文章编号:1004-0609(2013)08-2221-08
电沉积Ni-S/LaNi5多孔复合电极的电催化析氢性能
王森林,段钱花
(华侨大学 材料科学与工程学院 应用化学系,厦门 361021)
摘 要:
采用复合电沉积结合碱溶制备Ni-S/LaNi5多孔复合电极,并采用SEM、EDS 和XRD等技术表征电极的表面形貌、元素组成和镀层的晶形结构。运用阴极极化曲线研究该电极在20%(质量分数)NaOH溶液中的析氢电催化性能,通过间断恒电位电解、长时间恒电位电解研究电极的稳定性,用长时间电解后开路电位和阳极极化探讨电极稳定性的原因。结果表明:与电沉积Ni-S多孔电极相比,Ni-S/LaNi5多孔复合电极具有较低的析氢过电位和电化学反应阻抗及较大的交换电流密度和比表面积。在Ni-S多孔及Ni-S/LaNi5多孔复合电极上,析氢反应的表观活化自由能分别为78.08和49.41 kJ/mol,Ni-S/LaNi5多孔复合电极表现出较高的析氢电催化活性和稳定性。
关键词:
中图分类号:TQ153.2 文献标志码:A
Electrocatalytic hydrogen evolution characters of electrodeposited Ni-S/LaNi5 porous composite electrode
WANG Sen-lin, DUAN Qian-hua
(Department of Applied Chemistry, College of Materials Science and Engineering, Huaqiao University, Xiamen 361021, China)
Abstract: The Ni-S/LaNi5 porous composite electrodes were prepared by composite electrochemical deposition technique in combination with alkaline dissolving. The corresponding micrographs, element component and structures of the Ni-S/LaNi5 porous composite electrode were characterized by scanning electron microscopy (SEM), energy dispersive X-ray (EDS) and X-ray diffractometry (XRD). Their electrocatalytic characteristics for hydrogen evolution reaction (HER) in 20% (mass fraction) NaOH solution were investigated by cathodic polarization curves (CPC). Their stabilization was studied by constant potential with discontinuity electrolysis and constant potential electrolysis. The reason of the electrode stabilization was discussed by open-circuit potential (OCP) after constant potential electrolysis and anodic polarization curves. The results show that the Ni-S/LaNi5 porous composite electrode for HER exhibits a lower overpotential and a higher exchange current