
Effects of increase extent of voltage on wear and corrosion resistance of micro-arc oxidation coatings on AZ91D alloy
L? Wei-ling(吕维玲)1, CHEN Ti-jun(陈体军)1, MA Ying(马 颖)1, XU Wei-jun(徐卫军)1, 2,
YANG Jian(杨 健)1, HAO Yuan(郝 远)1
1. State Key Laboratory of Gansu Advanced Nonferrous Metal Materials, Lanzhou University of Technology,
Lanzhou 730050, China;
2. College of Chemical and Industry, Gansu Lianhe University, Lanzhou 730000, China
Received 12 June 2008; accepted 5 September 2008
Abstract:
The effects of increase extent of voltage on the wear resistance and corrosion resistance of micro-arc oxidation (MAO) coatings on AZ91D magnesium alloy were investigated in silicate electrolyte. The results show that with increasing extent of voltage, both of the thickness and bonding force of MAO coatings first increase, and then decrease. These parameters are all up to their maximum values when the increase extent of voltage is 20 V. The roughness of the coatings always increases. The coating has the best corrosion resistance when the increase extent of voltage is not below 25 V, and the coating has the best wear resistance when the increase extent of voltage is 10 V. The wear mechanisms for the micro-arc oxidation are abrasive wear and micromachining wear. These are related to their microstructures.
Key words:
AZ91D magnesium alloy; micro-arc oxidation; increase extent; corrosion resistance; wear resistance;
1 Introduction
Magnesium alloys have many advantages, but they suffer from poor corrosion resistance, which has seriously limited their applications[1-3]. To overcome this disadvantage, many surface treatment techniques have been carried out. But conventional conversion coating and anodizing techniques coating are thin, the corrosion and was resistance of them are poor, and chromium pollution were brought into the environment, the requests of corrosion and wear resistance and environmental protection are too difficult to satisfy[4]. So micro-arc oxidation (MAO) is considered as the most potential method for magnesium alloys[5].
The coatings formed by MAO technology are not only with good corrosion and wear resistance but also with good insulation and good cohesiveness with the matrix and so on[6-8]. Magnesium MAO has been given great attention in the recent years, and domestic and foreign scholars have done many researches on this area and remarkable fruits have been gained[1, 9-11]. However, the MAO coatings are always of porous microstructures and they are not directly used without painting. So MAO is only a pretreatment prior to painting. Therefore, shortening treatment time and improving efficiency become very important for MAO.
According to the characteristic of magnesium MAO and its application status, MA et al[12] have invented one technique of rapid forming (RF) MAO coatings on magnesium alloys. A coating with thickness of 20- 30 μm that is equivalent to that formed by using the present technique treating for 30 min, could be obtained within 4-5 min using this RF technique. This signifies that this technique significantly increases the MAO efficiency. The main characteristic of this technique is to increase the voltage by stages. But only HOU et al[13] had carried out some preliminary research. In this paper, the effects of the increase extent of voltage on the wear and corrosion resistance of RF MAO coatings on AZ91D alloy were investigated.
2 Experimental
2.1 Preparation of sample
The material was commercial AZ91D alloy. The preparation process was as follows: put certain amounts alloy into the electric resistance crucible furnace. After it melted, put it into cover medicinal RJ2, use C2Cl6 to refine and reduce the dregs when the temperature was up to 680 ℃. Pour the molten alloy into the metal mold at 710 ℃ and get the round rods with diameter of 55 mm. Then it were processed into a round pie with diameter of 40 mm, height of 8 mm, which will be used as the MAO sample. All the samples should be polished by 1200# sand paper, washed by distilled water, finally washed by acetone before it was dried by hot air.
2.2 MAO experiment and properties tests of MAO coatings
MAO experiment was carried out in the silicate electrolyte[14]. Firstly, the voltage should be increased slowly to get arc, then using the getting arc voltage as the criterion, increase a certain amounts voltage by degrees. The specific experiment scheme was that the increase extents of voltage were respectively 5, 10, 15, 20, 25 and 30 V at 2 min voltage increase interval and 4 times of voltage increase.
