
Effect of Nb in (La0.5Ce0.5)64-xAl16Ni5Cu15Nbx (x=0-5) bulk metallic glasses
ZHANG Guo-qing(张国庆)1,2, LIU Jin-fang(刘金芳)1, ZENG Qiao-shi(曾乔石)1,
WANG Li-na(汪丽娜)2, LIU Jin-qiang(刘今强)2, JIANG Jian-zhong(蒋建中)1
1. Laboratory of New-Structured Materials, Department of Materials Science and Engineering, Zhejiang University, Hangzhou 310027, China;
2. Key Laboratory of Advanced Textile Materials and Manufacturing Technology, Ministry of Education,
Zhejiang Sci-Tech University, Hangzhou 310018, China
Received 20 April 2006; accepted 30 June 2006
Abstract:
The effect of Nb in (La0.5Ce0.5)64-xAl16Ni5Cu15Nbx (x=0-5, mole fraction) bulk metallic glasses was investigated by X-ray diffractometry, differential scanning calorimetry, and scanning electron microscopy. Fully amorphous rods up to 5 mm in diameter were obtained using copper mold. Their lower glass transition temperatures are of about 401-407 K and wide supercooled liquid regions are up to 75 K. The oxidation resistance of the LaCe-based glassy alloys can be largely enhanced by adding tiny Nb, which makes the developed LaCe-based bulk metallic glasses more attractive for potential industrial applications.
Key words:
(La0.5Ce0.5)64AlNi5Cu15-Nb; bulk metallic glasses; oxidation resistance; casting;
1 Introduction
Since 1990 many bulk metallic glasses (BMGs) have been prepared by conventional casting at a critical cooling rate of less than 100 K/s[1]. The materials usually have excellent mechanical properties to be superior to those of their crystalline counterparts. They often have large supercooled liquid region and good corrosion resistance [2]. Bulk metallic glasses with a critical casting thickness of 10 mm in rod shape have been found in several systems, i.e. Pd-Ni-P[3], Zr-Ti(Nb)- Cu-Ni-Al[4], Pd-Cu-Ni-P[5], Y-Sc-Al-Co(Ni)[6], Zr-Al- Cu-Ni[7], Zr-Ti-Al-Cu-Ni[8], Zr-Ti-Cu-Ni-Be, Nd-Fe- Al[9], La-Al-Ni-Cu-Co[10], Ca–Mg–Ni(Zn)[11] and Fe-Cr-Co-Mo(Mn)-C-B-Y[12,13].
Rare-earth-based metallic alloys are known for their chemical simplicity and fairly good glass forming ability (GFA). Ternary La-Al-Ni [14], La-Al-Cu [15] and Nd–based alloys can form amorphous rods with diameters up to 6 mm. Recently, a series of Ce-, La- and Pr-based BMGs were found with relative good GFA[16-20]. They have very low glass transition temperatures (below 450 K). ZHANG et al[18-20] reported that Ce70Al10Cu20 and Ce70Al10Cu20Nb2 systems have glass transition temperature, Tg=341 K and wide supercooled liquid region, ΔT=Tx-Tg= 60-81 K, where Tx is crystallization temperature. The lowest glass transition temperature of 326 K was reported in a (Ce0.72Cu0.28)97.5Al2.5 alloy by BIAN and INOUE[21]. BMG alloys with low glass transition temperatures and stable supercooled liquid state are ideal systems for studying glass transition phenomenon. Recently, we developed a (La0.5Ce0.5)64Al16Ni5Cu15 bulk metallic glass with diameters up to 10 mm, synthesized by a copper mold casting method. The BMG alloy shows a glass transition temperature of 403 K and a supercooled liquid region of 60 K. The hardness of the BMG alloy is around 1.9 GPa at ambient temperature. The material exhibits large plasticity and imprint ability feature in the supercooled liquid region. However, the BMG alloys exhibit relative poor oxidation resistance. In this paper, we report the effect of Nb on glass forming ability, thermal stability and oxidation resistance in (La0.5Ce0.5)64-xAl16Ni5Cu15Nbx (x=0-5) bulk metallic glasses.
