
![]()

Trans. Nonferrous Met. Soc. China 22(2012) 2554-2561
Topology optimization of microstructure and selective laser melting fabrication for metallic biomaterial scaffolds
XIAO Dong-ming1,2, YANG Yong-qiang1, SU Xu-bin3, WANG Di1, LUO Zi-yi4
1. School of Mechanical and Automotive Engineering,South China University of Technology, Guangzhou 510641, China;
2. School of Electromechanical Engineering, Hunan University of Science and Technology, Xiangtan 411201, China
3. School of Materials Science and Engineering,South China University of Technology, Guangzhou 510641, China;
4. Guangzhou Research Institute of Nonferrous Metals, Guangzhou 510650, China
Received 6 July 2012; accepted 6 August 2012
Abstract:
The precise design and fabrication of biomaterial scaffolds is necessary to provide a systematic study for bone tissue engineering. Biomaterial scaffolds should have sufficient stiffness and large porosity. These two goals generally contradict since larger porosity results in lower mechanical properties. To seek the microstructure of maximum stiffness with the constraint of volume fraction by topology optimization method, algorithms and programs were built to obtain 2D and 3D optimized microstructure and then they were transferred to CAD models of STL format. Ti scaffolds with 30% volume fraction were fabricated using a selective laser melting (SLM) technology. The architecture and pore shape in the metallic biomaterial scaffolds were relatively precise reproduced and the minimum mean pore size was 231μm. The accurate fabrication of intricate microstructure has verified that the SLM process is suitable for fabrication of metallic biomaterial scaffolds.
Key words:
topology optimization; selective laser melting (SLM); microstructure; metallic biomaterial scaffolds;
1 Introduction
Load-bearing bones are sometimes damaged due to diseases or injuries so severely that they have to be replaced by prosthetics. For example, replacement of hip and knee bone, reconstruction of femur, vertebra, mandible and skull are often found in surgeries. The metallic biomaterial scaffolds are central to bone reconstruction since they provide a 3D framework for delivering reparative cells and enhance implants fixation as well as withstand the load-bearing demands. In order to obtain the desirable and targeted properties, optimal design approaches as well as fabrication methods that reproduce the designed architecture are indispensable.
The porous materials have an additional desired effect on promoting bone ingrowth. A number of researchers have presented design approaches of scaffolds materials and developed some techniques to fabricate scaffolds. van CLEYNENBREUGEL et al [1] designed internal lattice-structured scaffolds which matched the effective axial trabecular bone stiffness. YOO [2] proposed computer-aided porous scaffolds materials design method based on triply periodic minimal surfaces. LIN et al [3] presented a way to transform scaffold model by Boolean operation.
The implant materials should be designed to meet the multiple requirements such as sufficient stiffness and large porosity. However, the increased porosity will reduce stiffness. Conventional designs may not be able to meet the multiple requirements which are required for the load-bearing bone implants. Topology optimization is a design method that provides optimal material layout to satisfy maximized stiffness objective with specific porosity under the given boundary conditions, the optimal base cell is designed so that the final periodical structure meets specified design targets. HOLLISTER and LIN [4] adapted topology optimization method to find base cell and introduce the effective permeability to the optimization scheme. KANG et al [5] achieved the effective bulk modulus and diffusivity by optimal design for porous scaffold microstructures.
Conventional fabrication methods of metallic scaffolds include sintering metal powders [6], space-holder method [7], spark plasma sintering [8], templated vapor deposition [9] and decomposition of foaming agents [10]. However, there are inherent limitations for these conventional methods, such as long fabrication period, poor repeatability, irregularly shaped pores and insufficient interconnectivity of pores. In addition, the porosity and spatial distribution of pores is uncontrollable.
Recently several investigations have been carried out for the fabrication of porous scaffolds with high porosity and intricate architecture. The basic requirement of the available fabrication method is to have capability of producing an intricate microarchitecture. Rapid prototyping (RP) and rapid manufacturing (RM) technologies have provided solution for fabrication of porous scaffolds with intricate architecture. RP and RM technologies are considered a viable alternative for achieving precise control over the scaffolds architecture, pore shape and interconnectivity. Furthermore, the optimal scaffold model obtained from computational analysis can be reproduced by RM.