Use TT230 digital coating thickness gauge, 2206 surface roughness measuring instrument (E34-001) and W-92 coating adhesion scratch testing machine to measure thickness, roughness and bonding force. JSM-6700F scanning electron microscope was used to observe the surface and worn morphologies. X-ray diffractometer test (D8ADVANCE) was used to analyze the composition. To estimate the corrosion resistance with CHI600 electrochemistry workstation, Tafel curves were measured in the 3.5% NaCl aqueous solution. The wear resistance was tested by UMT-2MT reciprocating sliding wear apparatus (ball-on-block (disk)) under dry conditions, the counterpart was a ball with diameter of 3 mm made by Si3N4 at load of 2 N, the frequency is 1 Hz and time is 30 min.
3 Results and discussion
3.1 Effect of increase extent of voltage on thickness, roughness and bonding force of MAO coatings
Fig.1(a) shows the relationship among the thickness, roughness and bonding force of MAO coatings. It can be seen that the thickness first increases then decreases with increase extent of voltage, it reaches the maximum value when the increase extent is 20 V. The termination voltage is enhanced by increasing extent, but the termination voltage is decided by thickness[15]. Current density increases with the increase of termination voltage. The large current density signifies high efficiency of MAO, so the thickness increases. When the extent is above 20 V, the voltage is higher, corresponding current density is so big, and the arc density diminishes with increasing thickness. The single arc intensity is enhanced, the inclination of coating breaking off is also enhanced, which leads to the increase of thickness relatively slow. Even when the treatment time is 10 min, the increase extent of voltage is only 5 V, the thickness of MAO coating can also reach 28 μm, which vividly contrasts with the traditional craftwork with treatment time of 30 min[16]. So it shows the necessity of pretreatment before painting.
Fig.1(b) shows that the roughness of MAO coating increases with the increase of extent of voltage, all the roughness is below 2 μm since the time of MAO is not very long. With the increase of extent of voltage, on one hand, the size of hole increases quickly, which leads to the increase of big melted pellet on the coating surface. On the other hand, the emerging big hole increases, which leads to the increase quantity of big pellets on the coating surface. So this craft can control the roughness by controlling the extent. This can increase the roughness of coating by increasing the extent of voltage. Similarly it can decrease the roughness of coating by decreasing the extent, which can enhance the wear resistance, corrosion resistance and fatigue resistance[17].
Bonding force of coating consists of coating-matrix bonding force and cohesion which takes on a decrease trend with increasing current density and electrolyte conductivity[18]. Fig.1(c) shows that the bonding force of MAO coating takes on a trend that first increases then decreases with the increase of extent of voltage, it reaches the maximum value as the extent of voltage is 20 V. The main reason is that when the extent is below 20 V, the energy of oxidation increases with the enhancement voltage, which leads to a high degree of micro metallurgy combination of coating and high bonding force of coating matrix. When the extent of voltage is above 20 V, the excessive energy leads to the decrease of density, consequently the cohesion of the MAO coating is reduced.