2 Experimental
All ingots of (La0.5Ce0.5)64-xAl16Ni5Cu15Nbx (x=0, 2, 3, 4 and 5, mole fraction, the same below if not mealioned) alloys were prepared by arc-melting a mixture of pure La (99.5%), Ce (99.5%), Al (99.95%), Ni (99.98%) and Cu (99.9%) in a Zr-gettered argon atmosphere. Each of the master ingots was melted five times, mechanically polished and sucked into a copper mold. From these ingots, ribbon samples of about 30 μm thickness and 2 mm width were prepared by melt-spinning on a single copper roller at a speed of 20 m/s. The structure of the samples was examined by X-ray diffractometry(XRD) with monochromatic Cu Kα radiation. The XRD patterns were recorded from 20°to 80°with a step of 0.02°and integration time of 1 s. Phase transformations were studied by differential scanning calorimetry (DSC) at a heating rate of 0.33 K/s.
Oxidation treatments of (La0.5Ce0.5)64Al16Ni5Cu15 and (La0.5Ce0.5)62Al16Ni5Cu15Nb2 BMGs were carried out by an isothermal annealing at 393 K in DSC in air. The air-annealed samples were further examined by XRD, scanning electron microscopy (SEM) equipped with an energy-dispersive spectroscopy (EDS).
3 Results and discussionFig.1 shows the typical XRD patterns of 10 mm-diameter (La0.5Ce0.5)64Al16Ni5Cu15 BMG rod and a series of (La0.5Ce0.5)64-xAl16Ni5Cu15Nbx (x=2, 3, 4 and 5) BMG rods (with 5 or 4 mm in diameter). It is found that the LaCe-based BMG alloys containing Nb still have good GFA although Nb addition decreases the critical

Fig.1 XRD patterns for (La0.5Ce0.5)64-xAl16Ni5Cu15Nbx (x=0, 2, 3, 4 and 5) bulk metallic glasses with various diameters
size for forming fully amorphous rods.
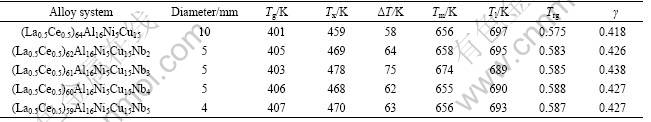
Fig.2 shows the DSC curves of (La0.5Ce0.5)64-xAl16-Ni5Cu15Nbx (x=0, 2, 3, 4 and 5) BMG alloys at a heating rate of 0.33 K/s. The thermal parameters such as glass transition temperature(Tg), crystallization temperature(Tx), melting (Tm) and liquidus temperature (Tl), together with supercooled liquid region, ΔT=Tx-Tg, reduced glass transition temperature Trg=Tg/Tl and γ value (γ= Tx/(Tg+Tl))[22], are listed in Table 1. The addition of Nb strongly affects supercooled liquid region from 58 K for (La0.5Ce0.5)61Al16Ni5Cu15 to 75 K for (La0.5Ce0.5)61Al16-
Ni5Cu15Nb3. By adding 3% Nb(mole fraction), the alloy exhibits single melting peak, indicating that this composition is close to an eutectic composition. Nb addition also slightly decreases liquidus temperature in the system and increases Trg and γ.
Heating treatments at a temperature below glass transition can relax amorphous alloys. In order to study the relaxation effect on structure, two BMG alloys, (La0.5Ce0.5)64Al16Ni5Cu15 and (La0.5Ce0.5)62Al16Ni5Nb2-
alloys, were annealed in silicone oil at 373 K for various times.
Fig.3 shows the DSC patterns for (La0.5Ce0.5)64Al16-Ni5Cu15 and (La0.5Ce0.5)62Al16Ni5 Cu15Nb2 samples after

Fig.2 DSC curves for (La0.5Ce0.5)64-xAl16Ni5Cu15Nbx glassy alloys at heating rate of 0.33 K/s in pure argon flow: (a) Low temperature part; (b) High temperature part
Table 1 Values of Tg, Tx, Tl, Tm, Trg, γ, ΔT and critical diameters of fully amorphous rods of LaCe-based BMGs prepared by copper mold
heat treatments at 373 K for various times. Both alloys annealed at 373 K for 12 h still remain amorphous, as confirmed by the XRD patterns shown in the insert in Fig.3. It is found that relaxation results in the recovery of enthalpy of metastable glassy alloys, the reduction of Tx, ΔT and the splitting of crystallization peak. In addition, we further performed XRD measurements for two (La0.5Ce0.5)64Al16Ni5Cu15 BMG alloys, one is as-prepared sample heated to 459 K (Tx) in DSC and the other is pre-annealed at 373 K for 12 h which was annealed at 455 K (Tx) in DSC. Both XRD patterns show almost the same crystallization phases. More experiments are

Fig.3 DSC patterns for (La0.5Ce0.5)64Al16Ni5Cu15(a) and (La0.5Ce0.5)62Al16Ni5Cu15Nb2(b) glassy alloys annealed at 373 K for different time(Inserts are XRD patterns of samples pre-annealed at 373 K for 12 h)required to understand the splitting of the crystallization peak due to relaxation.