Until now, the RP technology development mainly focused on polymer and ceramic materials, SINGARE et al [11] served stereolithography model as the casting pattern for construction of a silicon mould, and subsequent casting of an identical wax model as an expendable pattern for titanium mandible implants by investment casting. Recently, RM technologies have been developed to fabricate metallic scaffolds. LI et al [12] used 3D fiber deposition (3DF) technology to fabricate Ti-6Al-4V scaffold with controllable porosity and pore size. MURR et al [13] reported the fabrication of porous titanium with periodic microstructures by electron beam melting (EBM). van BAEL et al [14] investigated the biological behavior of cells seeded on selective laser-melted Ti-6Al-4V bone scaffolds with pore size of 500-1000 μm.
This paper explores the possible topology optimized microstructure scaffolds and verifies SLM process for the fabrication of titanium scaffolds. The paper starts with an introduction of bone scaffolds to find out the implants material requirements, conventional and current design approaches to design scaffolds are listed and compared with topology optimization method. Algorithms and programs are built to obtain 2D and 3D optimized microstructure and the solution is transferred to CAD models for STL format in FORTRAN. Scaffolds with 8×8×16 periodic microstructure of 30% volume fraction are fabricated using a selective laser melting (SLM) technology.
2 General desirable properties for metallic biomaterials scaffolds
The scaffolds for bone reconstruction are three dimensional architectures which are used as support structures allowing the cell and soft bone tissue to adhere. In most cases, scaffolds for hard tissue repair in a load-bearing area are not temporary but permanent. Bone replacement should be biocompatible with surrounding tissue. Smooth surfaces exhibit less cell adhesion and bioactivity than rough surface. So, surface modification is a necessary procedure after the scaffolds is fabricated.
In addition, the structural requirement is a major factor, such as the architecture, pore size, interconnectivity and the maximum feasible porosity. These factors can affect how many cells and tissue can penetrate and grow into the scaffolds. Previous research has shown that the optimum pore size for promoting bone ingrowth is in the range of 100-500 μm [15]. Scaffolds with pore size of less than 100 μm fail to support the growth of capillaries and allow the bone cell to bridge pores, on the contrary, scaffolds with pore size lager than 500 μm do promote bone in-growth but at a reduced rate and volume.
In mechanical properties, scaffolds materials aiming at bone repair should provide mechanical support in order to avert deformation and ultimately facilitate tissue regeneration. The most critical mechanical properties are stiffness and strength. When the scaffolds are not as stiff as bone, the remaining bone surrounding the implants will be insert under the increased stress; on the contrary, a stress-shielding effect will occur. In addition to matching bone stiffness, the strength of scaffolds should be close to or match that of natural bone. The implantation site should have equal or excess strength and stiffness while the in vivo tissue ingrowth is filled. An equal or excess strength ensures that the implantation site has equivalent or better load bearing capabilities than nature bone.
Anatomically, the external geometry and size of the scaffolds should match to the bone defect of each patient so that the scaffolds can fit and anchor into the defect site.
3 Methods and materials
3.1 Inverse homogenization theory
The mathematical theory of inverse homogenization is used to estimate the effectively macroscopic properties of porous materials, assuming periodicity from an analysis of representative microstructure (also called base cell) [16]. A microstructure is defined as a mixture of solid-void phase. If the microcosmic level is sufficiently small, an averaged behavior or property of the global scale can be described at local scale by computational or experimental investigation. The inverse homogenization method here provides a method to investigate the microcosmic effect on the macroscopic description. While the macroscopic level represents the scaffold, the microscopic level represents the base cell. Firstly, the appropriate local properties of base cell are solved and the effective properties are solved. Then, the boundary value problem for global properties with spatial repetition of base cells is obtained.
The inverse homogenization theory is applied to estimating the effective mechanical properties of scaffold materials which are arrayed by periodical base cells in this study. Thus, the properties of scaffold materials are obtained at the macroscopic scale while the design is conducted at the microcosmic level.
3.2 Topology optimization problem statement
Topology optimization often aimed at seeking to the stiffest structure with a given-volume material, so the compliance-volume index is used as a performance criterion and the evolutionary procedure is stopped once the performance criterion drops dramatically. A structure is optimized by removing and adding elements. Elements with near the lowest von Mises stresses are removed and elements near the highest von Mises stresses are switched on as solid elements [17]. The compliance, which is proportional to the strain energy, can be written as:
![]() (1)
(1)
where C is the global compliance; fT is the applied load; U is the global displacement vector; K is the global stiffness matrix.
