- Abstract:
- 1 Introduction▲
- 2 Experimental▲
- 3 Results and discussion▲
- 4 Conclusions▲
- References
- Figure
- Fig.1 Effect of pH on flotation recovery of chalcopyrite and pyrite (c(BX)=0.1 mmol/L; ρ(LSC)=100 mg/L; ρ(2# oil)= 10 mg/L)
- Fig.2 Effect of LSC concentration on flotation recovery of chalcopyrite and pyrite (pH=5.9-6.1; c(BX)=0.1 mmol/L; ρ(2# oil)=10 mg/L)
- Fig.3 FTIR spectrum of LSC
- Fig.4 FTIR spectra of pyrite in presence and absence of reagents: (a) Pyrite; (b) Pyrite +BX; (c) Pyrite +LSC+BX
- Fig.5 FTIR spectra of chalcopyrite in presence and absence of reagents: (a) Chalcopyrite; (b) Chalcopyrite+BX; (c) Chal- copyrite+LSC+BX
J. Cent. South Univ. Technol. (2009) 16: 0753-0757
DOI: 10.1007/s11771-009-0125-0
![]()
Effect of organic depressant lignosulfonate calcium on separation of chalcopyrite from pyrite
LIU Run-qing(刘润清), SUN Wei(孙 伟), HU Yue-hua(胡岳华), WANG Dian-zuo(王淀佐)
(School of Resources Processing and Bioengineering, Central South University, Changsha 410083, China)
Abstract:
In order to selectively separate chalcopyrite from pyrite,the effect of organic depressant lignosulfonate calcium (LSC) on the flotation separation of chalcopyrite from pyrite was investigated by flotation tests. The depression mechanism was studied by Fourier-transform-infrared (FTIR) analysis. The flotation tests of single mineral show that LSC can depress the flotation of pyrite in a certain pH range, but it has little effect on chalcopyrite flotation. Flotation separation of a mixture of chalcopyrite and pyrite can be completed to obtain a copper concentrate grade up to 24.73% with a recovery of 80.36%. IR analysis shows that LSC and butyl xanthate compete in absorption on pyrite surface, and there exists an LSC characteristic peak on pyrite surface. There is little adsorption of LSC on chalcopyrite.
Key words:
flotation; chalcopyrite; pyrite; depression; separation;
1 Introduction
Pyrite is the most widespread and abundant of naturally occurring metal sulfide, which is often associated with other valuable metal sulfides such as chalcopyrite, galena and sphalerite [1-2]. Hence, separation of pyrite from other associated metal sulfides is desirable for the economical extraction of these valuable metals. Because pyrite has good electric conductivity and similar floatability with chalcopyrite, the separation of chalcopyrite from pyrite becomes difficult, and also pyrite misreports into the copper concentrate because of its accidental activation by copper released from chalcopyrite during grinding or conditioning [3-7].
During the past years, many researches on the separation of chalcopyrite from pyrite have been carried out. CHEN and FENG [8] studied the effect of CTP on flotation separation of chalcopyrite and pyrite, and the results showed that CTP could effectively separate pyrite and chalcopyrite at pH 9 with CaO as adjusting reagent. XIONG et al [9] investigated the effect of calcium containing compounds on the flotation behavior of chalcopyrite and pyrite. But their study is restricted to high pH values, long conditioning time and extensive surface oxidation to realize the depressing of pyrite flotation. Most of the reagents are inorganics, such as cyanides, polymers, ferrocyanides, zinc sulphate, sulphite, hydroxide, calcic compounds, or a combination of these reagents [10-13]. Of all depressants, cyanides and lime are the most common. However, cyanides have raised concern on environment [14]. Lime is apt to form the dirt and jam the pipeline. Hence, natural, bio- degradable, and non-toxic reagents receive importance and held promise to function as a selective depressant [15-16].
LSC is derived from the paper pulp waste liquor by simple treatment. The relative molecular mass of LSC is 1 000-20 000. Its molecule includes hydroxyl group (—OH) and sulfonic group (—SO3H). LSC, as a flotation depressant, both in the laboratory and in commercial processes, has been reported [17]. LSC has ever been used to depress the calcite and barite, but it has seldom been reported to be used as depressant of sulfides.