3.2 Effect of increase extent of voltage on surface morphologies of MAO coatings
Fig.2 shows surface morphologies of the MAO coatings formed under different increase extents of voltage. Fig.2(a) shows that when the extent is 5 V, the surface is flat and consists of a large amount of small holes (less than 2 μm) with uniform distribution. Accordingly, the size of melted pellets on the surface are

Fig.1 Relationship among thickness, roughness and bonding force of MAO coatings and increase extent of voltage
small, and the pellets distribute uniformly. Fig.2(b) shows that when the extent of voltage is up to 15 V, the size of pellets increases, and the big hole of 6μm emerges, the quantity of such big holes increases. Fig.2(c) shows that the size of pellet increases rapidly as well as the size of hole, which is above 10 μm. When the increase extent of voltage is small, the voltage is low, the energy of reaction is also low, so the reaction is mildness, except the rapid cooling of the micro-arc area, therefore there are a large number of holes and the distribution of holes is homogeneous. With the increase of extent of voltage, the voltage is high, the reaction is drastic, and the amount of deflation increases as well as the deflation resistance, so the size of holes also increases. Big holes appear and increase due to the single arc intensity emerging and increase which leads to the increase of pellets size on the surface as well as the quantity of big pellets. No crack exists on the surface, since the extent which is selected and used still satisfies the conditions of thermodynamics and dynamics[19].

Fig.2 Surface morphologies of MAO coatings formed under different increase extents of voltage: (a) 5 V; (b) 15 V; (c) 25 V
3.3 Effect of increase extent of voltage on phase composition of MAO coatings
Fig.3 shows the XRD patterns of the MAO coatings formed under different increase extents of voltage. The coatings mainly consist of Mg2SiO4, MgO and Mg0.97Zn0.03 as well as a little amount of Mg17Al12. With the increase of extent of voltage, the diffraction intensity of Mg0.97Zn0.03 decreases dramatically while Mg2SiO4 and MgO gradually increase, Mg17Al12 gradually decreases and disappears subsequently when the increase extent of voltage is 20 V.
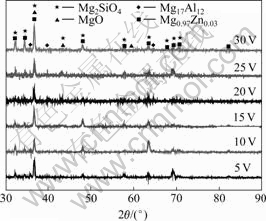
Fig.3 XRD patterns of MAO coatings formed under different increase extents of voltage
This has nature difference compared with the phase of non-crystal coating produced by anode oxidation. In the MAO process, the instant sintering interaction under high temperature and pressure convert the non-crystal MgO into the cubic structure MgO. This structure makes the coatings compaction[20], thereby improving its hardness, which is good for improving the wear resistance. The emergence of Mg2SiO4 shows that the electrolyte ions are also involved in the MAO reactions. Mg0.97Zn0.03 is α phase of matrix while Mg17Al12 is β phase which mainly relates to the thickness of coatings. The depth of X-ray diffraction is about a few micrometers to several tens micrometers[21], while the thickness of coatings ranges from 25 μm to 55 μm, so the inspected phase contains phase of matrix and some amounts of it decreases with increasing thickness.
3.4 Effect of increase extent of voltage on corrosion resistance of MAO coatings
Fig.4 shows the relation between the MAO coatings and the increase extent of voltage. Compared with matrix, when the extent is below 25 V, the corrosion electrical potentials are a bit negative displacement, but corrosion electricity gradually decreases which shows the gradual increase of the corrosion resistance. When the extent is not below 25 V, the corrosion electrical potential moves dramatically to the positive direction (170-200 mV), and the corrosion electricity decreases apparently (3.5-5.0 order of magnitude), so the corrosion resistance becomes much stronger.
MAO process consists of breaking down, melting, oxidation, solidification, breaking down again which comparatively occurs on the weakness area, the coatings combining with matrix in the metallurgical way can be gained[22]. The corrosion resistance of MAO coatings

Fig.4 Relationship between Tafel curves of MAO coatings and increase extent of voltage (96 h)
has relation with the density, the larger the density, the better the corrosion resistance[23]. So it is necessary to produce compact ceramic coatings in order to ensure good corrosion resistance. The analysis shows that when the increase extent of voltage is not below 25 V, the coatings are produced under a comparatively high energy, the holes rate of coatings are relatively small, there exists some big holes, but the quantity of through-hole is few. Therefore, the MAO coatings are relatively compact, the corrosion resistance is well. When the increase extent of voltage is below 25 V, the coatings are produced under low energy, the energy of breaking down is low, the quantity of big holes is few, but there are through-holes, which provides a channel to the corrosion of the medium and leads to the deteriorate corrosion, so the corrosion resistance is relatively weak.