To achieve a near solid-void design, elastic modulus of the intermediate densities is interpolated as:
![]() (2)
(2)
where E1 is the elastic modulus of the solid element; p>1 is a penalty factor which penalizes intermediate density to solid or void. The global stiffness matrix K is comprised of the elemental stiffness and design variables xi as:
![]() (3)
(3)
where ![]() is the elemental stiffness matrix of a solid element.
is the elemental stiffness matrix of a solid element.
Thus, the topology optimization problem with the volume constraint is stated as:
Min: ![]() (4)
(4)
Subject to:
![]()
![]() (5)
(5)
where V* is the prescribed design volume; Vi is the volume of an solid element, with contribution of local element in terms of xi; f is the volume fraction; N is the number of solid elements. The binary design variable of xi denotes the density of the ith element, and a very small value of xmin is used to denote the void element.
When the element is removed from the design domain, the change of the mean compliance or total strain energy is equal to the elemental strain energy. This change is defined as the elemental sensitivity number:
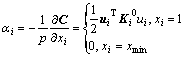 (6)
(6)
3.3 Materials
There are several biocompatible metallic materials which are frequently used as implanting materials in dental and orthopedic surgery to repair or replace bone defects. The main disadvantage of metallic scaffolds is their lack of biological recognition on surface. So the subsequently surface modification is necessary to preserve the mechanical properties and enhance surface biocompatibility.
Tantalum was shown to be highly biocompatible in several animal models [9]. Although tantalum is in its early evolution stages, it is an alternative of traditional orthopedic implant biomaterials in clinical use. Magnesium and its alloys for surgical application have been paid attention in recent years because of their fully biodegradable and matched mechanical properties with bone [18]. Titanium and its alloy Ti-6Al-4V [19] and Ni-Ti [20] are found to be well tolerated and nearly an implant material in the human body. They are not ferromagnetic and do not cause harm to patient. The current gold standard for load-bearing defect site reconstruction maintains titanium meshes and titanium scaffolds. Pure Ti powder with a mean particle size of 25 μm was used in this work.
3.4 Selective laser melting fabrication for metallic scaffolds
Figure 1(a) shows the operation principle of SLM machine Dimetal-280 which was developed independently by South China University of Technology. The machine equipped with a fiber laser with a power of 200 W and a focus diameter of 30-50 μm. The powder-laying roller spreads metallic powder on the build platform. The x-y scanner directs the laser beam according to the scanning paths generated by computer data. Then the build platform descends one layer thickness. Repeat above steps through layer-by-layer process until the whole processing ends [21]. Figure 1(b) shows the inter-layer staggered and orthogonal scanning strategy in process.

Fig. 1 Operation principle of Dimetal-280 (a) and inter-layer staggered and orthogonal scanning strategy (b)
In SLM process, scaffolds are constructed via three-dimensional layer structures from metallic powder in a relatively short time. Firstly, the corresponding CAD model is generated; next, the model is converted as a STL data; then the STL model is sliced into thin layers and generate scanning path; finally, the specimen is fabricated layer-by-layer through directly fusing and solidifying metal powder.
The SLM fabrication method for metallic scaffolds provides excellent control of intricate external shape and internal interconnectivity. The processing time is dependent on the solid volume of parts rather than the complexity of parts. The processing speed based on Dimetal-280 is 5-20 cm?/h. Layer manufacturing technology facilitates the fabrication of highly intricate structures which are hardly or impossible to be fabricated by other conventional methods. The processing parameters in this work are as follows: layer thickness of 20 μm, laser power of 160 W, scanning spacing of 40 μm, scanning speed of 800 mm/s, alternating x- and y-direction orthogonal scanning strategy.
4 Numerical experiments and topology optimization procedure
4.1 Checkerboard problem
The sensitivity numbers could become discontinuous across element boundaries, which could lead to checkerboard patterns in the final optimal structure. The presence of checkboard pattern causes difficult in manufacturing. To suppress the checkboard pattern in topology optimization, a numerical filter is applied to the elemental sensitivity number. The filter scheme which was originally developed for structural optimization [22] is adopted in this procedure. The elemental sensitivity number is modified as follows:
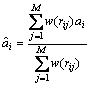 (7)
(7)
where rij denotes the distance between the centre of the ith and jth element; w(rij) is the weight factor given as:
![]() (8)
(8)
where rmin is the filter radius.