It is known that the addition of Nb can often improve the oxidation resistance of metallic glasses. Thus, in order to enhance oxidation resistance of the LaCe-based BMGs, we add Nb in our system. Oxidation processes of (La0.5Ce0.5)64Al16Ni5Cu15 and (La0.5Ce0.5)62-Al16Ni5Cu15Nb2 were examined by isothermal DSC measurements at 393 K in air. It is clear that an exothermic peak in Fig.4(a), which is attributed to oxidation-induced crystallization in Figs.4(b) and (c), was detected after about 75 min in the Nb-free BMG alloy while only a flat DSC curve in Fig.4(a) was observed for Nb-containing BMG alloy. Amorphous structure still remains for at least 120 min for the Nb-containing BMG alloy in Figs.4(d) and (e). Oxygen element distribution in fracture surface of (La0.5Ce0.5)64-Al16Ni5Cu15 and (La0.5Ce0.5)62Al16 Ni5Cu15Nb2, annealed for 60 min in air, are shown in Figs.5(b) and (c). The oxygen content vs distance is shown in Fig.5(d). The deeper into inside of the specimens, the less oxygen concentration is. It is clear that Nb addition can retard oxidation.
4 ConclusionsThe effect of Nb in the (La0.5Ce0.5)64-xAl16Ni5Cu15-Nbx (x=0, 2, 3, 4 and 5%) system was investigated by XRD, DSC, and SEM. It is found that the Nb addition increases the supercooled liquid region of (La0.5Ce0.5)64Al16Ni5Cu15 bulk glassy alloy from 58 K to 75 K while fully amorphous rods with diameter up to 5 mm are still obtained. The oxidation resistance of the LaCe-based glassy alloys can be largely enhanced by the addition of 2% Nb, which makes the developed LaCe-based bulk metallic glasses more attractive for potential industrial applications.

Fig.4 Isothermal DSC potterns for (La0.5Ce0.5)64Al16Ni5Cu15 and (La0.5Ce0.5)62Al16Ni5Cu15Nb2 glassy alloys at 393 K in air(a), SEM micrographs (b) and (d) and XRD patterns (c) and (e) for samples after oxidation at 393 K for various time, ((b) and (c) are from Nb-free (La0.5Ce0.5)64Al16Ni5Cu15 sample annealed at 393 K in air for 80 min, (d) and (e) are from Nb-containing (La0.5Ce0.5)62Al16Ni5Cu15Nb2 sample annealed at 393 K in air for 120 min)

Fig.5 SEM image of fracture plane(a) and mappings of oxygen element for (La0.5Ce0.5)64Al16Ni5Cu15(b) and (La0.5Ce0.5)62-
Al16Ni5Cu15Nb2(c), and oxygen contents as function of distance from surface to inside of specimen(d)
References[1] INOUE A. High Strength Bulk Amorphous Alloys with Low Critical Cooling Rates[J]. Materials Transactions, JIM, 1995, 36(7): 866-875.
[2] INOUE A. Stabilization of metallic supercooled liquid and bulk amorphous alloys [J].Acta Materialia, 2000, 48: 279-306.
[3] KUI H W, GREER A L, TURNBULL D. Formation of bulk metallic glass by fluxing [J]. Applied Physics Letters, 1984, 45: 615-616.
[4] LIN X H, JOHNSON W L, RHIM W K. Effect of oxygen impurity on crystallization of an undercooled bulk glass forming Zr-Ti-Cu-Ni-Al Alloy [J]. Materials Transactions, JIM, 1997, 38(5): 473-477.