density than the Ni-S porous electrode. The apparent activation energies of HER on the Ni-S porous and Ni-S/LaNi5 porous composite electrode are 78.08 and 49.41 kJ/mol, respectively. Thus, the Ni-S/LaNi5 porous composite electrode possesses a superior electrocatalytic activity and stability for HER corresponding to the Ni-S porous electrode.
Key words: LaNi5; porous composite electrode; composite electrodeposition; hydrogen evolution; electro-catalysis
能源可持续发展是当今社会共同关心的问题,氢能作为一种优越的新能源,在21世纪有可能在世界能源舞台上成为一种举足轻重的二次能源。其主要优点有燃烧热值高,燃烧的产物是水,是世界上最干净的能源;资源丰富,氢气可以由水制取,而水是地球上最为丰富的资源。氢由于具有以上特点可以同时满足资源环境和可持续发展的要求是其他能源所不能比拟的,所以备受重视[1]。
工业上生产氢的方式很多,常见的有水电解制氢、煤炭气化制氢、重油及天然气水蒸气催化转化制氢等。水电解是生产氢能的重要技术,只要提供一定形式和一定能量,则可使水分解。提供电能使水分解制得氢气的效率一般在75%~85%,其工艺过程简单,无污染,但消耗电量大,使得其应用受到一定的限制。为了降低制氢成本,就得寻找更加有效的析氢电极材料来降低析氢过电位和提高交换电流密度。
过渡族元素具有特殊的d电子层结构,是目前公认的电催化性能优良的电极材料[2],因而,该族元素是研究的主流,尤其是镍合金电极以制备方法简单、成本低,同时具有良好的电化学性能和较好的耐蚀性而得到广泛研究。近年来,具有吸/放氢能力的储氢合金,尤其是AB5型储氢合金,用作电解水析氢阴极受到了较大的关注,其原理在于储氢合金可以在电解过程中实现储氢功能,而在断电时这些吸附氢又可以在阴极发生放电反应,替代阴极合金组分的溶出,从而起到保护作用[3]。近年来,人们采用电沉积的方法制备许多二元及三元合金电极,如Ni-S[4-6]、Ni-P[7]、Ni-B[8-9]、Ni-Mo-Co[10]、Ni-Mo-P[11]、Ni-Mo-La[12]等,这些合金电极在电解析氢反应中均表现出较高的析氢活性和较好的稳定性。本文作者先通过一步复合电沉积将LaNi5和Al颗粒囊嵌到镀层中得到了Ni-S/ (LaNi5+Al)复合镀层, 然后采用碱溶法将镀层中的铝溶解掉制得Ni-S/LaNi5多孔复合镀层。采用SEM和XRD等表征电极结构,运用电化学手段研究电极的析氢电催化性能及电化学稳定性,并与相应的Ni-S多孔电极进行对比。
1 实验
1.1 电极制备
镀镍溶液组成及工艺条件如下:NiSO4·6H2O, 300 g/L;NiCl2·6H2O, 50 g/L;H3BO3, 40 g/L;硫脲(TU), 50 g/L。电流密度50 mA/cm2, pH为4~5,温度为45 ℃。所用试剂均为分析纯,镀液由蒸馏水配制。 阴极材料为1.7 cm×1.7 cm的黄铜片, 阳极为6 cm×9 cm的纯镍板, 将阴极和阳极平行置于镀液中。在未加LaNi5颗粒的镀液中加入处理好的铝粉制备Ni-S多孔镀层,在加有LaNi5和铝粉的镀液中制备Ni-S /LaNi5多孔复合镀层。