4.2 2D and 3D design domain for stiffness optimization
A design domain of square plate with L=30 is shown in Fig. 2(a). The plate is fixed on the below and loaded with F=100 on the other three directions.
The objective of the optimization problem is to find the minimum compliance structure with a volume fraction constraint of 10%, 20%, 30%, 40% and 50% respectively. The material considered here is titanium, and its properties are as follows: elastic modulus E=1, Poisson ratio v=0.3.
The cubic domain with side length of 40 is shown in Fig. 2(b). The cubic is fixed on the centre of bottom and loaded with F=200 in the other five directions. The objective function, volume constraint and material properties considered in 3D base cell are equal to above 2D base cell.
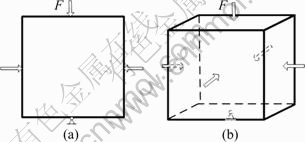
Fig. 2 2D (a) and 3D (b) design domain
4.3 Topology optimization procedure
The square plate domain is discretized into quadrilateral elements of 120×120, and the plane strain problem is assumed so that the plate thickness is not taken into account. The cubic domain is discretized into 8-node cubic elements of 40×40×40. The filter radius rmin is set as 3 and 4 respectively. The volume gradually decreases until the volume fraction constraint is achieved and the objective function convergence criterion is satisfied.
The topology optimization procedure for unit cell design is as follows:
Step 1 Define the above parameters.
Step 2 Discretize the design domain using a finite element mesh and assign initial values (1 in this procedure) for the elements.
Step 3 Perform the finite element analysis and then calculate the elemental sensitivity numbers ai according to Eq. (6).
Step 4 Calculate the modified elemental sensitivity number ![]() according to Eqs. (7) and (8).
according to Eqs. (7) and (8).
Step 5 Remove a number of elements with the lowest sensitivity number according to the evolutionary volume ratio RE.
Step 6 Repeat steps 3-5 until both the volume constraint is satisfied and the objective function is convergent.
5 Results and discussion
5.1 2D base cell for stiffness optimization
When the volume fraction constraints of solid materials are set to be respectively 10%, 20%, 30%, 40% and 50% of the square plate domain, the corresponding final base cells are given in Fig. 3. The mean compliances are 91.5, 32.8, 11.5, 6.8, 2.7 corresponding to total iterations of 89, 82, 71, 55 and 51, respectively.
Figure 4 shows the evolution histories of mean compliance as the volume fraction constraint is 30%. Generally, the mean compliance decreases as the total volume of the solid elements decreases. Once the volume constraint is satisfied, the objective function (mean compliance) and structure topologies stably converge to their final solutions. The final base cell can be interpreted as near two-dimension cross with smooth transitions in intersection.
The macrostructure of material can be constructed periodically by 4×4 base cell as shown in Figs. 3(a2)-(e2). According to inverse homogenization theory, the macroscopic properties can be solved from the microcosmic properties of base cell. Therefore, the strength of material in macrostructure equals that of their base cell.
5.2 3D base cell for stiffness optimization
For the volume fraction constraint of solid by imposing different volume fractions (corresponding to different porosities), the optimal layout of materials within the base cell model is evaluated through topology optimization in order to obtain the minimal compliance (corresponding to maximized stiffness) structure. It must be pointed out that different initial base cells and boundary conditions will result in different optimal structures of scaffolds.
Volume fraction constraints of materials are set to be respectively 10%, 20%, 30%, 40% and 50% of the cubic domain, the corresponding final base cells are given in Fig. 5, and the mean compliances are 58.1, 12.2, 6.8, 4.2, 2.9 respectively. The final base cell can be also interpreted as near three-dimension cross with smooth transitions in intersection.
5.3 Conversion of design solutions to CAD model
For fabrication with an RM process, it is not enough to know the geometry details in the form of coordinate vertices of the points of element. There is a need to construct a CAD model from these vertices in an STL (stereolithography) format developed by 3D systems. FORTRAN was used to convert the material distribution information into coordinate points and subsequently into surface patches connecting these vertices using self-built function without smooth processing. Figure 6 shows the STL format CAD model of base cell with 30% volume fraction.