[5] INOUE A, NISHIYAMA N, KIMURA H. Preparation and thermal stability of bulk amorphous Pd40Cu30Ni10P20 alloy cylinder of 72 mm in diameter [J]. Materials Transactions, JIM, 1997, 38(2): 179-183.
[6] GUO F Q, JOSEPH POON S, GARY J. Metallic glass ingots based on yttrium [J]. Applied Physics Letters, 2003, 83: 2575-2577.
[7] INOUE A, ZHANG T, NISHIYAMA N, OHBA K, MASUMOTO T. Preparation of 16 mm diameter rod of amorphous Zr65Al7.5Ni10Cu17.5 alloy [J]. Materials Transactions, JIM, 1993, 34(12): 1234-1237.
[8] XING Q L, OCHIN P, HARMELIN M, FAUDOT F, BIGOT J. Alloys of high glass-forming ability[J]. Journal of Non-Crystalline Solids, 1996, 205-207: 597-601.
[9] Inoue A, Zhang T. Thermal stability and glass-forming ability of amorphous Nd-Al-TM (TM = Fe, Co, Ni or Cu) alloys[J].Mater Sci Eng A, 1997, A226-228: 393-396.
[10] TAN H, LU Z P, YAO H B, YAO B, FENG Y P, LI Y. Glass forming ability of La-rich La-Al-Cu ternary alloys [J]. Materials Transactions, JIM, 2001, 42(4): 551-555.
[11] PARK E S, KIM D H. Effect of atomic configuration and liquid stability on the glass-forming ability of Ca-based metallic glasses [J]. Applied Physics Letters, 2005, 86: 201912
[12] SHEN J, CHEN Q J, SUN J F, FAN H B, WANG G. Exceptionally high glass-forming ability of an FeCoCrMoCBY alloy[J]. Applied Physics Letters, 2005, 86: 151907.
[13] LU Z P, LIU C T, THOMPSON J R, PORTER W D. Structural amorphous steels[J]. Physics Review Letter, 2004, 92: 245503.
[14] Inoue A, YAMAGUCHI H, ZHANG T, MASUMOTO T. Al-La-Ni amorphous alloys with a wide supercooled liquid region[J]. Materials Transactions, JIM, 1989, 30(12): 956-972.
[15] INOUE A, ZHANG T, MASUMOTO T. Al-La-Cu amorphous alloys with a wide supercooled liquid region [J]. Materials Transactions, JIM, 1990, 31(2): 104-109.
[16] ZHANG Y, TAN H, LI Y. Bulk glass formation of 12 mm rod in La-Cu-Ni-Al alloys [J]. Mater Sci Eng A, 2004, A375-377: 436-439.
[17] TAN H, ZHANG Y, MA D, FENG Y P, LI Y. Optimum glass formation at off-eutectic composition and its relation to skewed eutectic coupled zone in the La based La-Al-(Cu,Ni) pseudo ternary system[J]. Acta Materialia, 2003, 51: 4551-4561.
[18] ZHANG B, ZHAO D Q, PAN M X, WANG W H, GREER A L. Amorphous metallic plastic [J]. Physics Review Letter, 2005, 94: 205502.
[19] ZHANG B, WANG R J, ZHAO D Q, PAN M X, WANG W H. Properties of Ce-based bulk metallic glass-forming alloys[J]. Physical Review B, 2004,70: 224208.
[20] ZHAO Z F, ZHANG Z, WEN P, PAN M X, ZHAO D Q, WANG W H, WANG W L. A highly glass-forming alloy with low glass transition temperature[J]. Applied Physics Letters, 2003, 82: 4699-4701.
[21] BIAN Z, INOUE A. Ultra-low glass transition temperatures in Ce-Based bulk metallic glasses [J]. Materials Transactions, JIM, 2005, 46 (8): 1857-1860.
[22] LU Z P, LIU C P. Glass formation criterion for various glass-forming systems[J]. Physics Review Letter, 2003, 91: 115505.
Foundation item: Projects(50341032; 50425102) supported by the National Natural Science Foundation of China; projects(2004/249/37-14; 2004/250/31-01A) supported by the Ministry of Science and Technology of China; project supported by the Key Laboratory of Advanced Textile Materials and Manufacturing Technology, Zhejiang Sci-Tech University
Corresponding author: JIANG Jian-zhong; Tel: +86-571-87952107; E-mail: jiangjz@zju.edu.cn