固体微粒预处理:铝粉30 g/L (平均粒径为10 μm),LaNi5, 25 g/L (平均粒径为20 μm)。将以上固体粉末混合放入pH值为8.0的弱碱中除油、过滤,用大量的蒸馏水进行冲洗,然后放在1 g/L的聚乙二醇中充分搅拌1.0 h,过滤,蒸馏水洗净,烘干[13]。复合电极制备:将处理好的固体微粒倒入电解液中,控制电镀液pH值在4.0~5.0,充分搅拌使得固体粉末均匀地悬浮于电解液中,进行电镀,分别得到了Ni-S/Al复合镀层和Ni-S/(Al+LaNi5)复合镀层。再将其置于60 ℃ 6 mol/L NaOH溶液中除铝,待无气泡冒出,将其取出,用蒸馏水清洗,吹干得到Ni-S多孔和Ni-S/LaNi5多孔复合镀层。
1.2 电极结构及性能测试
用日本Hitachi公司生产的S-3500N扫描电镜(SEM)观察电极的表面形貌,镀层成分分析用该扫描电镜附带的ISIS-300能谱仪(英国牛津公司)测定。结构分析在Panalytical X′pert PRO粉末X射(XRD)线衍射仪上进行,Cu Kα射线,测试所用基体为1.7 cm×1.7 cm×0.2 cm黄铜片,而镀层组成和表面形貌测试所用基体为1.7 cm×1.7 cm×0.05 cm紫铜片。
电化学测试在CHI-630D电化学综合测定仪上进行。采用三电极体系进行测量,使用玻璃三室电解槽(150 mL)。工作电极是自制的多孔复合电极,辅助电极使用3 cm×3 cm大面积铂电极,参比电极选用Hg/HgO电极(20% (质量分数) NaOH),电解液为20% (质量分数) NaOH溶液。
2 结果与分析
2.1 电极的表面形貌及结构
图1所示为不同镀层的表面形貌。由图1(a)可以看出,镀层表面不是非常平整;由图1(b)也可看到,在镀层表面很明显的看到很多大小不一的孔洞;由图1(c)可以看出,LaNi5复合在镀层表面,并也有很多孔洞,这些复合的LaNi5使得镀层的比表面积大大增大,由于LaNi5的复合,其带来的正协同效应[14]和大的比表面积使电催化活性大大增强。
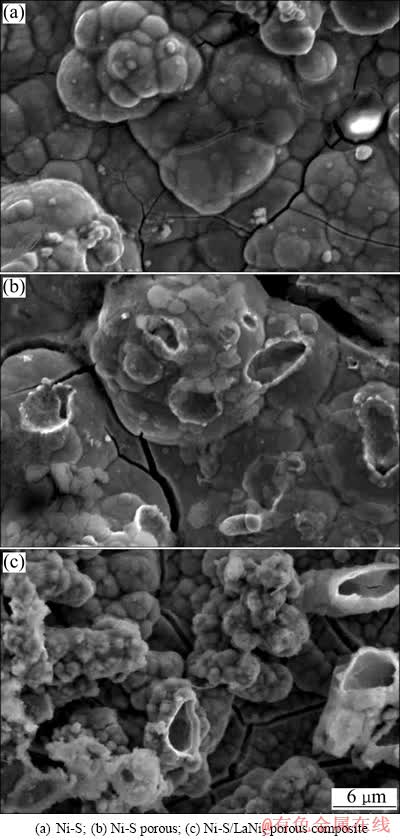
图1 不同镀层的表面形貌
Fig. 1 Surface morphologies of different coatings
图2所示分别为Ni-S多孔和Ni-S/LaNi5多孔复合镀层的能谱。由图2可看出,Ni-S多孔镀层中各元素摩尔分数分别为Ni 75.8%、S 24.2%;而Ni-S/LaNi5多孔复合镀层中各元素摩尔分数分别为Ni 76.78%、S 21.22%、La 2.0%。由La的含量可以推出,Ni-S/LaNi5多孔复合镀层中LaNi5相中的Ni占10%, 故Ni-S合金相中Ni占66.78%,S占21.22%。
图3所示为不同镀层的XRD谱。由图3可看出,Ni-S多孔镀层在44.758°时出现了Ni的衍射峰,空间晶形为面心立方(Fm3m),对应PDF卡号为65-2865;Ni-S/LaNi5多孔复合镀层在22.207°、30.234°、35.907°和42.5°的位置出现了LaNi5的衍射峰,空间晶形为六方密堆积(P6/mmm),对应PDF卡号为65-0093,在44.758°的位置同样出现Ni的衍射峰。两样品的XRD谱中均未出现S的衍射峰,说明S在样品中没有独立成相,而是与Ni一起形成了新相。两谱中均出现了馒头峰,说明随着S的加入,Ni和S的结构由晶体逐渐转化为晶体和非晶体的混合形式[15-16]。通过Jade软件分析,推测在49.3°、58.6°、72.3°的位置可能出现了Ni3S2的衍射峰,即在电沉积过程中,Ni和S可能形成金属间化合物。这种晶体和非晶体的混合结构,非晶体的短程有序长程无序晶形,使得致密、无序的原子堆积形式及所具有的空间结构使其表现出较高的化学活性。另外,非晶态合金的表面自由能较高,处于亚稳状态,这种亚稳态结构能有效地降低氢原子在金属表面吸附的活化能,这些都使非晶态合金较之同成分的晶态材料具有更高的催化活性[17]。
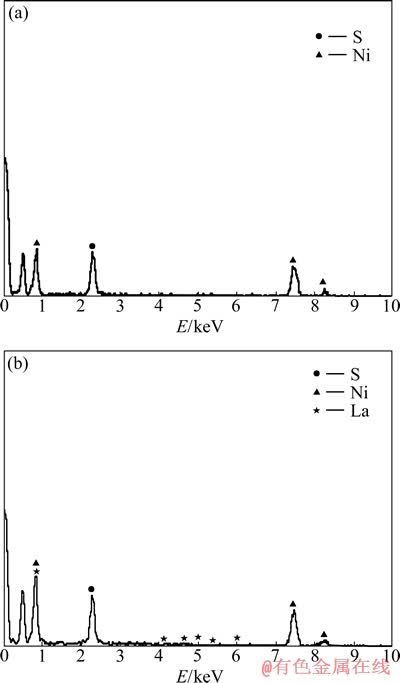
图2 Ni-S和Ni-S/LaNi5多孔复合镀层的EDS谱
