
Characterization of irregular seeds on gibbsites precipitated from caustic aluminate solutions
CHEN Guo-hui(陈国辉), CHEN Qi-yuan(陈启元), YIN Zhou-lan(尹周澜), YIN Zhi-min(尹志民)
School of Chemistry and Chemical Engineering, Central South University, Changsha 410083, China
Received 3 June 2005; accepted 15 September 2005
Abstract:
The irregular surface of seeds on which gibbsites are precipitated from caustic aluminate solutions, was investigated according to the fractal theory. Two kinds of fractal dimensions were used to characterize these irregularity. Box-dimension and spectral dimension are based on the SEM images of seeds and diffusive dynamic equation of the precipitation respectively. Both these two dimensions are affected by the reaction temperature, evolved with different reaction conditions and can reflect the influence of irregularity of seeds on the precipitation rate. Box dimension is fit for the characterization of the irregular morphology of seeds, while spectral dimension can explain the fractal dynamic behavior.
Key words:
gibbsite; fractal theory; box-dimension; spectral dimension; caustic aluminate solution; fracted dynamic behavior;
1 Introduction
Because of the great viscosity and surface tension of caustic aluminate solutions, industrial aluminum trihydroxide products cannot be precipitated from caustic aluminate solutions directly. An amount of gibbsites seeds should be added to induce crystallization. Actually, the precipitation of caustic aluminate solutions is carried out on the surface of the seeds and includes four main procedures: secondary nucleation, growth, brokenness and aggregation[1, 2]. The surface of all industrial seeds is irregular, and all experiments show that the irregular surface plays an important role on the gibbsites precipitation from caustic aluminate solutions[3]. As a result this reaction is a kind of heterogeneous reaction.
Fractal theory was first established by American scientist Mandelbrot in 1973 to elucidate irregular structure, then broadly expanded in scientific fields such as physics, chemistry, computer, geology and material science. Heterogeneous reactions carried out on solid surface are substantially fractal based on the work of AVNIR et al[4], FARIN et al[5] and PFEIFER et al[6]. There are many different fractal dimensions, such as Hausdorff dimension , box-dimension, correlation dimension and spectral dimension, which can be used to characterize the irregularity of reactive surface of heterogeneous reactions. For example, SEM images were used by JI et al[7] to determine the box-dimension of catalyst and those fractal dimensions were found to connect with the preparation conditions, different processing methods and the activation of the catalyst. A kind of second order chemical reaction equation based on spectral dimension was given by Kopelman[8] to explain the fractal reaction on solid-liquid surface. In this article, these two kinds of fractal dimensions were used to characterize the irregular surface of gibbsites seeds and to evaluate the influence of irregularity on the precipitation of caustic aluminate solutions.
2 Experimental
2.1 Gibbsites precipitation from supersaturated caustic aluminate solutions
1) Common procedure
Fresh, supersaturated caustic aluminate solutions(αK: 1.42-1.62, c(NaOH): 5.16 mol/L) were obtained by dissolving Al(OH)3(industry grade from the Great Wall Aluminum Company) into NaOH (chemistry grade), then ploughed quickly into stainless steel reactor(2.5 L). Other experiment conditions were: reaction temperature 45-65 ℃; agitation rate 400 r/min; primary seeds content 600 g/L.
2) Precipitation with ultrasound
The caustic aluminate solutions were processed for 10 min by low frequency ultrasound at the reaction temperature, and the output power of ultrasound was about 200 W, other procedures were the same as 1).
2.2 SEM images analysis
The SEM images of the seeds dried by infrared light were observed by KYKY2800 scanning electron micro- scopy.
3 Fractal characterization of gibbsites precipitation from caustic aluminate solutions
3.1 Box-dimension characterization of seeds
The SEM images of different seeds are shown in Fig.1. Every grain is the aggregation of small crystals, and the surface is very irregular. These SEM images can be used to determine the box-dimension of the seeds. First, they were converted to digital images, and condensed into 128×128 digital images. The gray scale of the images were treated as the third dimension, and then 128×128×128 cubic images were set up. Boxes were used to cover these cubic images and the number and grade of boxes(Bij) were summed up, at last, the box-dimensions (Df) were calculated by the following equation:
![]()
According to this method, the box-dimensions of seeds A and B are 1.8 and 1.85 respectively. These decimal data indicate that the surface dimensions of seeds are not integral, but irregular and fractal. Seeds B are precipitated from the caustic aluminate solution quickly, therefore a little more irregular than seeds A.
