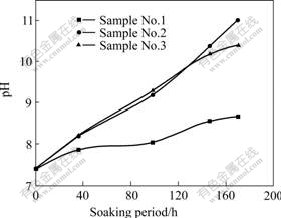
Fig.4 Change of pH value of SBF
After 170 h, a white substance was observed on the surface of all samples. The composition of the substance was analyzed by a D/MAX-1200 type diffractometer. In organism environment, the corrosion of magnesium and its alloys is the process mainly caused by the reaction: Mg+2H2O→Mg(OH)2+H2↑, thus the solid substance of the main corrosion product is Mg(OH)2 (Fig.5).
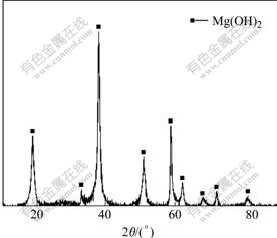
Fig.5 X-ray diffraction pattern of corrosion product
The equilibrium potential of magnesium is -2.34 V, and that in conventional media is also very low. Moreover, the oxide film of magnesium is generally loose and porous, therefore, magnesium and its alloys normally have fairly poor corrosion resistance. The electrochemical corrosion of Mg and its alloys is mainly the process of releasing hydrogen with its quick dissolution and finally powdering in the form of spot or plane corrosion[11-12]. The overall reaction for magnesium in aqueous solutions is
Mg+2H2O→Mg(OH)2+H2↑ (1)
This reaction can be expressed as the summation of the following fractional reactions:
Negative pole reaction:
Mg2++2e-→Mg (2)
Pole potential:
![]() (3)
(3)
Positive pole reaction:
2H2O+2e-=H2↑+2OH- (4)
Pole potential:
![]() (5)
(5)
Thus the electromotive force is
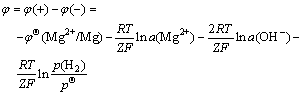 (6)
(6)
Assume p(H2)=pΘ, therefore,
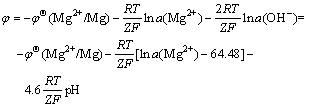 (7)
(7)
According to Table 1, the concentration of Mg2+ in organisms is 1.5 mmol/L and considering the SBF as the weak solution, we obtain a(Mg2+)=2.7×10-5. In the above formula, R=8.314 4 J·K-1·mol-1, F=9.648 5×104 C/mol, T=311 K, Z=2, and φΘ(Mg2+/Mg)=-2.363 V.
It is thus very clear, from the above formula, that the electromotive force of the primary cell involved in the corrosion has linear relations with the pH value, and decreases with its rise, which means that, with the increase of pH value, the driving force of corrosion reaction falls down. As a result, the corrosion rate drops and corrosion becomes slower and slower.
Zn is the major hardening element in Mg-Zn-Zr alloy with the reinforcing phase MgZn that is electrochemically negative. Zr can remarkably improve the corrosion resistance of the Mg-Zn alloy due to the high potential needed to release hydrogen. The addition of Y increases the solubility of the matrix due to its high solubility in Mg, which enables the harmful elements to dissolve into the matrix and therefore slows down corrosion.
Alloying is an essential step to improve mechanical properties and corrosion resistance of magnesium [13-14]. Protective coatings and surface treatments[15] can also be applied to improve the corrosion resistance and potentially improve biological compatibility and biological activity of magnesium -based implants, which will make magnesium alloys be likely to become the next generation of hard texture materials with wide applications.
4 Conclusions
1) After 242 h soaking in SBF, the mass loss rate of pure magnesium is 0.9%, the best in corrosion resistance among the measured materials, which indicates that fewer impurities result in better corrosion resistance.
2) After 242 h soaking in SBF, Mg-Zn-Zr (ZK60) has very serious mass loss, while that of Mg-5.6Zn-0.55Zr-0.9Y alloy is merely 1.7%, which means that the addition of the alloying element Y improves the corrosion resistance of Mg-Zn alloys.
3) Under this experimental condition, the corrosion current densities of pure Mg, ZK60 and Mg-5.6Zn- 0.55Zr-0.9Y are 2.03, 10.14 and 4.42 mA/mm2, respectively.
4) With the time of soaking in SBF prolonging, the pH value of the solution increases, which decreases the electromotive force, leading to the reduction in corrosion rate.
References
[1] NIINOMI M. Recent metallic materials for biomedical applications [J]. Met Mater Trans A, 2002, 33: 477-86.
[2] DEGARMO P E. Materials and processes in manufacturing. 5th ed[M]. New York: Collin Macmillan, 1979.
[3] PORTER R S, KAPLAN J L, HOMEIER B P, BEERS M H. Merk manual of diagnosis and therapy (chapter 12) Endocrine & metabolic disorders (section 2): Water, electrolyte mineral and acid/base metabolism [M]. Whitehouse Station: Merck & Co. Inc. 1995-2004.
[4] SARIS N E L. Magnesium: An update on physiological, clinical and analytical aspects [J]. Clin Chim Acta, 2000, 294: 1-26.
[5] OKUMA T. Magnesium and bone strength [J]. Nutrition, 2001, 17: 679-80.
[6] VORMANN J. Magnesium: Nutrition and metabolism [J]. Mol Aspects Med, 2003, 24: 27-37.
[7] WOLF F I, CITTADINI A. Chemistry and biochemistry of magnesium [J]. Mol Aspects Med, 2003, 24: 3-9.
[8] HARTWIG A. Role of magnesium in genomic stability [J]. Mutat Res/Fund Mol Mech Mutagen, 2001, 475: 113-21.
[9] GAO J C, LI L C, WANG Y. Surface improvement of magnesium and its corrosion behavior in simulated body fluids [J]. The Chinese Journal of Nonferrous Metals, 2004, 14(9): 1508-1513. (in Chinese)
[10] SONG Y J, LI M S, WEN S L. The latest research technique in preparing bioceramic film by simulation method [J]. Chinese Journal of Prosthodontics, 2003, 4(1): 0049-0053.
[11] DU Q Z, YANG J S. Physical chemistry [M]. Chongqing: Chongqing University Press, 1999: 386.
[12] SONG Guang-ling, SONG Shi-zhe. Corrosion behavior of pure magnesium in simulated body fluid [J]. Acta Phys-Chim Sin, 2006, 22(10): 1222-1226. (in Chinese)
[13] SHAW B A. Corrosion resistance of magnesium alloys[M]// STEPHEN D. ASM handbook(Volume 13a). Corrosion: fundamentals, testing and protection. UK: ASM Int. 2003.
[14] KAESEL V T, BACH P T, HAFERKAMP H, WITTE F, WINDHAGEN H. Approach to control the corrosion of magnesium by alloying[C]// KAINER K U. Proceedings of the sixth international conference magnesium alloys and their applications. New York: Wiley-Vch. 2004: 534-539.
[15] GREY J E, LUAN B. Protective coatings on magnesium and its alloys—A critical review [J]. J Alloys Compounds, 2002, 336: 88-113.
Foundation item: Project(30670562) supported by the National Natural Science Foundation of China
Corresponding author: WU Sha; Tel: +86-13594080957; E-mail: wusha818@163.com