Fig. 3 Optimized 2D base cells (a1-e1) and their periodic structure (a2-e2)
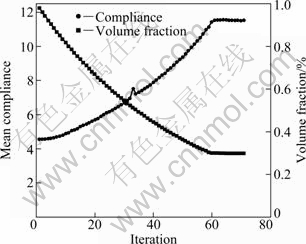
Fig. 4 Evolution histories of mean compliance
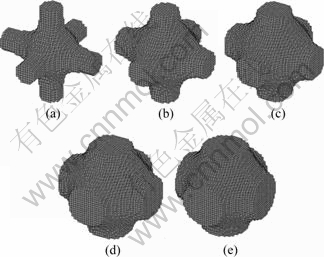
Fig. 5 Optimized 3D base cells with volume fractions of 10% (a), 20% (b), 30% (c), 40% (d) and 50% (e)
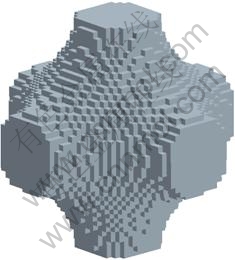
Fig. 6 CAD model for STL format
5.4 Specimens of titanium scaffolds
The scaffolds with periodic base cells of 8×8×16 are fabricated on a SLM machine Dimetal-280. The different dimensions of scaffolds can be obtained by multiplying a scaling factor.
Their SEM images of scaffolds with microstructure sizes of 500, 750 and 1000 μm are shown in Fig. 7. The measured mean values of pore sizes are 231, 387, 542 μm respectively. The architecture and pore shape of microstructure are relatively precise reproduced. The rough surface morphology of these scaffolds enhances osteoblast response. These results suggest that SLM fabricated metallic biomaterials scaffolds may be potentially used in bone tissue engineering with high bone regenerative efficacy.
New tissue ingrowth into titanium scaffolds depends on several factors, including pore size, pore architecture and pore interconnectivity. There are some studies [15] in which the influence of scaffolds porosity and pore size on the biological behavior has been investigated, and their results demonstrated that bone ingrowth was not only dependent on pore size between 100 and 500 μm, but dependent on pore architecture and pore aspect ratio. Based on different porosities and dimensions, the current pore sizes of scaffolds presented in this work were between 231 μm and 542 μm. The minimum feature of 100 μm can be fabricated in Dimetal-280 which was presented in our previous paper [23].
The high interconnectivity in random directions is beneficial for cell attachment and bone formation. Although increased pore size and porosity are obviously beneficial for new bone ingrowth, but the pore size and porosity increase will result in reduction of mechanical properties, thus a balance between the mechanical properties and the biological performance should be found depending on the implantation sites. Meanwhile, the strength will increase gradually with the growth of new bone tissue. The implantation site will have equal or excess strength while the in vivo tissue ingrowth is filled. An equal or excess strength ensures that the implantation site has equivalent or better load-bearing capabilities than nature bone.
6 Conclusions
1) This research presents the processes of optimal design for 2D and 3D microstructures and selective laser melting fabrication for 3D metallic biomaterial scaffolds. The theoretical foundation of the work is the topology optimization method. Topology optimization method was used to seek for microstructure with a maximum stiffness under the constraint of specific volume fraction. That is, with an initial objective, the highly porous structures can be obtained.

Fig. 7 SEM images of scaffolds with microstructure sizes of 500 μm (a, d); 750 μm (b, e) and 1000 μm (c, f)
2) A topology optimization procedure for 3D microstructure with a maximized stiffness is proposed. To suppress the checkboard pattern in topology optimization, a filter scheme is applied to the elemental sensitivity number. Algorithms and programs were built to obtain the microstructure of topology optimization and transfer the solution to CAD models with STL format in FORTRAN.
3) Specimens of scaffolds are fabricated using a SLM machine, the architecture and pore shape of microstructure are relatively precise reproduced, and a minimum mean pore size is 231 μm. The accurate fabrication of intricate titanium scaffolds has verified that the SLM process is suitable for fabrication of biomaterial scaffolds.
4) Although initial attempts have been made in combination of topology optimization method and SLM process, future work will be carried out to evaluate the precise relation between desired mechanical properties and that of the topology optimized scaffolds by SLM process.