The box-dimensions of gibbsites during precipitation are listed in Table 1(all primary seeds are seeds A). These decimal data show that the precipitations are carried out on the fractal surface of seeds. All fractal dimensions are different from those of primary seeds A, and evolve with the decomposition of caustic aluminate solutions. It can also be seen that the box-dimensions are obviously affected by different precipitation temperature. Although, the seeds of four reactions are the same, the box-dimensions at 60 ℃ are smaller than those at 55 ℃. This is because the growth rate of crystals increases with temperature, the higher temperature, the more regular the surface, while secondary nucleation benefits from lower temperature. These secondary nuclei make the surface of seeds more irregular. It can be indicated that there are some important relationship between temperature and irregular surface during precipitation of caustic aluminate solutions. On the other hand, it can also be found that the box-dimensions of gibbsites during the precipitation of caustic aluminate solutions processed by ultrasound are similar to those common precipitations. But, as shown in Fig.2, the precipitation ratio of gibbsites at 55 ℃ with ultrasound is quicker than that without ultrasound, which means that ultrasound can fasten the precipitation of caustic aluminate solution at 55 ℃. Obviously, the box-dimension cannot explain this, and spectral fractal dimension was introduced to evaluate the reaction.

Fig.1 SEM images of gibbsite produced by different methods: (a) Seeds A, industry grade Al(OH)3 from the Great Wall Aluminum Company; (b) Seeds B, Al(OH)3 precipitated from caustic aluminate solution promoted by inputting CO2 at room temperature
Table 1 Box-dimension of seeds during process of gibbsites precipitation from caustic aluminate solution


Fig.2 Precipitation rate of gibbsite precipitated from liquids with common seeds
3.2 Spectral dimensions of heterogeneous reaction
The spectral dimension is based on the theory of diffusion. Diffusion of particles is regarded as the result of Brown motion, so the diffusive action of particles on fractal media is related with their random walk on the fractal surface. This unclassical diffusive action can induce bizarre chemical dynamics. Based on the work of Kopelman[8], Misra and White[9] and Audet and LAROCQUE[10], CHEN et al[11, 12] set up a fractal equation of gibbsites precipitated from sodium aluminate solutions:
![]() (1)
(1)
where cA is the concentration of Al(OH)3 at time t; cAe is the equilibrium concentration of Al(OH)3 at time t; cAe=Nktexp(6.210 6-2 486.7/T+1.097 53 Nkt/T) and Nkt is the instantaneous concentration of NaOH at time t during the reaction; T is the absolute temperature; ds is the spectral dimension, closely related with diffusion on fractal surface; Sd is the instantaneous surface of seeds at time t during the reaction, Sd=mt×S0(mt is the instantaneous mass of seeds; S0 is the the primary surface of seeds).
After integration, Eqn.(2) is yielded:
ln[(cA0-cAe)/(cA-cAe)-1] -lnmt=
lnk+2/ds-ln(cA0-cAe)+lnS0+0.5dslnt (2)
where cA0 is the beginning concentration of Al(OH)3.
Because ln k, 2/ds, ln(cA0-cAe) and ln S0 are constants, it is obvious that ln[(cA0-cAe)]/(cA-cAe)-1]-lnmt is proportional to lnt with the slope equal to 0.5ds. So, if regression lines can be drawn from experimental data, the ds of the reactions can also be calculated.
1) Influence of temperature and ultrasound
Based on the equation above, the spectral dimensions of gibbsite precipitation from caustic aluminate solutions were yielded with all correlation coefficient data equal to or above 0.99.
Some interesting results of spectral dimensions of the effect of temperature during gibbsites precipitation from caustic aluminate solution are given in Table 2. The spectral dimensions at 60 ℃ are larger than those at 50 ℃(before 4.4 h) and 55 ℃. This acts in accordance with the facts that both diffusion of liquids on surface and the precipitation rate of liquids increase with the reaction temperature. Although with the temperature increases, the last precipitation ratio decreases, the higher temperature quickens the diffusion of liquids on fractal surface of seeds and hastens the instantaneous precipitation rate of the precipitation. There is close relation between the practical dynamic behavior of the precipitation and the diffusion, and the dynamic equation must be partly controlled by the fractal diffusion. It must also be noted that there are two different spectral dimensions at 50 ℃ in Fig.3. The spectral dimension is 1.12 during the primary 4.4 h of the precipitation, and then changes to 1.88. This phenomenon is related with secondary nucleation of liquids. At lower temperature, the precipitation of liquid is slower than that at higher temperature, but the secondary nucleation is easier. It creats more secondary nuclei. These new nuclei are highly active and can accelerate the precipitation of liquids and increase the spectral dimension. This can be proved by the precipitation rate in Fig.2. The precipita- tion rate at 50 ℃ at first 5 h is much smaller than that at 55 ℃, but after that, it increases quickly.