In this work, the effect of LSC on flotation behavior of pyrite and chalcopyrite in the butyl xanthate (BX) system was investigated, and the flotation separation of chalcopyrite and pyrite was conducted in the mixed mineral system. FTIR was also used to explain the flotation results and investigate the adsorption mechanism of LSC on pyrite surface.
2 Experimental
2.1 Samples and reagents
Pure handpicked mineral samples of pyrite and chalcopyrite were obtained respectively from Tongling mine of Anhui Province and Chenzhou of Hunan Province. The mineral samples were crushed, handpicked and dry-ground with a porcelain ball mill and dry-sieved to obtain different size fractions. The fraction less than 106 ?m was used for flotation studies. The fraction less than 37 ?m was used for FTIR studies. The minerals were stored in a desiccator under nitrogen atmosphere. The purity of the mineral samples was ascertained by mineralogical studies. Chemical analysis of two mineral samples indicated that pyrite purity was 87.56% and chalcopyrite purity was 72.65%.
The collector was butyl xanthate, which was industrial grade product from Chemical Factory of Zhuzhou, China. 2# oil was employed as a frother, which was also industrial grade product. HCl and NaOH were adopted to adjust the pulp pH value, and organic depressant LSC is a kind of chemical product.
2.2 Flotation test
In the flotation test, flotation machine of hitch groove of XFG-1600 type with volume of 40 mL was used. In each test, 2.0 g of sample was taken, then the surface was cleaned for 10 min by using supersonic cleaner in order to clean the oxide. The upper layer liquid was settled and decanted, and then flushed in groove of 40 mL and pH value was adjusted. Depressant, collector and frother were added by order. The conditioning time for the depressant, collector and frother was 3, 2 and 1 min, respectively. The flotation was performed for 4 min. The floating and non-floating fractions were filtrated, dried and weighed for the recovery calculation.
The 40 mL groove was used in flotation separation test of mixture minerals, 1.5 g chalcopyrite and 1.5 g pyrite were taken, and the surface was cleaned by using supersonic cleaner, respectively. Then they were mixed to float. Products were filtrated, dried and weighed for assessing the recovery by chemical analysis.
2.3 Infrared spectrum analysis
1.0 g sample was immersed in 25 mL solutions containing corresponding reagent, ground for 30 min in an agate mortar, settled for 30 min, and filtrated. The solid obtained was dried under vacuum. Infrared spectra were recorded in an NEXUS-470 infrared spectrum apparatus.
3 Results and discussion
3.1 Effect of pH on flotation of chalcopyrite and pyrite
In order to selectively separate chalcopyrite from pyrite, the effect of LSC on flotation of chalcopyrite and pyrite at various pH values was studied. The results are shown in Fig.1. In the flotation test, the concentration of LSC is 100 mg/L, butyl xanthate concentration is 0.1 mmol/L, and 2# oil concentration is 10 mg/L.
Fig.1 shows that both chalcopyrite and pyrite have good floatability in wide pH region with butyl xanthate as a collector and the recovery is more than 70%. At pH>10, pyrite recovery drops because pyrite surface oxidation form hydroxyl compound, resulting in hydrophilic surface in high pH. It can be seen that it may be difficult to separate chalcopyrite from pyrite in absence of depressant. Fig.1 also shows that depressant LSC has good depression action on pyrite flotation in whole pH range with butyl xanthate as a collector, while LSC has no or little effect on chalcopyrite flotation. According to the above results it can be inferred that the flotation separation of pyrite from chalcopyrite may be possible in wide pH range by using LSC as a depressant.
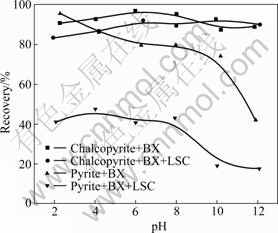
Fig.1 Effect of pH on flotation recovery of chalcopyrite and pyrite (c(BX)=0.1 mmol/L; ρ(LSC)=100 mg/L; ρ(2# oil)= 10 mg/L)
3.2 Effect of LSC concentration on flotation of chalcopyrite and pyrite
Effect of LSC concentration on the mineral floatability was investigated at pH 6 and butyl xanthate concentration of 0.1 mmol/L. The results are shown in Fig.2. It follows that pyrite recovery sharply decreases with the increase of LSC concentration. When LSC concentration is 150 mg/L, pyrite recovery is 10.17%, showing strong depression effect on the pyrite. However, LSC has weak effect on chalcopyrite flotation. This demonstrates the selective function of LSC on pyrite.