3.5 Effect of increase extent of voltage on wear resistance of MAO coating
Fig.5(a) shows the relationship between the wear rate of MAO coatings and the increase extent of voltage. It can be seen that the wear rate decreases rapidly when compared with the matrix with the increase extent. The wear rate decreases close to 1 order of magnitude when it is 5 V. The wear rate decreases close to 2 order of magnitude while the increase extent of voltage increases to 10 V; then the wear rate displays the slow growing trend, but it is still close to 1 order of magnitude. Fig.5(b) shows the relationship between the friction coefficients of MAO coatings and the increase extent of voltage. It assumes a prompt linearity increase-slow growing-stable trend.
The wear and friction properties of MAO coatings are related to the microstructure and thickness. When the increase extent of voltage is 5 V, both the surface pellets and the thickness are small, the carrying capability to load is low, the wear rate appears increase trend when the load reaches a certain value, therefore its wear rate reduces obviously compared with the matrix, but it is relatively higher than those of other coatings, the friction coefficients increases promptly. The dimension of surface pellets and holes increases homogeneously, the thickness also increases when it is 10 V. As the increase extent of voltage is between 10 V and 20 V, bigger holes and pellets appear on the surface although the thickness continuously increases, the proportion of loosened layer on the surface increases, the internal stress of coatings also becomes bigger, leading to the loosened layer too easy to exfoliate under the outside force, and the horniness granule is formed. The wear accelerates with the increase of wear rates, the friction coefficients increase slowly to the maximum value. When the increase extent of voltage is above 20 V, the coating has larger holes and particles, the extortionate voltage affects the thickness of loosened layer on the surface, leading to the wear rates continuous increase, the friction coefficients become stable. Thereby, the wear resistance of MAO is good as the increase extent of voltage is 10 V.
Fig.6 shows the worn surface morphologies of the MAO coatings which forms under different increase extents of voltage. Fig.6(a) shows that the surface of
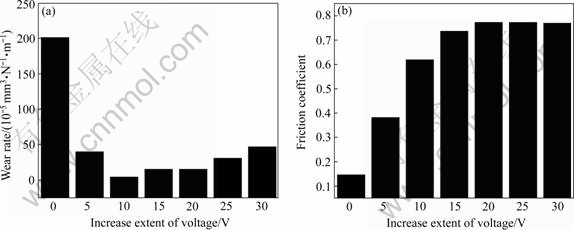
Fig.5 Relationship between wear rate(a), friction coefficient(b) of MAO coatings and increase extent of voltage

Fig.6 Worn surface morphologies of MAO coatings formed under different increase extents of voltages:(a) Matrix; (b) 5 V; (c) 10 V; (d) 15 V
matrix without coating has wide grooves and straight scratches. Fig.6(b) shows that the surface of coating has clear grooves which become relative narrow when it is 5 V, there exist regular scratches on the grooves along the friction movement direction, and some irregular debris adhere scratches, the surface layer has the peeling phenomenon. Fig.6(c) shows that the pellets are gritted and crushed, the holes are incompletely filled with the powder of pellets when the increase extent of voltage is 10 V. Fig.6(d) shows that the holes are completely filled with the powder of the abrasive and crushed pellets, and there are cracks appearing on the surface loosened layer when the increase extent of voltage is 15 V. The main reason is that the matrix wear mechanism is micromachining wear[24], the wild grooves and straight scratches imply bigger wear rate and smaller friction coefficients (see Fig.5). It is not difficult to find that the relative narrow grooves and regular scratches imply the decrease of wear rate, meanwhile the friction coefficients increase when the increase extent of voltage is 5 V, and some irregular debris adhering scratches are involved in the wear process. The peeling phenomenon is the character of micromachining wear, so the wear mechanism is the same as that of matrix because the thickness of the coating is relative thin(see Fig.1(a)), but it is also abrasive wear. Due to the increase of thickness, big pellets appear as the increase extent of voltage is 10 V, the wear mechanism is abrasive wear. Then the thickness of the coatings increases further, the quantity of the big pellets and the proportion of surface loosened layer increase, there appear cracks which implies the wear mechanism changes from abrasive wear to micromachining wear.