Table 2 Spectral dimension of gibbsite precipitation from caustic aluminate solutions at different temperatures

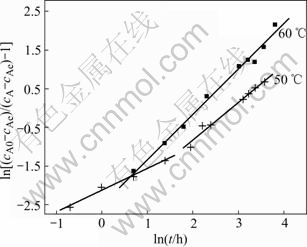
Fig.3 Precipitation of aluminate sodium liquor with common seeds
The spectral dimension with ultrasound at 55 ℃ is a little larger than that without ultrasound in Table 2, while the spectral dimensions at 60 ℃ are almost the same. The reason is that, at lower temperature(≤55 ℃), there is small variation of species balance of caustic aluminate solution compared with common liquids after it being processed by low frequency ultrasound[13]. This change can increase the diffusion and hasten precipitation; while at higher temperature, because of the strong influence of temperature, the effect of ultrasound decrease quickly and its effect to solution can be ignored. Compared with box-dimension, the influence of both temperature and ultrasound to the precipitation kinetics can be reflected by spectral dimension better.
2) Influence of different seeds
Spectral dimension is a kind of dimension based on the diffusion on fractal surface. It can be imaged that different seeds with different reactive surfaces will have different ds and result different precipitation rate. In industrial process, common seeds contain lots of alkaline(w(Na2O)%: 3.32). After being washed with distilled water three times, w(Na2O)% will decrease to 0.10.
The spectral dimensions of these two kinds of seeds at different temperatures are listed in Table 3 and their precipitation ratios are listed in Table 4. The precipitation ratio curves are shown in Fig.5. It can been found that the spectral dimensions are very different from those precipitated from common seeds. Compared with gibbsites precipitated with common seeds at 50 ℃ in Table 2, there are also two spectral dimension(ds) in the same precipitation reactions at 45 ℃ and 55 ℃ with washed seeds, but on the contrary, the ds at the beginning period of dissolution are larger than that at the later. The reason is that washed seeds attach less alkaline than common seeds, when they are ploughed into liquids, the huge concentration deviation of alkaline between the surface of washed seeds and the liquid makes the liquid diffuse much quicker than that with common seeds, and hastens the precipitation in Fig.3. The higher the temperature, the more the precipitation rate at the primary few hours of the reaction (in Table 4) and the shorter time the seeds deactivation. It can be indicated that the datum at 0.5 h at 65 ℃ in Fig.4 is still under the regression line, but there is no inflexion point any more. After the washed seeds deactivation, the increase of the precipitation ratio becomes very small. This can also be reflected by the later ds. It can easily be yielded that the spectral dimensions can clearly characterize the activation of different seeds.
Table 3 Spectral dimesions(ds) of precipitation reaction of caustic aluminate solutions with washed seeds

Table 4 Precipitation ratio of caustic aluminate solutions with washed seeds
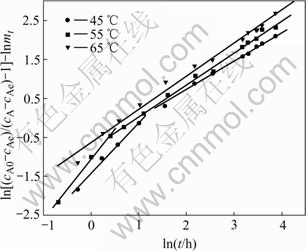
Fig.4 Precipitation of aluminate sodium liquor with washed seeds at different temperatures
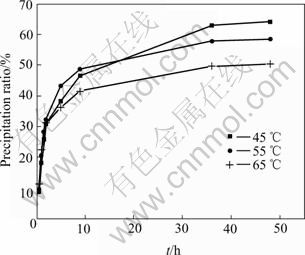
Fig.5 Dissolution rate of gibbsite precipitated from liquids with washed seeds
4 Conclusions
To sum up, the dynamic property of gibbsites precipitation from caustic aluminate solutions is fractal. Both box-dimension and spectral dimension can be used to characterize the fractal. The activation of seeds is affected by temperature and surface morphology. The box-dimension is beneficial to characterizing the irregular morphology of seeds, while the spectral dimension can connect the fractal surface with the diffusion and the precipitation behavior of liquids. The higher the temperature, the quicker the growth of crystals on the surface of seeds, and the less the box dimension; the lower the temperature, the quicker the secondary nucleation on surface of seeds, and the more irregular and the bigger of the box dimension. With the increasing temperature, the diffusion of ions in liquids increases, so does the spectral dimension. As a result, the precipitation of liquids is quickened. When the liquids are intensified by low frequency ultrasound at lower temperature, the balance of aluminate anions is changed to a certain extent. This can change the diffusion of liquids and the spectral dimensions and can hasten the reaction. When washed seeds are used, because of much less alkaline, their spectral dimensions are much larger than common seeds, and much more active, and can quicken the precipitation.