3.3 Separation of mixture mineral
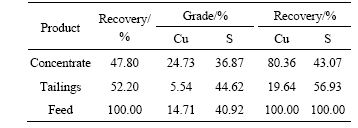
On the basis of single mineral flotation test, adopting sodium hydroxide to adjust pulp, the separation test of manual mixed mineral was performed under the condition of pH=9-10, LSC concentration of 150 mg/L, butyl xanthate concentration of 0.1 mmol/L, 2# oil concentration of 15 mg/L. The results are listed in Table 1. From Table 1, it can be seen that LSC can effectively separate pyrite from chalcopyrite with grade of copper concentrate up to 24.73% with the recovery of 80.36%. From the results it is indicated that the separation of chalcopyrite and pyrite is possible in alkaline condition with LSC as a depressant and butyl xanthate as a collector.
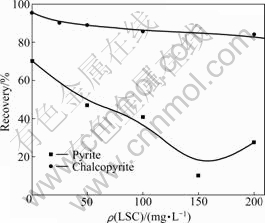
Fig.2 Effect of LSC concentration on flotation recovery of chalcopyrite and pyrite (pH=5.9-6.1; c(BX)=0.1 mmol/L; ρ(2# oil)=10 mg/L)
Table 1 Results of flotation separation of chalcopyrite-pyrite manual mixture mineral
3.4 Mechanism of mineral-LSC interaction
The FTIR spectrum of LSC presented in Fig.3 shows peaks at 2 936.9 and 1 415.9 cm-1 due to stretching vibration and in-plane bending vibration of alkyl, and peaks at![]() and
and ![]() corresponding to asymmetry and symmetry vibration of —SO3 group adsorption, respectively. The adsorption band of 619.4 cm-1 is corresponding to C—S stretching vibration. Peaks at 1 663.7 and
corresponding to asymmetry and symmetry vibration of —SO3 group adsorption, respectively. The adsorption band of 619.4 cm-1 is corresponding to C—S stretching vibration. Peaks at 1 663.7 and ![]() correspond to stretching vibration of —COO- and —OH, and peaks at
correspond to stretching vibration of —COO- and —OH, and peaks at ![]() and
and ![]() correspond to the characteristic adsorption of phenyl. The results indicate that there are a number of function groups such as —COO-, —SO3 and —OH in the molecule of LSC.
correspond to the characteristic adsorption of phenyl. The results indicate that there are a number of function groups such as —COO-, —SO3 and —OH in the molecule of LSC.
The FTIR spectra of different samples are shown in Fig.4. Fig.4(b) shows that there are obvious characteristic peaks at 2 963.6, 1 467.1, 1 372.2, 1 266.0,

Fig.3 FTIR spectrum of LSC
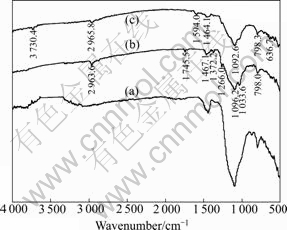
Fig.4 FTIR spectra of pyrite in presence and absence of reagents: (a) Pyrite; (b) Pyrite +BX; (c) Pyrite +LSC+BX
1 096.2, 1 033.0 and 916.7 cm-1. As reported in Ref.[18], pyrite interacts with xanthate to form dixanthogen and the characteristic peaks of dixanthogen appear at 1 269 and 1 024 cm-1. By analyzing Fig.4(b) in wavenumber range of 1 000-1 300 cm-1, it can be found that there are adsorption peaks at 1 266.0 and 1 033.6 cm-1 on pyrite surface, which indicates that there is dixanthogen formation on pyrite surface. Fig.4(c) shows that there are adsorption of phenyl and C—S stretching vibration at 3 730.4, 1 594.0 and 636.7 cm-1. Also there are butyl xanthate characteristic peaks at 2 965, 1 464 and 1 092 cm-1. This indicates that LSC and butyl xanthate are adsorbed simultaneously on pyrite surface, but LSC carries numerous hydrophilic groups, which make the pyrite surface hydrophilic and hence depress the flotation of pyrite.