4 Conclusions
1) In silicate system, the RF MAO coatings craftwork of AZ91D magnesium alloy is stable, and it is feasible to increase MAO efficiency using the principle of elevating the increase extent of voltage during the same interval.
2) The thickness and bonding force of MAO coatings are all up to their maximum values when the increase extent of voltage is 20 V. The roughness increases. No crack exists on coating surface. And they mainly consist of Mg2SiO4, MgO and Mg0.97Zn0.03 as well as a little amount of Mg17Al12.
3) MAO coating has relative compact structure which leads to its best corrosion resistance when the extent is not less than 25 V in this craft, so magnesium alloy MAO technology has advantage to extensive use.
4) With the extent increases, the wear rate decreases rapidly compared with that of the matrix and reaches the minimum value as the extent of voltage is 10 V. The friction coefficients take on an increase trend and the trend become stable when the extent of voltage is 10 V. The wear resistance of MAO is well when the increase extent of voltage is 10 V in the RF MAO coatings craftwork. The wear mechanism is abrasive wear and micromachining wear.
References
[1] GRAY J E, LUAN B. Protective coatings on magnesium and its alloys-A critical review[J]. Journal of Alloys and Compounds, 2002, 336(1/2): 88-113.
[2] MORDIKE B L, EBERT T. Magnesium properties—applications— potential[J]. Mater Sci Eng, 2001, 302(2): 37-45.
[3] JIANG Bai-ling, LI Jun-ming, SHI Hui-ying, BAI Li-jing. Application of micro oxidation technology in magnesium alloy protective treatment[J]. Automobile Technologe and Material, 2003, 5: 24-27.
[4] ZHANG Rong-fa, SHAN Da-yong, HAN En-hou, ZENG Zhi-liang. Status and prospect of anodization on magnesium and its alloys[J]. The Chinese Journal of Nonferrous Metals, 2006, 16(7): 1136-1148. (in Chinese)
[5] JIANG Bai-ling, ZHANG Shu-fen, WU Guo-jian, LEI Ting-quan. Micro-flaw and phases constitution of ceramic coating formed by micro-arc oxidation on magnesium alloys and their influence on corrosion resistance[J]. The Chinese Journal of Nonferrous Metals, 2002, 12(3): 454-457. (in Chinese)
[6] DITTRICH K H, LEOARD L G. Microarc oxidation of aluminium alloy components[J]. Crystal Research and Technology, 1984, 19(1): 93-96.
[7] XUE Wen-bin, DENG Zhi-wei, LAI Yong-chun, et al. Review of micro arc oxidation technique on surface of non-ferrous metals[J]. Heat Treatment of Metals, 2000(1): 1-3. (in Chinese)
[8] HUANG Ping, HAN Yong, XU Ke-wei. Experimental study of microarc oxidation on the surface of titanium alloy[J]. Rare Metal Materials and Engineering, 2002, 31(4): 308-311. (in Chinese)
[9] GUO Hong-fei, AN Mao-zhong, XU Shen, HUO Hui-bin. Effect of current density on mechanism of micro-arc oxidization and property of ceramic coating formed on magnesium alloys[J]. Rare Metal Materials and Engineering, 2005, 34(10): 1554-1557. (in Chinese)
[10] LI Ke-jie, LI Quan-an. Research and application progress of micro-arc oxidization on alloys[J]. Rare Metal Materials and Engineering, 2007, 36(S3): 199-203. (in Chinese)
[11] BROWN R E. Application of magnesium alloy[J]. Light Metal Age, 1991, 49(7/8): 6-9.