Fig. 2 EDS spectra of Ni-S porous (a) and Ni-S/LaNi5 (b) porous composite
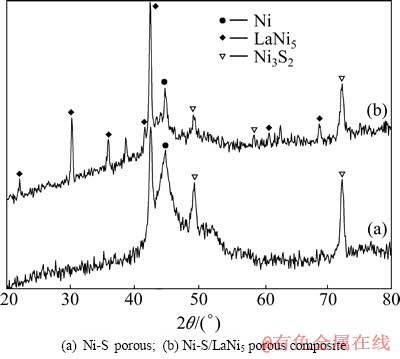
图3 不同镀层的XRD谱
Fig. 3 XRD patterns of different coatings
2.2 电极的电催化析氢性能
图4(a)所示为Ni-S多孔电极和Ni-S/LaNi5多孔复合电极常温下在20%的NaOH溶液中的阴极极化曲线。扫描范围为从开路电位到-1.3 V,其中Ni-S多孔电极的开路电位为-0.695 V,Ni-S/LaNi5多孔复合电极的开路电位为-0.745 V,扫描速度为1 mV/s。从图4(a)中的曲线B可以看出,Ni-S/LaNi5多孔复合电极从-1.02 V时开始大量析氢,而曲线ANi-S多孔电极在-1.08 V才开始大量析氢,即Ni-S/LaNi5多孔复合电极具有更正的起始析氢过电位。图4(b)所示为Ni-S/LaNi5多孔复合电极常温下在20%的NaOH溶液中不同扫描速度的阴极极化曲线。扫描范围为从开路电位到-1.3 V(vs Hg/HgO,以下相同),扫描速度分别为1、2、5、10、20和50 mV/s。从图中4(b)可以看出,随着扫描速度的加快,电流也随之增强。
为进一步测出两电极的析氢反应表观活化能,分别测定Ni-S多孔电极和Ni-S/LaNi5多孔复合电极在不同温度下的阴极极化曲线,扫描范围为从开路电位到-1.3 V,扫描速度为1 mV/s,温度测试范围30~60 ℃,如图5所示。图5(a)和(a′)所示为Ni-S多孔电极,图5(b)和(b′)所示为Ni-S/LaNi5多孔复合电极。从图5中可以看出,两电极的起始析氢电位都随着温度的升高而正移,即电极的电催化活性随着温度的升高而增强。图5(b′)曲线的斜率较图5(a′)的大,也说明了Ni-S/LaNi5多孔复合电极的电催化活性较Ni-S多孔电极高。
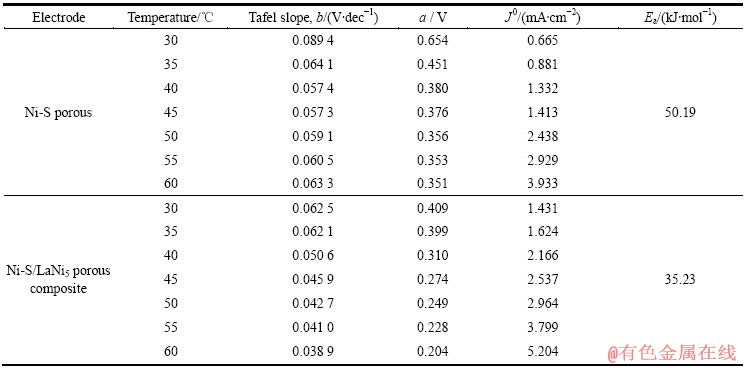
根据阴极极化曲线在极化较大时的数据,做φ—ln J的Tafel曲线,以电极的开路电位近似作为体系的平衡电位。根据Tafel关系式:φ = a + bln J,当阴极极化时,a=-[(RT)/(αnF)]lnJ 0,b=(RT)/(αnF),式中φ为过电位(φ=φocp-φ′,式中φocp为开路电位,φ′是电极电位),J为电流密度,T为温度,R为气体常数,n为转移电子数,F为法拉第常数,α为传递系数。通过Tafel曲线线性拟合,从而得到析氢过程的表观交换电流密度J 0,而J 0=Fkαexp[-Ea/(RT)],其中k为常数,Ea为表观活化自由能。通过测量不同温度下的交换电流密度J 0,将ln J 0对1/T作图则有dln J 0/d(1/T)= -Ea/R。通过计算可以得到电极析氢反应的表观活化能Ea,数据见表1。显然,随着温度的升高,Ni-S多孔电极和Ni-S/LaNi5多孔复合电极的交换电流密度都不断增强,也证明电极的析氢电催化活性随着温度的升高而增强。计算得到Ni-S/LaNi5多孔复合电极的表观活化能为35.23 kJ/mol,比Ni-S多孔电极表观活化能50.19 kJ/mol要低。这进一步说明Ni-S/LaNi5多孔复合比Ni-S多孔电极具有更好的电催化析氢活性。
2.3 电极的电解HER稳定性
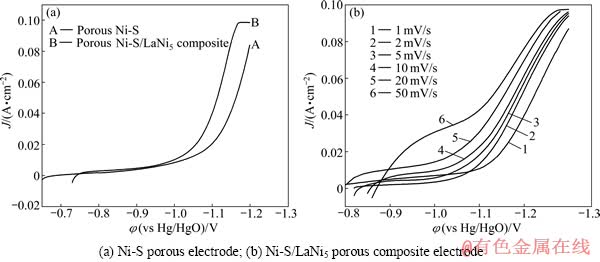
图4 不同电极的阴极极化曲线
Fig. 4 Cathodic polarization curves of different electrodes
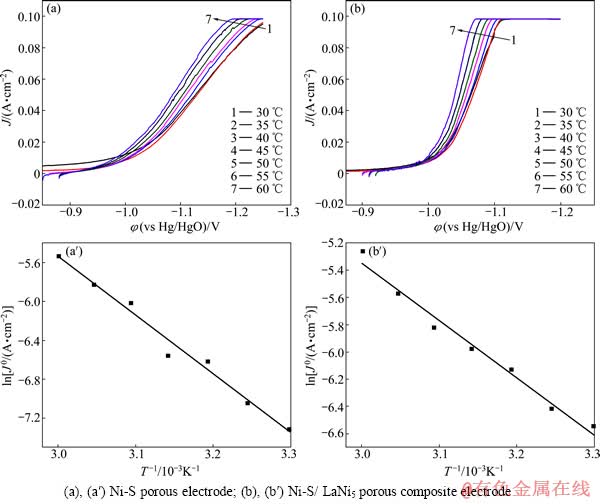
图5 不同电极在不同温度下的阴极极化曲线
Fig. 5 Cathodic polarization curves of different electrodes at different temperatures