References
[1] Fleming S, Rohl A, lee M Y, GALE J, PARKINSON G. Atomistic modelling of gibbsite: surface structure and morphology[J]. Journal of Crystal Growth, 2000, 209: 159-66.
[2] Seyssiecq I, Veesler S, Mangin D, KLEIN J P, BOISTOLLE R. Modelling gibbsite agglomeration in a constant supersaturation crystallizer[J]. Chemical Engineering Science, 2000, 55: 5565-5578.
[3] Rossiter D S,Fawell P D,Ilievski D,PARKINSON G M. Investigation of the unseeded nucleation of gibbsite Al(OH)3 from synthetic bayer liquors[J]. J Crys Growth, 1998, 191: 525-536.
[4] Avnir D, Farin D, Pfeifer P. Surface geometric irregularity of particulate materials: A fractal approach[J]. J Colloid Interface Sci, 1985, 103: 112-1123.
[5] Farin D, Peleg S, Yavin D, AVNIR D. Applications and limitations of boundary line fractal analysis of irregular surfaces: protein, aggregates and porous materials[J]. Langmuir, 1985, 1(4): 399-407.
[6] Pfeifer P, Avnir D, Farin D. Scaling behavior of surface irregularity in the molecular: from adsorption studies to fractal catalysts[J]. J Stat Phys, 1984, 36: 699-716.
[7] JI Hong-bing, LIN Wei-ming. Application of fractal in catalytic selection and catalytic activity evaluation[J]. Journal of Chemical Industry and Engineering, 1997, 48: 453-456.(in Chinese)
[8] Kopelman R. Fractal reaction kinetics[J]. Science, 1988, 241: 1620-1624.
[9] Misra C, White E T. Crystallization of bayer aluminium trihydroxide[J]. J Crystal Growth, 1971, 8: 172-178.
[10] Audet D R, Larocque J E. Development of model for prediction of productivity of alumina hydrate precipitation[A]. Light Metals1989[C]. Warrendale PA: TMS, 1989. 21-26.
[11] CHEN Guo-hui, CHEN Qi-yuan, YIN Zhou-lan. Spectral dimension during the precipitation of gibbsite from the seeded sodium aluminate solutions[J]. J Cent South Univ Technol, 2002, 33(2): 157-159.(in Chinese)
[12] CHEN Guo-hui, CHEN Qi-yuan, YIN Zhou-lan, ZHANG Bin, LI Jie, CHEN Jin-qing, LIU Ji-bo. Fractal kinetics study on precipitation of sodium aluminate liquor under ultrasound[J]. The Chinese Journal of Nonferrous Metals, 2002, 12(3): 607-610.(in Chinese)
[13] ZHAO Ji-hua. The Study of the Precipitation of Bayer Method with Ultrasound[D]. Changsha: Central South University, 2001.
Foundation item: Project(59874031) supported by the National Priority Development Fundamental Research; Project(G19906492-3) supported by China Post-Doctor Program
Corresponding author: CHEN Guo-hui; Tel: +86-731-8879616; E-mail: gh-ch@163.com
Abstract: The irregular surface of seeds on which gibbsites are precipitated from caustic aluminate solutions, was investigated according to the fractal theory. Two kinds of fractal dimensions were used to characterize these irregularity. Box-dimension and spectral dimension are based on the SEM images of seeds and diffusive dynamic equation of the precipitation respectively. Both these two dimensions are affected by the reaction temperature, evolved with different reaction conditions and can reflect the influence of irregularity of seeds on the precipitation rate. Box dimension is fit for the characterization of the irregular morphology of seeds, while spectral dimension can explain the fractal dynamic behavior.