Fig.5 shows the FTIR spectra of chalcopyrite in solution of butyl xanthate and LSC. It can be seen from Fig.5(b) that the adsorption of butyl xanthate on chalcopyrite occurs at 1 022.5 cm-1, showing the formation of dixanthogen. Fig.5(c) shows that there is no obvious absorption of LSC. This indicates that there is no or little adsorption of LSC on chalcopyrite. This also indicates that butyl xanthate is absorbed on the chalcopyrite surface prior to LSC. The existence of LSC cannot prevent butyl xanthate from being adsorbed on chalcopyrite surface, so LSC has litter influence on the chalcopyrite flotation.

Fig.5 FTIR spectra of chalcopyrite in presence and absence of reagents: (a) Chalcopyrite; (b) Chalcopyrite+BX; (c) Chal- copyrite+LSC+BX
Different mechanisms of LSC interaction with chalcopyrite and pyrite were caused by different mineral surface atomic structures and different chemical properties. For pyrite, the existence of oxygen makes its surface oxidized in the pulp by the reaction:
FeS2+11H2O-15e=Fe(OH)3+2SO42-+19H+ (1)
O2+4e+4H+=2H2O (2)
After adding depressant LSC, oxidized product Fe(OH)3 interacts with —SO3 of LSC. LSC is covered on the pyrite surface, resulting in the reduction atmosphere of pyrite surface, suppressing oxidization of xanthate on the pyrite surface, and preventing the formation of dixanthogen to reduce the pyrite flotation.
For the chalcopyrite, oxidation-reduction reactions are as follows:
2CuFeS2+6H2O+6O2=2Fe(OH)3+Cu2S+3SO42-+6H+(3)
Cu2S+2X-=2CuX+S2- (4)
When xanthate interacts with chalcopyrite, cuprous or copper xanthogenate forms on chalcopyrite surface, which is adsorbed on the chalcopyrite surface and makes chalcopyrite hydrophobic. After adding depressant LSC, though the chalcopyrite surface has iron ion, the solubility of xanthogenate copper is very small. The reaction between copper and xanthate ions is faster than that between iron and sulfonic ions. The formation of xanthogenate on chalcopyrite surface hinders the contact of sulfonic and iron ions. Therefore, LSC has weak function on chalcopyrite. It may be the reason why LSC could selectively depress pyrite in flotation separation chalcopyrite and pyrite.
4 Conclusions
(1) Flotation tests show that LSC has good depression action on pyrite flotation in whole pH range with butyl xanthate as collector, while LSC has no or little effect on chalcopyrite flotation. LSC can effectively separate pyrite from chalcopyrite in wide pH range.
(2) The separation test of manual mixed mineral shows that LSC can separate pyrite from chalcopyrite at pH 9-10 with grade of copper concentrate up to 24.73% and recovery of 80.36%.
(3) FTIR spectrum indicates that LSC has a number function groups —OH and —SO3. Pyrite interacts with xanthate and LSC, and LSC is covered on the pyrite surface, and preventing the formation of dixanthogen on pyrite surface, which makes the pyrite surface hydrophilic, resulting in depressing the flotation. For chalcopyrite, the reaction between copper ion and xanthate ion is faster than that between iron ion and LSC, so LSC has no or little adsorption of LSC on chalcopyrite. The conclusion is in accordance with the flotation results.
References
[1] CHANDRAPRABHA M N, NATARAJAN K A, MODAK J M. Selective separation of pyrite and chalcopyrite by biomodulation [J]. Colloids and Surfaces, 2004, 379(3/4): 93-100.
[2] CHANDRAPRABHA M N, NATARAJAN K A, SOMASUNDARAN P. Selective separation of pyrite from chalcopyrite and arsenopyrite by biomodulation using Acidithiobacillus ferrooxidans [J]. International Journal of Mineral Processing,2005, 75(1/2): 113-122.
[3] HE S H, SKINNER W, FORNASIERO D. Effect of oxidation potential and zinc sulphate on the separation of chalcopyrite from pyrite [J]. International Journal of Mineral Processing, 2006, 80(2/4): 169-176.