表1 不同电极的电化学析氢反应动力学参数
Table 1 Kinetic parameters for electrochemical hydrogen evolution reaction of different electrodes
为了研究Ni-S/LaNi5多孔复合电极的稳定性,分别将Ni-S多孔电极和Ni-S/LaNi5多孔复合电极(两电极表观面积相同)常温下置于20% NaOH溶液中进行恒电位-1.3 V长时间电解,电解时间为20 h,得到图6(a)。由图6(a)中曲线A可以看出,Ni-S多孔电解的电流从0.05 A慢慢降低到0.03 A附近时趋于稳定, 电解16 h后又有所波动,电流再次稍有下降;而曲线B显示的Ni-S/LaNi5多孔复合电极的电解曲线较平稳,且电流也更大,基本维持在0.099 A附近。这说明Ni-S/LaNi5多孔复合电极具有更高的稳定性和电化学活性。图6(b)所示为Ni-S多孔电极和Ni-S/LaNi5多孔复合电极常温下在20% NaOH溶液中的恒电位-1.3 V下间断电解曲线。测试条件是电解2 h后间断1 h,以此循环,直到共间断电解10次结束。从图6(b)中可以看出, Ni-S多孔电极电解得到的曲线波动较大, 每次开始电解时表观电流都比较大,然后慢慢减小, 后趋于平稳,电流波动范围为0.03~0.05 A。而Ni-S/LaNi5多孔复合电极经过同样的10次间断电解,得到的曲线比较稳定,电流基本维持在0.099 A附近。说明相对于Ni-S多孔电极它具有更高的抗断电稳定性,这主要是因为LaNi5具有较强的吸附氢的能力,在断电时吸附的氢又释放出来,与空气中的氧气结合,有效抑制电极表面被氧气氧化,从而起到了保护电极的作用[18]。
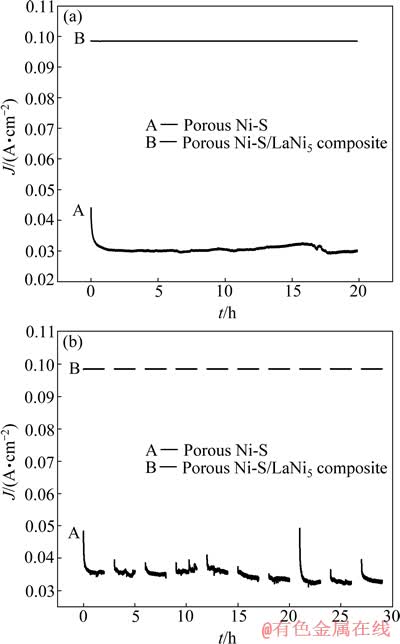
图6 不同电极的恒电位长时间电解和间断电解曲线
Fig. 6 Curves of different electrodes after constant potential electrolysis constant potential with long-time (a) and discontinuity (b) electrolysis
图7所示为Ni-S/LaNi5多孔复合电极常温下恒电位(-1.3 V)长时间电解后的开路电位随时间变化曲线,测试时间为30 min。由图7可看出,该电极的开路电位逐渐正移,随着时间的进行正移速度开始较快,15 min后变慢,曲线非常平缓;30 min后,开路电位仍保持在-0.88 V,这比电解前开路电位-0.32 V还要负,因为在电解的过程中电极不断析氢,使得在电解停止后该电极中LaNi5吸附的大量的原子氢而达到了饱和,这些吸附氢在电极断电后不断释放,因此电极的开路电位不断正移。且由于电极中LaNi5吸附大量氢,在断电后不断释放并与空气中的氧气结合,阻止电极金属相物质被空气氧化和腐蚀,维持电极的电催化活性,因此具有一定的抗断电性能[19]。
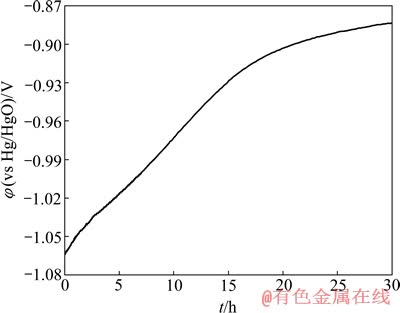
图7 Ni-S/LaNi5多孔复合电极的恒电位长时间电解后的开路曲线
Fig. 7 Open-circuit potential of Ni-S/LaNi5 porous composite electrode after constant potential electrolysis