[12] MA Ying, XU Wei-jun, CHEN Ti-jun, LI Yuan-dong, HOU Wei-ao, LV Wei-ling, HAO Yuan. The method of producing micro arc oxidation ceramic coating on the surface of magnesium alloy. CN 1928165[P]. 2007. (in Chinese)
[13] HOU Wei-ao, CHEN Ti-jun, HAO Yuan, LI Hai-hong. Contrast of electrical parameter between die-casting and semi-solid casting samples during AZ91D alloy microarc oxidation[J]. Hot Working Technology, 2006, 35(22): 39-43. (in Chinese)
[14] LIANG Yong-zheng. Study on micro-arc of magnesium alloy surfaces[D]. Lanzhou: Lanzhou University of Technology, 2004. (in Chinese)
[15] CHEN Hong, HAO Jian-min, WANG Li-jie. Influences of Mg alloy micro-arc oxidization voltage on ceramic layer[J]. Surface Technology, 2004, 33(3): 18.
[16] YAN Feng-yun, LIN Hua, WANG Sheng. Optimization of electrolyte formula of micro-arc oxidization for AZ91D magnesium alloys in silicate solutions[J]. New Technology and New Process, 2006(7): 68-70. (in Chinese)
[17] Surface roughness[BO/OL]. http://baike.baidu.com/view/55599.htm, 2006-08-09.
[18] XIA Tian. Study on bonding strength and compact-ability of ceramic coatings formed by micro-arc oxidation on magnesium alloys[D]. Xi’an: Xi’an University of Technology, 2005. (in Chinese)
[19] CHEN Xian-ming, LUO Cheng-ping, LIU Jiang-wen, LI Wen-fang. Thermodynamic and kinetic analysis of micro-arc oxidation on magnesium alloy[J]. Ordnance Material Science and Engineering, 2006, 29(3): 17-20. (in Chinese)
[20] CHEN Fei, ZHOU Hai, YAO Bin, YANG Ying-ge, L? Jun-xia, L? Fan-xiu. Study on the tribological behavior of micro-arc oxidized ceramic coatings on magnesium alloy surfaces[J]. Rare Metal Materials and Engineering, 2006, 35(5): 806-809. (in Chinese)
[21] ZUO Yan-sheng, CHEN Weng-zhe, LIANG Wei. Material modern methods of analysis[M]. Beijing: Beijing Industrial University Press, 2000: 53. (in Chinese)
[22] LI Jun-ming, JIANG Bai-ling, JING Xiao-tian, WEN Xiao-bin. Effect of conductivity of solution on the growth rate and compact of micro-arc oxidation coating of LY12 aluminum[J]. Transactions of Metal Heat Treatment, 2003, 24(1): 63-65.
[23] JIANG Bai-ling, XIA Tian, SHI Hui-ying, LEI Nian-min. Study of impedance characteristic and corrosion-resistance properties of ceramic coating by micro-arc oxidation on magnesium alloys[J]. Transactions of Metal Heat Treatment, 2005, 26(2): 82-85.
[24] CHEN Ti-jun, MA Ying, LI Bing, LI Yuan-dong, HAO yuan. Friction and wear properties of permanent mould cast AZ91D magnesium alloy[J]. Materials Science and Technology, 2007, 000(000): 1-8.
(Edited by LONG Huai-zhong)
Foundation item: Project(2007CB613700) supported by National Basic Research Program of China; Project(0702GKDA024) supported by Science and Technology Significant Special Item of Gansu Province, China; Project(0708WCGA151) supported by International Scientific and Technological Cooperation Plan Item between Provinces; Project(Z2006-1-62002) supported by Spring Light Plan Item of Ministry of Education, China
Corresponding author: L? Wei-ling; Tel: +86-13919804525; E-mail: linzi1107@163.com