[4] PENG Y J, GRANO S, FORNASIERO D, RALSTON J. Control of grinding conditions in the flotation of chalcopyrite and its separation from pyrite [J]. International Journal of Mineral Processing,2003, 69(1/4): 87-100.
[5] KUOPANPORTTI H, SUORSA T, DAHL O, NIINIMAIKI J. A model of conditioning in the flotation of a mixture of pyrite and chalcopyrite ores [J] International Journal of Mineral Processing, 2000, 59(4): 327-338.
[6] RICHARDSON P E, CHEN Z, TAO D P, YOON R H. Electrochemical control of pyrite activation by copper [C]// Proceedings of the Fourth International Symposium Electrochemistry in Mineral and Metal Processing. Amazon, 1996: 179-190.
[7] YUAN X M, PALSSON B I, FORSSBERG K S E. Flotation of a complex sulphide ore (II): Influence of grinding environments on Cu/Fe sulphide selectivity and pulp chemistry [J]. International Journal of Mineral Processing,1996, 46(3/4): 181-204.
[8] CHEN Jian-hua, FENG Qi-ming. Mechanism and depression behavior of CTP on pyrite and chalcopyrite in different alkaline media [J]. Nonferrous Metal, 1998, 50(1): 28-32. (in Chinese)
[9] XIONG Dao-ling, CHEN Xiang-qing, JIANG Yu-ren. Effect of calcium containing compounds on the flotation behavior of chalcopyrite and pyrite [J]. Hunan Nonferrous Metal, 2004, 20(6): 8-10. (in Chinese)
[10] CHANDRAPRABHA M N, NATARAJAN K A, MADAK J M. Selective separation of pyrite and chalcopyrite by biomodulation colloids and surface B [J]. Biointerfaces, 2004, 37(3/4): 93-100.
[11] XU Jing, SUN Wei, ZHANG Qin, LIU Hui, HU Yue-hua. Research on depression mechanism of pyrite and pyrrhotite by new organic depressant RC [J]. Mining and Metallurgical Engineering, 2003, 23(6): 29-32. (in Chinese)
[12] MUSTAFA S, HAMID A, NAEEM A. Xanthate adsorption studies on chalcopyrite ore [J]. International Journal of Mineral Processing, 2004, 74(1/4): 317-325.
[13] QIU Ting-sheng, FANG Xi-hui, ZHONG Chang-ming. Comparison of depression performance of several pyrite depressants [J]. Multipurpose Utilization of Mineral Resources, 2005(3): 6-9. (in Chinese)
[14] DEWET J R, HODGKINSON G, PISTORIUS P C, PRINSLOO L C, SANDENBERGH R F. The influence of cyanide on the flotation of pyrite from Witwatersrand gold leach residues [J]. Minerals Engineering, 1995, 8(11): 1333-1345.
[15] HU Yue-hua, ZHANG Shun-li, QIU Guan-zhou, WANG Dian-zuo. The mechanism of activation floatation of pyrite depressed by lime [J]. Journal of Central South University of Technology: Natural Science Edition, 1995, 26(2): 176-180. (in Chinese)
[16] LIU Zhi-lin, XU Fang. The research on the inhibitor of pyrite under the condition with low pH value [J]. Mining and Metallurgical Engineering, 2005, 25(5): 33-35. (in Chinese)
[17] JIAN Bai-xi. Flotation reagent [M]. Beijing: Metallurgical Industry Press, 1981: 333-336.
[18] MIELCZARSKI J A, MIELCZARSKI E, ZACHWIEJA J. Insitu and exsitu infrared studies of nature and structure of thiolmondayers absorbed on cuprous sulfide at controlled potential [J]. Langmuir, 1995, 11(7): 2787-2799.
(Edited by YANG You-ping)
Foundation item: Project(2006AA06Z120) supported by High-Technology Research and Development Program of China; Project(1343-74334000028) supported by the Graduate Student Education Innovation Project of Central South University, China
Received date: 2008-11-07; Accepted date: 2009-02-24
Corresponding author: HU Yue-hua, Professor; Tel: +86-731-88879815; E-mail: HYH@mail.csu.edu.cn
- Effect of organic depressant lignosulfonate calcium on separation of chalcopyrite from pyrite