为进一步测试该电极吸附氢的量,研究Ni-S/ LaNi5多孔复合电极常温下不饱和吸附氢和饱和吸附氢的阳极极化曲线如图8所示,曲线a为该电极不饱和吸附氢(制备态-电解前该电极在制备过程中也吸附了一定数量的氢)的阳极极化曲线,其开路电位为-0.32 V,阳极极化范围为-0.32 - 0.20 V,再将该电极在恒电位-1.3 V下电解30 min得到饱和吸附氢电极。曲线b为该电极饱和吸附氢后的阳极极化曲线,开路电位为-0.95 V,阳极极化范围为-0.95 - 0.20 V,扫描速度均为2 mV/s。从图8中可以看出,不饱和吸附氢电极只出现了一个氧化峰,在-0.03 V处;而饱和吸附氢电极在-0.68 V时出现了第一个氧化峰,在-0.015 V时出现了非常微弱氧化峰(Ni电极表面的氧化)[20]。第一氧化峰即为该电极吸附氢的氧化造成。从图8中还可以看出,饱和吸附氢电极具有很高的氧化峰电流,这是由于电解后该电极LaNi5吸附了大量的氢达到饱和状态,吸附氢在断电时不断的释放出来与氧气结合,阻止了Ni的氧化,电解前该电极在制备的过程中也吸附了一定的原子氢但没有达到饱和。
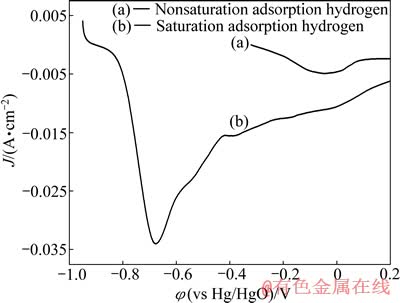
图8 Ni-S/LaNi5多孔复合电极饱和吸附氢和不饱和吸附氢的阳极极化曲线
Fig. 8 Anodic polarization curves of porous composite Ni-S/ LaNi5 electrode after nonsaturation hydrogen adsorption and saturation hydrogen adsorption
3 结论
1) 先复合电沉积制备复合Ni-S/Al和Ni-S/ (LaNi5+Al)镀层, 然后浓碱浸泡除铝分别得到Ni-S多孔和Ni-S/LaNi5多孔复合镀层。
2) Ni-S/LaNi5多孔复合电极比Ni-S多孔电极具有更低的析氢过电位、更高的电催化活性和更优异的抗断电稳定性.
REFERENCES
[1] 毛宗强. 氢能及其近期应用前景[J]. 科技导报, 2005, 23(2): 34-38.
MAO Zong-qiang. Moving towards hydrogen energy [J]. Science & Technology Review, 2005, 23(2): 34-38.
[2] 韩 庆, 魏绪均, 刘奎仁. 镍合金用作电解水析氢阴极的发展现状[J]. 中国有色金属学报, 2001,11(S1): 158-161.
HAN Qing, WEI Xu-jun, LIU Kui-ren. Development of nickel alloys as HER cathodes for water electrolysis [J]. The Chinese Journal of Nonferrous Metals, 2001, 11(S1): 158-161.
[3] XU Yan-hui, HE Guo-rong, WANG Xiao-lin. Hydrogen evolution reaction on the AB5 metal hydride electrode [J]. International Journal of Hydrogen Energy, 2003, 28(9): 961- 965.
[4] HAN Qing, JIN Yan, PU Nian-wen, LIU Kui-ren, CHEN Jian-she, WEI Xu-jun. Electro-chemical evolution of hydrogen on composite La-Ni-Al/Ni-S alloy film in water electrolysis [J]. Renewable Energy, 2010, 35(12): 2627-2631.
[5] YUAN Tie-chui, LI Rui-di, ZHOU Ke-chao. Electrocatalytic properties of Ni-S-Co coating electrode for hydrogen evolution in alkaline medium [J]. Transactions of Nonferrous Metals Society of China, 2007, 17(4): 762-765.