References
[1] van CLEYNENBREUGEL T, van OOSTERWYCK H, VANDER SLOTEN J, SCHROOTEN J. Trabecular bone scaffolding using a biomimetic approach [J]. Journal of Materials Science: Materials in Medicine, 2002, 13: 1245-1239.
[2] YOO D J. Computer-aided porous scaffold design for tissue engineering using triply periodic minimal surfaces [J]. International Journal of Precision Engineering and Manufacturing, 2011, 12(1): 61-71.
[3] LIN L, HU Q, HUANG X, XU G. Design and fabrication of bone tissue engineering scaffolds via rapid prototyping and CAD [J]. Journal of Rare Earths, 2007, 25: 379-383.
[4] HOLLISTER S J, LIN C Y. Computational design of tissue engineering scaffolds [J]. Computer Methods in Applied Mechanics and Engineering, 2007, 196: 2991-2998.
[5] KANG H, LIN C Y, HOLLISTER S J. Topology optimization of three dimensional tissue engineering scaffold architectures for prescribed bulk modulus and diffusivity [J]. Structural and Multidisciplinary Optimization, 2010, 42: 633-644.
[6] OH I H, NOMURA N, MASAHASHI N, HANADA S. Mechanical properties of porous titanium compacts prepared by powder processing [J]. Scripta Materialia, 2003, 49: 1197-1202.
[7] BRAM M. High-porosity titanium, stainless steel, and superalloy parts [J]. Advanced Engineering Materials, 2000, 2: 196-199.
[8] MIYAO R, OMORI M, WATARI F, YOKOYAMA A, MATSUNO H, HIRAI T, KAWASAKI T. Fabrication of functionally graded implants by spark plasma sintering and their properties [J]. Journal of the Japan Society of Powder and Powder Metallurgy, 2000, 47: 1239-1242.
[9] BOBYN J D, STACKPOOL G J, HACKING S A, TANZER M, KRYGIER J J. Characteristics of bone ingrowth and interface mechanics of a new porous tantalum biomaterial [J]. Journal of Bone & Joint Surgery, 1999, 81: 907-914.
[10] GU Y W, YONG M S, TAY B Y, LIM C S. Synthesis and bioactivity of porous Ti alloy prepared by foaming with TiH2 [J]. Materials Science and Engineering C, 2009, 29(5): 1515-1520.
[11] SINGARE S, LI D, LU B, LIU Y, GONG Z, LIU Y. Design and fabrication of custom mandible titanium tray based on rapid prototyping [J]. Medical Engineering & Phys, 2004, 26(8): 671-676.
[12] LI J P, DE WIJN J R, VAN BLITTERSWIJK C A, DE GROOT K. Porous Ti6Al4V scaffolds directly fabricated by 3D fibre deposition technique: Effect of nozzle diameter [J]. Journal of Materials Science: Materials in Medicine, 2005, 16: 1159-1163.
[13] MURR L E, ESQUIVEL E V, QUINONES S A, GAYTAN S M, LOPEZ M I, MARTINEZ E Y, MEDINA F, HERNANDEZ D H, MARTINEZ E, STAFFORD S W, BROWN D K, HOPPE T, MEYERS W, LINDHE U, WICKER R B. Microstructures and mechanical properties of electron beam-rapid manufactured Ti-6Al-4V biomedical prototypes compared to wrought Ti-6Al-4V [J]. Materials Characterization, 2009, 60: 96-105.
[14] van BAEL S, CHAI YC, TRUSCELLO S, MOESEN M, KERCKHOFS G, van OOSTERWYCK H, KRUTH JP, SCHROOTEN J. The effect of pore geometry on the in vitro biological behavior of human periosteum-derived cells seeded on selective laser-melted Ti6Al4V bone scaffolds [J]. Acta Biomaterialia, 2012, 8(7): 2824-2834.
[15] KARAGEORGIOU V, KAPLAN D. Porosity of 3D biomaterial scaffolds and osteogenesis [J]. Biomaterials, 2005, 26: 5474-5491.
[16] SIGMUND O, TORQUATO S. Design of materials with extreme thermal expansion using a three-phase topology optimization method [J]. Journal of the Mechanics and Physics of Solids, 1997, 45: 1037-1067.