[6] SHAN Zhong-qiang, LIU Yan-jie, CHEN Zheng, WARRENDER G, TIAN Jian-hua. Amorphous Ni-S-Mn alloy as hydrogen evolution reaction cathode in alkaline medium [J]. International Journal of Hydrogen Energy, 2008, 33(1): 28-33.
[7] FEHOHI A E, ABDEL HAMEED R M, EL-KHATIB K M, SOUAYA E R. Ni-P and Ni-Co-P coated aluminum alloy 5251 substrates as metallic bipolar plates for PEM fuel cell applications [J]. International Journal of Hydrogen Energy, 2012, 37(9): 7677-7688.
[8] GUO Yong, LIU Xiao-hui, AZMAT M U, XU Wen-jie, REN Jia-wen, WANG Yan-qin. Hydrogen production by aqueous- phase reforming of glycerol over Ni-B catalysts [J]. International Journal of Hydrogen Energy, 2011, 37(1): 227-234.
[9] FERNANDES R, PATEL N, MIOTELLO A, FILIPPI M. Studies on catalytic behavior of Co-Ni-B in hydrogen production by hydrolysis of NaBH4 [J]. Journal of Molecular Catalysis A: Chemical, 2009, 298(1): 1-6.
[10] 王国庆, 尉海军, 朱 磊, 简旭宇. Ni-Mo-Co合金电极制备工艺的研究[J]. 稀有金属与硬质合金, 2011, 39(1): 21-24.
WANG Guo-qing, YU Hai-jun, ZHU Lei, JIAN Xu-yu. Study on process for Ni-Mo-Co alloy electrode preparation [J]. Rare Metals and Cemented Carbides, 2011, 39(1): 21-24.
[11] 李 聪, 许洪胤, 杜朝军, 郑盛会. 电沉积Ni-Mo-P合金及其析氢电催化性能的研究[J]. 电镀与环保, 2005, 25(1):10-11.
LI Cong, XU Hong-yin, DU Chao-jun, ZHENG Sheng-hui. A study of Ni-Mo-P alloy electrodeposition and its electro-catalytic properties for hydrogen evolution [J]. Electroplating & Pollution Control, 2005, 25(1): 10-11.
[12] 高诚辉, 李 凝. 电沉积非晶/纳米晶Ni-Mo-La合金电极的析氢性能[J]. 中国有色金属学报, 2011, 21(11): 2819-2824.
GAO Cheng-hui, LI Ning. Hydrogen evolution reaction activity of electrodeposited amorphous/nanocrystalline Ni-Mo-La alloy electrode [J]. The Chinese Journal of Nonferrous Metals, 2011, 21(11): 2819-2824.
[13] 王森林, 张 艺. Ni-Mo/LaNi5多孔复合电极的制备及其电催化析氢性能[J]. 物理化学学报, 2011, 27(6): 1417-1423.
WANG Sen-lin, ZHANG Yi. Preparation and electrocatalytic performance of Ni-Mo/LaNi5 porous composite electrode toward hydrogen evolution reaction [J]. Acta Physico-Chimica Sinica, 2011, 27(6): 1417-1423.
[14] CHEBOTAREVA N, NYOKONG T.First-row transition metal phthalocyanines as catalysts for water electrolysis: A comparative study [J]. Electrochimica Acta, 1997, 42: 3519- 3524.
[15] 李爱昌, 骆鹏飞, 刘 瑛. 电沉积制备(Ni-W-P)-TiO2纳米复合电极的催化析氢性能[J]. 中国有色金属学报, 2010, 20(4): 712-717.
LI Ai-chang, LUO Peng-fei, LIU Ying. Hydrogen evolution properties of (Ni-W-P)-TiO2 composite coating as electrode materials prepared by electrolytic co-deposition [J]. The Chinese Journal of Nonferrous Metal, 2010, 20(4): 712-717.
[16] 曹寅亮, 王 峰, 刘景军, 王建军, 张良虎, 覃事永. 镍硫析氢活性阴极的电化学制备及其电催化机理[J]. 物理化学学报, 2009, 25(10): 1979-1984.
CAO Yin-liang, WANG Feng, LIU Jing-jun, WANG Jian-jun, ZHANG Liang-hu, QIN Shi-yong. Electrochemical preparation and electrocatalytic mechanisms of Ni-S active cathode for hydrogen evolution [J]. Acta Physico-Chimica Sinica, 2009, 25(10): 1979-1984.