[17] YANG X Y, XIE Y M, STEVEN G P, QUERIN O M. Bidirectional evolutionary method for stiffness optimization [J]. AIAA Journal, 1999, 37: 1483-1488.
[18] STAIGER M P, PIETAK A M, HUADMAI J, DIAS G. Magnesium and its alloys as orthopedic biomaterials: A review [J]. Biomaterials, 2006, 27: 1728-1734.
[19] DAVIES J E. Bone bonding at natural and biomaterial surfaces [J]. Biomaterials, 2007, 89: 5058-5067.
[20] PRYMAKA O, BOGDANSKIB D, K?LLERB M, ESENWEINB S A, MUHRB G, BECKMANN F, DONATH T, ASSAD M, EPPLE M. Morphological characterization and in vitro biocompatibility of a porous nickel-titanium alloy [J]. Biomaterials, 2005, 26: 5801-5807.
[21] YANG Yong-qiang, SU Xu-bin, WANG Di, CHEN Yong-hua. Rapid fabrication of metallic mechanism joints by selective laser melting [J]. Journal of Engineering Manufacture, 2011, 225(12): 2249-2256.
[22] HUANG X, XIE Y M. Convergent and mesh-independent solutions for bi-directional evolutionary structural optimization method [J]. Finite Elements in Analysis and Design, 2007, 43(14): 1039-1049.
[23] WANG Di, YANG Yong-qiang, SU Xu-bin, CHEN Yong-hua. Study on energy input and its influences on single-track, multi-track, and multi-layer in SLM [J]. International Journal of Advanced Manufacturing Technology, 2011, 54(9-12): 1-11.
金属生物材料支架的微结构拓扑优化设计及选区激光熔化制造
肖冬明1,2,杨永强1,苏旭彬3,王 迪1,罗子艺4
1. 华南理工大学 机械与汽车工程学院,广州 510641;
2. 湖南科技大学 机电工程学院,湘潭 411201;
3. 华南理工大学 材料科学与工程学院,广州 510641;
4. 广州有色金属研究院,广州 510650
摘 要:生物材料支架的精确设计和制造是骨组织工程系统研究的基础。生物材料支架应该同时满足大孔隙率和与骨组织匹配的力学性能要求。这两个目标相互制约,大的孔隙率会降低其力学性能。利用拓扑优化的方法,在体积分数的约束下,寻求刚度最大的最优材料分布微结构。建立算法,得到了不同体积分数的2D和3D最优微结构,并提取3D拓扑优化的结果,然后将其转化为STL格式的CAD模型文件。微结构在三维方向整列成支架结构,通过选区激光熔化方法制造30%(体积分数)的Ti支架样品。从SEM图像看出,支架样品的结构和孔径与CAD模型基本一致,500 μm微结构单元的平均孔径为231 μm。复杂形状金属生物材料支架的精确制造证实了选区激光熔化技术在金属生物医学材料制造中的可行性。
关键词:拓扑优化;选区激光熔化;微结构;金属生物材料支架
(Edited by YANG Hua)
Foundation item: Project (51275179) supported by the National Natural Science Foundation of China; Project (2010A090200072) supported by Industry, University and Research Institute Combination of Ministry of Education, Ministry of Science and Technology and Guangdong Province, China; Project (2012M511797) supported by China Postdoctoral Science Foundation; Project (2012ZB0014) supported by Fundamental Research Funds for the Central Universities of China
Corresponding author: SU Xu-bin; Tel: +86-20-87114484; E-mail: scutsxb@yahoo.com.cn
DOI: 10.1016/S1003-6326(11)61500-8
Abstract: The precise design and fabrication of biomaterial scaffolds is necessary to provide a systematic study for bone tissue engineering. Biomaterial scaffolds should have sufficient stiffness and large porosity. These two goals generally contradict since larger porosity results in lower mechanical properties. To seek the microstructure of maximum stiffness with the constraint of volume fraction by topology optimization method, algorithms and programs were built to obtain 2D and 3D optimized microstructure and then they were transferred to CAD models of STL format. Ti scaffolds with 30% volume fraction were fabricated using a selective laser melting (SLM) technology. The architecture and pore shape in the metallic biomaterial scaffolds were relatively precise reproduced and the minimum mean pore size was 231μm. The accurate fabrication of intricate microstructure has verified that the SLM process is suitable for fabrication of metallic biomaterial scaffolds.