[17] FEHOHI A E, ABDEL HAMEED R M, EL-KHATIB K M, SOUAYA E R. Study of different aluminum alloy substrates coated with Ni-Co-P as metallic bipolar plates for PEM fuel cell applications [J] International Journal of Hydrogen Energy, 2012, 37(14): 10807-10817.
[18] YANG Shu-qin, HAN Shu-min, SONG Jian-zheng, LI Yuan. Influences of molybdenum substitution for cobalt on the phase structure and electrochemical kinetic properties of AB5-type hydrogen storage alloys [J]. Journal of Rare Earths, 2011, 29(7): 692-697.
[19] 韩 庆, 陈建设, 刘奎仁, 魏绪钧. 复合型LaNi5/Ni-S合金镀层在碱液中的析氢反应[J]. 金属学报, 2008, 7(44): 887-891.
HAN Qing, CHEN Jian-she, LIU Kui-ren, WEI Xu-jun. Hydrogen evolution reaction of the composite LaNi5/Ni-S alloy film in alkaline medium [J]. Acta Metallurgica Sinica, 2008, 7(44): 887-891.
[20]  VEGA-BECERRA O, AGUILAR-
VEGA-BECERRA O, AGUILAR-  A. The synthesis of Ni-activated carbon nanocomposites via electroless deposition without a surface pretreatment as potential hydrogen storage materials [J]. International Journal of Hydrogen Energy, 2012, 37(14): 10743- 10749.
A. The synthesis of Ni-activated carbon nanocomposites via electroless deposition without a surface pretreatment as potential hydrogen storage materials [J]. International Journal of Hydrogen Energy, 2012, 37(14): 10743- 10749.
(编辑 李艳红)
收稿日期:2012-12-27;修订日期:2013-06-24
通信作者:王森林,教授,博士;电话:0595-22693746;E-mail: slwang@hqu.edu.cn
摘 要:采用复合电沉积结合碱溶制备Ni-S/LaNi5多孔复合电极,并采用SEM、EDS 和XRD等技术表征电极的表面形貌、元素组成和镀层的晶形结构。运用阴极极化曲线研究该电极在20%(质量分数)NaOH溶液中的析氢电催化性能,通过间断恒电位电解、长时间恒电位电解研究电极的稳定性,用长时间电解后开路电位和阳极极化探讨电极稳定性的原因。结果表明:与电沉积Ni-S多孔电极相比,Ni-S/LaNi5多孔复合电极具有较低的析氢过电位和电化学反应阻抗及较大的交换电流密度和比表面积。在Ni-S多孔及Ni-S/LaNi5多孔复合电极上,析氢反应的表观活化自由能分别为78.08和49.41 kJ/mol,Ni-S/LaNi5多孔复合电极表现出较高的析氢电催化活性和稳定性。
[1] 毛宗强. 氢能及其近期应用前景[J]. 科技导报, 2005, 23(2): 34-38.
MAO Zong-qiang. Moving towards hydrogen energy [J]. Science & Technology Review, 2005, 23(2): 34-38.
[2] 韩 庆, 魏绪均, 刘奎仁. 镍合金用作电解水析氢阴极的发展现状[J]. 中国有色金属学报, 2001,11(S1): 158-161.
[10] 王国庆, 尉海军, 朱 磊, 简旭宇. Ni-Mo-Co合金电极制备工艺的研究[J]. 稀有金属与硬质合金, 2011, 39(1): 21-24.
[11] 李 聪, 许洪胤, 杜朝军, 郑盛会. 电沉积Ni-Mo-P合金及其析氢电催化性能的研究[J]. 电镀与环保, 2005, 25(1):10-11.
[12] 高诚辉, 李 凝. 电沉积非晶/纳米晶Ni-Mo-La合金电极的析氢性能[J]. 中国有色金属学报, 2011, 21(11): 2819-2824.
[13] 王森林, 张 艺. Ni-Mo/LaNi5多孔复合电极的制备及其电催化析氢性能[J]. 物理化学学报, 2011, 27(6): 1417-1423.
[15] 李爱昌, 骆鹏飞, 刘 瑛. 电沉积制备(Ni-W-P)-TiO2纳米复合电极的催化析氢性能[J]. 中国有色金属学报, 2010, 20(4): 712-717.
[16] 曹寅亮, 王 峰, 刘景军, 王建军, 张良虎, 覃事永. 镍硫析氢活性阴极的电化学制备及其电催化机理[J]. 物理化学学报, 2009, 25(10): 1979-1984.
[19] 韩 庆, 陈建设, 刘奎仁, 魏绪钧. 复合型LaNi5/Ni-S合金镀层在碱液中的析氢反应[J]. 金属学报, 2008, 7(44): 887-891.


